Back to Journals » Cancer Management and Research » Volume 13
Esophagogastric Cancer After Sleeve Gastrectomy: A Systematic Review of Case Reports
Authors Chen W , Wang Y, Zhu J, Wang C, Dong Z
Received 24 January 2021
Accepted for publication 4 March 2021
Published 15 April 2021 Volume 2021:13 Pages 3327—3334
DOI https://doi.org/10.2147/CMAR.S303590
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Bilikere Dwarakanath
Wenhui Chen,* Yucheng Wang,* Jie Zhu, Cunchuan Wang, Zhiyong Dong
Department of Gastrointestinal Surgery, The First Affiliated Hospital of Jinan University, Guangzhou, 510630, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhiyong Dong
Department of Bariatric Surgery, The First Affiliated Hospital of Jinan University, No. 613 Huangpu Avenue West, Guangzhou, 510630, People’s Republic of China
Tel/Fax +86 20 38688608
Email [email protected]
Introduction: Sleeve gastrectomy (SG) is the most commonly performed bariatric procedure. It has been shown that bariatric surgery reduces cancer risk. However, the risk of esophagogastric cancer after SG has not been defined yet and the development of cancer in the esophagus and stomach remains a matter of concern.
Methods: Web of Science, PubMed and Embase databases were searched. Articles that described the diagnosis and management of esophageal or gastric cancer after SG were considered.
Results: Seventeen esophagogastric cancer patients after SG were included. The age of the patients ranged from 21 to 64 years. Tumors were diagnosed after an interval of 33.9 ± 22.8 months from SG (range 4 months– 96 months). There were 4 esophageal cancers,4 gastroesophageal cancers and 9 gastric cancers; adenocarcinoma was the most frequent tumor histology (88.2%). The most commonly reported symptoms were food intolerance/dyspepsia (50.0%), vomiting/nausea/regurgitation (35.7%). Upper gastrointestinal endoscopy (UGIE) with biopsy was used for diagnosis in most of the patients. Surgery was performed in 10 patients (58.8%), while 4 patients were treated by endoscopic procedures (23.5%). The mean follow-up length was 12.2 months (range 3 months– 36 months) and the overall disease-free survival rate was 88.9%.
Conclusion: The development of esophagogastric cancer after SG is still not well defined but it may occur at any time. Preoperative and follow-up UGIE are essential in order to allow for prevention, early diagnosis. Further epidemiologic studies are needed to investigate the post-SG-related risk of esophagogastric cancer development.
Keywords: sleeve gastrectomy, esophageal cancer, gastric cancer, obesity
Introduction
Obesity is one of the major health problems over all the world. Epidemiologic studies have identified high body mass index substantially increases the risk of several cancers, including esophageal cancer, gastric cancer.1 Some underlying mechanisms, involving adipose tissue inflammation, endocrine and immune alterations, link obesity to cancer development.2,3 Bariatric surgery reduces cancer risk along with weight reduction.4 However, some studies showed that bariatric surgery is associated with decreased risk of hormone-related cancers, such as breast, endometrium and prostate cancer, whereas gastric bypass might increase the risk of colorectal cancer.5 Another study also showed that bariatric surgery is associated with a persistent increase in early-onset colorectal cancer trends.6 In fact, a number of esophageal and/or gastric cancer after bariatric surgery have been also reported in literature,7,8 but the influence of bariatric surgery on the cancers is unclear.
Sleeve gastrectomy (SG) has become the most commonly performed bariatric procedure for the treatment of morbid obesity worldwide.9 Its main strengths are technical simplicity, effective resolution of co-morbidities, the low rate of complications, the lack of gastrointestinal anastomosis and malabsorption,10 but there is concern over some possible complications such as gastroesophageal reflux disease (GERD), which is the main risk factors of esophagogastric cancer. Clinical practice guidelines endorsed by the International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) recommend preoperative upper gastrointestinal endoscopy (UGIE) should be considered in possibly all patients eligible for SG,11,12 but there are still controversies after surgery. The malignancies after SG are considered rare, but some esophageal or gastric neoplasms are still reported.7 It is not clear whether there is a relation between SG and the development of esophagogastric cancers.
The purpose of this systematic review is to summarize epidemiological, histological and clinical feature about esophagogastric cancers after SG, and to determine prevention strategies for high-risk populations.
Materials and Methods
A systematic review was performed using the PubMed, EMBASE and Web of Science databases to identify all English-written published articles about the development of Esophagogastric Cancer after SG up to December 10, 2020. The retrieval strategy was conducted using the following search terms: (neoplasia OR neoplasm OR Tumor* OR Cancer* OR malignancy OR adenocarcinoma) AND (esophageal OR esophagus OR gastric OR stomach) AND (sleeve gastrectomy OR SG OR LSG). Additionally, we also manually screened the references of eligible articles and reviews for other potential articles. All abstracts retrieved were screened and full text acquired for relevant studies. Reviews, comments, conference abstract, nonhuman studies and reports of cancer after bariatric surgery different from SG were excluded. Tumors existing before surgery or at the time of surgery were omitted.
Data extraction from eligible studies was completed independently by two authors (CWH and WYC) using a pre-specified data extraction form: first author, publication year, country, clinical and demographic characteristics of patients’ population, type of surgical procedure, and outcomes.
Results
The flow diagram of study selection procedure is displayed in Figure 1. A total of 1068 potentially relevant articles were retrieved in different databases. After removal of duplicate articles, 795 articles were left. Afterwards, we evaluated titles and abstracts, and reviewed full-text. Finally, 17 patients reported cancer arising in the esophagus or the stomach after SG were found in 15 articles.13–27 There were 4 esophageal cancers,4 gastroesophageal cancers and 9 gastric cancers. The characteristics of patients are summarized in Tables 1 and 2. Eleven patients were female (64.7%) and six were male (35.3%); the age of the patients ranged from 21 to 64 years. The body mass index (BMI) prior to SG were available in 16 patients, mean BMI was 47.0 (35.8–75.6) kg/m2. Cancer was diagnosed after an interval of 33.9 ±22.8 months from SG, ranging 4 months to 96 months. The duration of symptoms was reported in 9 patients and ranged from 2 months to 60 months. The BMI at the time of cancer diagnosis was 36.3 (23.8–70.6) kg/m2 in 10 patients.
 |
Table 1 The Basic Condition of the Patient Before Sleeve Gastrectomy |
 |
Table 2 Demographic, Clinical, and Operative Data of the Patient Population |
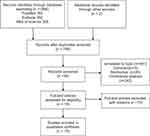 |
Figure 1 PRISMA flow diagram for selection of studies. |
The adenocarcinoma was reported in 4 patients who had not performed the preoperative upper gastrointestinal endoscopy (UGIE). Preoperative detection of Helicobacter pylori (Hp) was available only in 9 patients, but HP was positive in one patient. Two patients had chronic gastritis like chronic atrophic gastritis, erosive gastritis before SG. Three cases of cancers after SG occurred in patients who had previously undergone other gastric procedures (gastric pacemaker implantation and intragastric balloon).14,18,25 One patient had a history of pancreas transplant (immunocompromised). Five of the patients were smokers. Other reported risk factors for cancer included family history of upper gastrointestinal cancer, alcohol abuse, immune insufficiency. Most frequent reported comorbidities were obstructive sleep apnea (OSA), type 2 diabetes mellitus (T2DM), hypertension, dyslipidemia, GERD, hyperlipidemia, and gastritis (Table 1).
The tumor was located in the distal esophagus in four patients, in the gastroesophageal junction in four patients, in the gastric body and/or antrum in six patients, in the gastric cardia in two patients and in the gastric pylorus in one patient. The adenocarcinoma was the most frequent tumor histology (88.2%), followed by carcinoid (11.7%). The pathological tumor stage was reported in 15 patients and five cases had invaded the adjacent structures or distant metastasis (Table 2).
The most commonly reported symptoms were food intolerance/dyspepsia (50.0%), vomiting/nausea/regurgitation (35.7%), abdominal/epigastric pain (35.7%), dysphagia (28.6%), Iron deficiency/anemia (21.4%), asthenia, bloating and back pain (Table 3). Reported examinations for diagnosis and staging were upper gastrointestinal endoscopy (UGE) with biopsy, abdominal CT scan, positron emission tomography (PET). Following cancer diagnosis, surgery was the treatment of choice for 10 patients, while 4 patients were treated by endoscopic procedures. Another 2 patients underwent only chemotherapy and/or radiotherapy. One patient died during completion of diagnostic assessment.
 |
Table 3 Patients’ Symptoms |
The follow-up was reported in 9 patients; mean follow-up length was 12.2 months (range 3 months– 36 months). Disease-free survival was reported in 8 patients, while one patient died 5 months after surgery.
Discussion
This is the first study to review the available evidence for esophagogastric cancer after SG, summarizing currently available evidence for clinical and demographic characteristics of cancer patients, risk factors, treatment, outcomes. In the literature, despite it shows that the number of cancer case after SG is small (17 cases), but we can gain the experience in every case to be summarized, allowing for a comprehensive analysis.
The main drawback of SG is that it may induce or aggravate GERD. A recent meta-analysis based on 46 studies totaling 10,718 patients showed that the increase of postoperative GERD after SG was 19% and de novo reflux was 23% and the long-term prevalence of esophagitis was 28% and Barrett’s esophagus was 8%.28 However, sleeve gastrectomy associated to anterior fundoplication (D-SLEEVE) can control and/or prevent mild GERD by recreating a functional LES pressure.29 In addition, Pizza et al30 evaluated the clinical-endoscopic results of postoperative One Anastomosis Gastric Bypass(OAGB) in 241 patients and showed that the severity and incidence of esophagitis after OAGB were lower compared to SG. Hence, SG may increase the incidence of GERD. The association between HP infection and GERD remains unclear. A prospective study conducted by Emile et al31 showed that morbidly obese patients with Hp infection may be more prone to develop GERD symptoms. Another study showed HP infection seems not to influence postoperative outcome of SG.32 The main risk factors for esophageal adenocarcinoma are reflux, obesity, smoking and male sex.33 A meta-analysis based on 5 studies showed that compared with individuals without reflux symptoms, experiencing symptoms at least weekly increased the odds of developing esophageal adenocarcinoma nearly 5-fold (odds ratio [OR], 4.9; 95% confidence interval [95% CI], 3.9–6.2), while daily symptoms increased the odds more than 7-fold (OR, 7.4; 95% CI,4.9–11.1).34 The pathophysiological pathway of esophageal adenocarcinoma is likely to be chronic gastroesophageal reflux disease, causing metaplasia from the native squamous cell mucosa to a specialized columnar epithelium (Barrett’s esophagus), which develops into esophageal cancer.35 In this review, three patients did not undergo UGIE before SG and two of the patients with a history of smoking after SG developed cancer in four months and eight months post-operation. From this point, we can infer that these patients had been probably present early or premalignant esophageal lesions before SG. One patient developed GERD symptoms 15 months after SG, but he refused to be studied and was lost to follow-up.19 Another cancer patient also developed GERD after SG was the presence of Hp infection.26 Therefore, it is vital to thoroughly screen obese patients before SG to rule out the presence of potential esophageal lesions. Those with GERD is present, gastric bypass should be recommended instead of SG. More importantly, considering the risks of postoperative GERD, esophagitis, and Barrett’s esophagus, UGIE is important to use to monitor esophageal lesions of patients during long-term follow-up after SG.
The pathological mechanism for the development of cancer in remnant stomach remains unclear. Some important risk factors are infection of the gastric mucosa by HP, previous chronic gastritis, a family history of upper gastrointestinal cancer, obesity, smoking, and previous gastric surgeries.36 In this study, two patients had chronic gastritis,18,22 and one patient was a pancreas transplant case (immunocompromised),16 which augment the chance of developing gastric cancer. In addition, intestinal metaplasia and dysplasia will also increase risk of cancer in remnant stomach. The most important for early detection of these lesions is the use of UGIE during follow-up.
Carcinoid tumor is the most common neuroendocrine tumor of the stomach. Gastric carcinoids are regarded as rare but their incidence has gradually increased over the past decades, due to environmental factors, diet alterations, longer life expectancy and increased upper endoscopy screening.37,38 This incidence is higher in patients undergoing upper gastro-intestinal endoscopic evaluations before bariatric surgery.15 Furthermore, obesity may increase the prevalence of malignant gastric carcinoid tumors.39 The underlying mechanisms behind above higher incidence may attribute to hormonal changes that happen in obese people. Masuda et al conducted an animal study on obese diabetic rats undergone SG and found antral G cells hyperplasia led to hypergastrinemia.40 Hypergastrinemia plays a major role in pathogenesis of gastric carcinoid tumor.38 Hence, the risk of gastric carcinoid tumor may be increased after SG. However, only two cases of gastric carcinoid were found after SG, so there is not conclusive evidence to support the hypothesis of a higher incidence of gastric carcinoid after SG. Therefore, it is extremely important to detect this type of tumor early through strict follow-up.
The diagnosis of esophagogastric cancer after SG is usually late since these patients underwent UGIE when they were present with the new onset of symptoms. The vague or absent signs and symptoms developed by patients might be the reasons of a delayed diagnosis. Furthermore, upper gastrointestinal symptoms such as food intolerance/dyspepsia and vomiting/nausea/regurgitation are usually attributable to bariatric surgery itself and are for this reason often overlooked. In this literature, the duration of symptoms ranged from 2 months to 60 months, which meant that many patients had symptoms after surgery, but they did not take it seriously. Although these new symptoms are not specific, attention must be paid by doctors and patients. More importantly, the follow-up UIGE after SG is necessary, especially for certain patients (males, smokers, those with upper GI symptoms, those with GERD, family history of upper GI cancer, evidence of previous H pylori)
Conclusion
Data on the development of esophagogastric cancer after SG are scarce and the real incidence is unknown, but it may occur at any time. Because of the increasing number of SG, surgeons should be aware of this potential event. Preoperative and follow-up UGIE are essential in order to allow for prevention, early diagnosis. Further epidemiologic studies are needed to investigate the post-SG-related risk of esophagogastric cancer development.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
A Statement of Formal Consent
For this type of study, formal consent is not required.
Disclosure
The authors declare that they have no conflict of interest.
References
1. Sung H, Siegel RL, Torre LA, et al. Global patterns in excess body weight and the associated cancer burden. CA Cancer J Clin. 2019;69(2):88–112. doi:10.3322/caac.21499
2. Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA. Obesity and cancer mechanisms: tumor microenvironment and inflammation. J Clin Oncol. 2016;34(35):4270–4276. doi:10.1200/JCO.2016.67.4283
3. Park J, Morley TS, Kim M, Clegg DJ, Scherer PE. Obesity and cancer – mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol. 2014;10(8):455–465. doi:10.1038/nrendo.2014.94
4. Ebrahimi R, Kermansaravi M, Khalaj A, et al. Gastro-intestinal tract cancers following bariatric surgery: a narrative review. Obes Surg. 2019;29(8):2678–2694. doi:10.1007/s11695-019-04007-y
5. Mackenzie H, Markar SR, Askari A, et al. Obesity surgery and risk of cancer. Br J Surg. 2018;105(12):1650–1657. doi:10.1002/bjs.10914
6. Hussan H, Patel A, Akinyeye S, et al. Bariatric surgery is associated with a recent temporal increase in colorectal cancer resections, most pronounced in adults below 50 years of age. Obes Surg. 2020;30(12):4867–4876. doi:10.1007/s11695-020-04902-9
7. Musella M, Berardi G, Bocchetti A, et al. Esophagogastric neoplasms following bariatric surgery: an updated systematic review. Obes Surg. 2019;29(8):2660–2669. doi:10.1007/s11695-019-03951-z
8. Tornese S, Aiolfi A, Bonitta G, et al. Remnant gastric cancer after Roux-en-Y gastric bypass: narrative review of the literature. Obes Surg. 2019;29(8):2609–2613. doi:10.1007/s11695-019-03892-7
9. Angrisani L, Santonicola A, Iovino P, et al. Bariatric surgery and endoluminal procedures: IFSO worldwide survey 2014. Obes Surg. 2017;27(9):2279–2289. doi:10.1007/s11695-017-2666-x
10. Angrisani L, Santonicola A, Iovino P, et al. IFSO worldwide survey 2016: primary, endoluminal, and revisional procedures. Obes Surg. 2018;28(12):3783–3794. doi:10.1007/s11695-018-3450-2
11. Mechanick JI, Apovian C, Brethauer S, et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures - 2019 update: cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, the Obesity Society, American Society for Metabolic & Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Surg Obes Relat Dis. 2020;16(2):175–247. doi:10.1016/j.soard.2019.10.025
12. Brown WA, Johari Halim Shah Y, Balalis G, et al. IFSO position statement on the role of esophago-gastro-duodenal endoscopy prior to and after bariatric and metabolic surgery procedures. Obes Surg. 2020;30(8):3135–3153. doi:10.1007/s11695-020-04720-z
13. Scheepers AF, Schoon EJ, Nienhuijs SW. Esophageal carcinoma after sleeve gastrectomy. Surg Obes Relat Dis. 2011;7(4):e11–e12. doi:10.1016/j.soard.2010.09.019
14. Angrisani L, Santonicola A, Iovino P. Gastric cancer: a de novo diagnosis after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2014;10(1):186–187. doi:10.1016/j.soard.2013.09.009
15. Erim T, Colak Y, Szomstein S. Gastric carcinoid tumor after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2015;11(6):e51–e52. doi:10.1016/j.soard.2015.06.016
16. Masrur M, Elli E, Gonzalez-Ciccarelli LF, Giulianotti PC. De novo gastric adenocarcinoma 1 year after sleeve gastrectomy in a transplant patient. Int J Surg Case Rep. 2016;20:10–13. doi:10.1016/j.ijscr.2015.12.045
17. Sohn S, Fischer J, Booth M. Adenocarcinoma of the gastro-oesophageal junction after sleeve gastrectomy: a case report. ANZ J Surg. 2017;87(10):E163–e164. doi:10.1111/ans.13064
18. Vladimirov M, Hesse U, Stein HJ. Gastric carcinoma after sleeve gastrectomy for obesity. Surg Obes Relat Dis. 2017;13(8):1459–1461. doi:10.1016/j.soard.2017.04.020
19. Wright FG, Duro A, Medici JR, et al. Esophageal adenocarcinoma five years after laparoscopic sleeve gastrectomy. A case report. Int J Surg Case Rep. 2017;32:47–50. doi:10.1016/j.ijscr.2017.01.054
20. El Khoury L, Benvenga R, Romero R, et al. Esophageal adenocarcinoma in Barrett’s esophagus after sleeve gastrectomy: case report and literature review. Int J Surg Case Rep. 2018;52:132–136. doi:10.1016/j.ijscr.2018.10.015
21. Sista F, Abruzzese V, Carandina S, et al. Which is the correlation between carcinoid tumor and laparoscopic sleeve gastrectomy? A case series and literature review. Ann Med Surg. 2018;36:252–255. doi:10.1016/j.amsu.2018.09.010
22. Seki Y, Kasama K, Tanaka T, et al. Early gastric cancer successfully treated by endoscopic submucosal resection 1 year after laparoscopic sleeve gastrectomy with duodenal-jejunal bypass. Asian J Endosc Surg. 2019;12(3):357–361. doi:10.1111/ases.12630
23. Yamashita T, Tan J, Lim E, et al. A case of gastric cancer after sleeve gastrectomy. Asian J Endosc Surg. 2020;13(4):586–591. doi:10.1111/ases.12777
24. Guzel K. A gastric cancer following sleeve gastrectomy with transit bipartition surgery. Indian J Surg. 2020. doi:10.1007/s12262-020-02291-y
25. Muamar AS, Ammori BJ. Non-cardia gastric adenocarcinoma more than 5 years after laparoscopic sleeve gastrectomy: a case report and literature review. Asian J Endosc Surg. 2020. doi:10.1111/ases.12850
26. Aljarboo A, Alghamdi F, Alzahrani A, Ali B. A case of gastroesophageal cancer after laparoscopic sleeve gastrectomy. J Gastr Surg. 2020;2(4):127–129.
27. Genco A, Castagneto-Gissey L, Lorenzo M, et al. Esophageal adenocarcinoma after sleeve gastrectomy: actual or potential threat? Italian series and literature review. Surg Obes Relat Dis. 2020. doi:10.1016/j.soard.2020.11.023
28. Yeung KTD, Penney N, Ashrafian L, Darzi A, Ashrafian H. Does sleeve gastrectomy expose the distal esophagus to severe reflux?: a systematic review and meta-analysis. Ann Surg. 2020;271(2):257–265. doi:10.1097/SLA.0000000000003275
29. Del Genio G, Tolone S, Gambardella C, et al. Sleeve gastrectomy and anterior fundoplication (D-SLEEVE) prevents gastroesophageal reflux in symptomatic GERD. Obes Surg. 2020;30(5):1642–1652. doi:10.1007/s11695-020-04427-1
30. Pizza F, D’Antonio D, Lucido FS, et al. Postoperative clinical-endoscopic follow-up for GERD and gastritis after one anastomosis gastric bypass for morbid obesity: how, when, and why. Obes Surg. 2020;30(11):4391–4400. doi:10.1007/s11695-020-04805-9
31. Emile SH, Elshobaky A, Elbanna HG, Elkashef W, Abdel-Razik MA. Helicobacter pylori, sleeve gastrectomy, and gastroesophageal reflux disease; is there a relation? Obes Surg. 2020;30(8):3037–3045.
32. Rossetti G, Moccia F, Marra T, et al. Does helicobacter pylori infection have influence on outcome of laparoscopic sleeve gastrectomy for morbid obesity? Int J Surg. 2014;12(Suppl 1):S68–S71. doi:10.1016/j.ijsu.2014.05.051
33. Lagergren J, Lagergren PJ. Recent developments in esophageal adenocarcinoma. CA Cancer J Clin. 2013;63(4):232–248. doi:10.3322/caac.21185
34. Rubenstein JH, Taylor JB. Meta-analysis: the association of oesophageal adenocarcinoma with symptoms of gastro-oesophageal reflux. Aliment Pharmacol Ther. 2010;32(10):1222–1227. doi:10.1111/j.1365-2036.2010.04471.x
35. Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer [J]. Lancet. 2017;390(10110):2383–2396. doi:10.1016/S0140-6736(17)31462-9
36. Ohira M, Toyokawa T, Sakurai K, et al. Current status in remnant gastric cancer after distal gastrectomy [J]. World J Gastroenterol. 2016;22(8):2424–2433. doi:10.3748/wjg.v22.i8.2424
37. Mottin CC, Cruz RP, Gomes Thomé G, Padoin AV. Carcinoid tumors and morbid obesity. Obes Surg. 2009;19(2):247–249. doi:10.1007/s11695-008-9541-8
38. Grozinsky-Glasberg S, Alexandraki KI, Angelousi A, et al. Gastric carcinoids. Endocrinol Metab Clin North Am. 2018;47(3):645–660. doi:10.1016/j.ecl.2018.04.013
39. Aminian A, Schauer PR, Brethauer SA. Malignant gastric carcinoid tumor and morbid obesity. Surg Obes Relat Dis. 2014;10(6):1237. doi:10.1016/j.soard.2014.08.009
40. Masuda T, Ohta M, Hirashita T, et al. A comparative study of gastric banding and sleeve gastrectomy in an obese diabetic rat model. Obes Surg. 2011;21(11):1774–1780. doi:10.1007/s11695-011-0512-0
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
