Back to Journals » Clinical and Experimental Gastroenterology » Volume 16
Esophageal Mucosal Admittance: A New Technique to Diagnose Gastroesophageal Reflux Disease – Is It Feasible?
Authors Dao HV, Hoang LB , Nguyen BP, Nguyen HL, Goldberg R , Allison J, Dao TMA, Matsumura T, Dao LV
Received 30 November 2022
Accepted for publication 28 March 2023
Published 7 April 2023 Volume 2023:16 Pages 45—54
DOI https://doi.org/10.2147/CEG.S399764
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Koulaouzidis
Supplementary video of "Esophageal mucosal admittance for GERD" [ID 399764].
Views: 316
Hang Viet Dao,1– 3 Long Bao Hoang,3 Binh Phuc Nguyen,3 Hoa Lan Nguyen,4 Robert Goldberg,4 Jeroan Allison,4 Thi Minh An Dao,5,6 Tomoaki Matsumura,7 Long Van Dao1,3
1Internal Medicine Faculty, Hanoi Medical University, Hanoi, Vietnam; 2Endoscopy Center, Hanoi Medical University Hospital, Hanoi, Vietnam; 3Institute of Gastroenterology and Hepatology, Hanoi, Vietnam; 4Department of Population and Quantitative Health Sciences, University of Massachusetts Medical School, Worcester, MA, USA; 5School of Public Health, University of Queensland, Brisbane, Australia; 6Epidemiology Department, Hanoi Medical University, Hanoi, Vietnam; 7Department of Gastroenterology, Graduate School of Medicine, Chiba University, Chiba, Japan
Correspondence: Hang Viet Dao, Institute of Gastroenterology and Hepatology, Floor 10, VCCI Tower, No. 9, Dao Duy Anh Street, Dong Da District, Hanoi City, 10000, Vietnam, Tel +84987988075, Email [email protected]
Purpose: Esophageal mucosal admittance (MA) is a promising diagnostic method for gastroesophageal reflux disease (GERD). We conducted a study to describe the esophageal MA in patients with reflux symptoms and determine its diagnostic accuracy.
Patients and Methods: We recruited 92 patients with ambulatory pH-impedance monitoring, upper gastrointestinal endoscopy, and MA measured by the tissue conductance meter. MA was measured during endoscopy at 5cm (distal esophagus) and 15cm above the Z line (middle esophagus), repeated at least five times at each position, and median MA was obtained. Afterwards, two biopsies were taken 5cm above the Z line for histopathological evaluation using the Esohisto criteria. Patients were classified as GERD or non-GERD according to the 2018 Lyon consensus.
Results: The mean age was 43.2 years, and 42 patients were males. The most common symptoms were regurgitation (75.0%), belching (65.2%), and heartburn (46.7%). Twenty-three (32.3%) were diagnosed with GERD using the Lyon consensus, and 24 (26.1%) had esophagitis on histopathology. The median MA at the distal and middle esophagus was moderately correlated. The median MA at both positions was higher in the GERD group but only statistically significant in the middle esophagus. MA was not associated with pH-impedance parameters and esophagitis on histopathology. The diagnostic model developed using the logistic regression did not have good accuracy.
Conclusion: MA was not different between GERD and non-GERD patients.
Keywords: mucosal permeability, tissue conductance meter, pH-impedance monitoring, Lyon consensus
Introduction
The prevalence of gastroesophageal reflux disease (GERD) is increasing worldwide,1,2 resulting in a greater burden due to persistent symptoms and complicated functional evaluation.3 Conclusive GERD can be diagnosed using endoscopy or 24-hour pH-impedance monitoring.4,5 However, endoscopic criteria are strict, thus limiting the number of patients being diagnosed with GERD, especially in the Asian population.6 Also, despite its high sensitivity and specificity, pH-impedance monitoring is expensive, complicated, and inconvenient for routine practice.7,8
The Lyon consensus suggests using other diagnostic modalities in difficult cases, such as histopathology.4 Histopathological assessment of esophageal mucosa using the Esohisto guideline is helpful, especially in patients without endoscopic lesions.9,10 However, this method requires experienced histopathologists and biopsies at multiple sites in the esophagus. Another approach is evaluating esophageal mucosal permeability. Data have demonstrated the correlation of esophageal mucosal permeability with acid exposure time (AET), the main pH-impedance parameter for GERD diagnosis.11,12 This result suggests a novel approach to overcome the difficulty of 24-hour pH-impedance monitoring by new technologies to evaluate mucosal admittance (MA) during endoscopy.
In physics, admittance, the reciprocal of impedance, measures how well electricity is conducted. Injuries to the esophageal mucosa alter the characteristics of linking structures between cells, such as the tight junctions, leading to changes in electrical conductance and thus changes in MA.13 Therefore, measuring the MA of the esophageal mucosa (esophageal MA) is thought to be able to detect early GERD. MA measurement can be done by devices such as the tissue conductance meter (TCM) system, which uses a catheter with an electrode on the tip to place on the surface of the esophageal lumen during endoscopy. Studies have suggested the ability of these devices in discerning between GERD and functional heartburn.14,15
We conducted this study to describe the esophageal MA in patients with reflux symptoms and determine the accuracy of a diagnostic model for GERD integrating esophageal MA.
Materials and Methods
We conducted a cross-sectional study from September 2020 to February 2022 on patients above 18 years old admitted to the Institute of Gastroenterology and Hepatology (Hanoi, Vietnam). The selection criteria included patients with reflux symptoms (heartburn, regurgitation, suspected extraesophageal reflux symptoms) who underwent the following procedures: (1) upper gastrointestinal endoscopy with anesthesia; (2) measurement of esophageal MA by TCM system during endoscopy; (3) two biopsies at 5cm above the Z line during endoscopy; and (4) 24-hour esophageal pH-impedance monitoring.
Data Collection
Demographic and clinical data were collected before administering study procedures. Height and weight were collected to calculate the body mass index (BMI). According to the recommendation of the WHO’s experts for the Asian population, a BMI value <18.5 was considered underweight, 18.5–22.9 normal, 23–24.9 overweight, and >25 obese.16 GERD questionnaires (Gastroesophageal reflux disease questionnaire—GerdQ and Frequency scale for the symptoms of GERD—FSSG) were administered to evaluate the severity of GERD. A GerdQ score ≥8 or FSSG ≥8 was suggestive of GERD.15,17
All patients were off-PPI before undergoing upper gastrointestinal endoscopy under anesthesia. Mucosal breaks detected on endoscopy were classified by the Los Angeles (LA) classification (grades A-D).18
We used the Ohmega system (Laborie) for 24-hour esophageal pH-impedance monitoring using a catheter with six impedance channels and one pH channel. For patients who were on proton-pump inhibitors (PPI), the technique was performed after stopping PPI for at least five days. Data collected from pH-impedance monitoring included AET, DeMeester score, and mean nocturnal baseline impedance (MNBI). AET was defined as the percentage of the time that esophageal mucosa is exposed to an environment of pH <4.0.19 MNBI was measured by manually calculating the mean baseline impedance at two positions (3cm and 5cm above the lower esophageal sphincter) at 10-minute periods around 1 AM, 2 AM, and 3 AM without the disturbance of reflux events and swallowing.20 The DeMeester score was automatically calculated from a six-item scoring system (percent and the total amount of time esophageal pH <4 in upright and supine positions, number of reflux episodes, number of reflux episodes more than five minutes, and the duration of the longest episode).21
Measurement of Mucosal Admittance
The TCM system was used to measure esophageal MA during the withdrawal phase of endoscopy (Supplementary Video). Two electrode pads were attached to the flexor side of the arms. After cleaning the mucosal surface of the measurement site to avoid fluid and bubbles, a small catheter with a diameter of 1.9mm was introduced into the endoscopic biopsy channel. During measurements, the endoscopist placed the electrode tip of the catheter onto the visibly normal mucosa (no mucosal break) in a stable position for 2 to 3 seconds to form a close circuit (Figure 1). During measurements, the system loaded two electrical frequencies of 320Hz and 37kHz into the circuit. The device analyzed measurements from both currents to calculate MA.
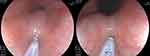 |
Figure 1 MA measurement at the esophagus. |
MA was measured at two sites: 5cm (distal esophagus) and 15cm (middle esophagus) above the Z line (the squamocolumnar junction). Each position was measured at least five times. The median value of the measurements at each position was used for data analysis. We also calculated the ratio of median MA measured at the distal esophagus to that at the middle esophagus.
Histopathological Assessment
After measuring esophageal MA, the endoscopist collected two biopsies at the distal esophagus (5cm above the Z line) for histopathology. The specimens were stained with hematoxylin-eosin, and severity was graded according to the Esohisto consensus guidelines based on the following criteria: basal cell layer hyperplasia, papillary elongation, dilation of intercellular spaces (ICS), and infiltration of inflammatory cells (eosinophil, neutrophils, and monocytes) in the epithelium (Table S1).9 The overall score was calculated by dividing the total scores of the first three criteria and intraepithelial eosinophils by the number of criteria (four).10 An overall score of 0 or 0.25 was considered normal, 0.5 or 0.75 mild esophagitis, and ≥1 severe esophagitis. In our study, a patient with a score of 0.5 or more would be considered to have esophagitis on histopathology.
Diagnosis of GERD (Primary Outcome)
According to the 2018 Lyon Consensus,4 we would diagnose GERD if one of the following criteria was met: on endoscopy (1) erosive esophagitis LA grades C to D; (2) long-segment Barrett’s esophagus; (3) esophageal stricture; or on 24-hour pH-impedance monitoring (4) AET >6%. Patients who did not meet any criteria were considered to have no GERD (non-GERD).
Statistical Analysis
Patient characteristics were presented as counts and percentages (%) for qualitative variables and as mean (standard deviation, SD) or median (interquartile range, IQR) for quantitative variables, and were compared between GERD and non-GERD patients by Chi-squared test, Fisher’s exact test, two-sample t-test, or Kruskal–Wallis test, where appropriate. A P-value of 0.05 was considered statistically significant.
Logistic regression was used to evaluate the association between related factors (sex, body mass index (BMI), erosive esophagitis, and MA) with GERD diagnosis. Based on the logistic model, we predicted the probability of having GERD, plotted the receiver operating characteristic (ROC) curve, and calculated the C-statistic or area under the curve (AUC) of the ROC curve.
Data were cleaned and analyzed by the Python programming language version 3.9.12. Packages tableone was used to make summary tables, and statsmodels were used for logistic regression.22,23
Ethical Considerations
The study was conducted in compliance with the Declaration of Helsinki and Good Clinical Practice. It has been approved by the institutional review board of the Dinh Tien Hoang Institute of Medicine (no. IRB-1909 dated 01st March 2020). Informed consent was obtained from all study patients.
Results
Patient’s Characteristics
From September 2020 to February 2022, we recruited ninety-two patients (42 males, mean age 43.2). About one-third of patients were overweight (30.4%). The most common clinical symptoms were regurgitation (75.0%), belching (65.2%), bloating (51.1%), and heartburn (46.7%) (Table S2). The mean (SD) FSSG score was 11.7 (7.5), with 66.3% of patients having an FSSG score ≥8. The mean (SD) GerdQ score was 7.5 (2.5), with 50% having a GerdQ score ≥8. About eighty percent of patients had erosive esophagitis on endoscopy, mostly LA grade A (94.6%). On histopathology, 24 (26.1%) patients had esophagitis. On 24-hour pH monitoring, MNBI had weak correlation with AET (r = −0.47, p < 0.001) (Figure S2).
Based on the 2018 Lyon consensus, 23 (25.0%) patients were diagnosed with GERD. Patients who did and did not have GERD diagnosis had similar prevalence of clinical symptoms, erosive esophagitis on endoscopy, and esophagitis on histopathology (Table 1).
 |
Table 1 Characteristics of GERD and Non-GERD Patients |
Mucosal Admittance
The median (IQR) MA at the distal and middle esophagus were 37.1 (22.1–60.6) and 39.4 (25.9–61.2), respectively. The median MA measured at the two positions appeared to correlate with each other (Figure 2); the correlation was stronger in the non-GERD group (Pearson’s r = 0.82).
 |
Figure 2 Correlation of the mucosal admittance at distal esophagus and middle esophagus. |
In GERD patients, the median MA at the distal esophagus appeared higher than at the middle esophagus. The median MA in the GERD group was higher than in the non-GERD group at both measured positions (Figure 3), but the difference was only significant at the middle esophagus (p = 0.006). The ratio of MA was also not different between GERD and non-GERD patients. The median MA had no correlation with AET, DeMeester score, and MNBI in both GERD and non-GERD groups (Figures 4–6). On histopathology, the esophageal MA at both positions was not different between patients with and without esophagitis.
 |
Figure 3 Mucosal admittance measured at the distal and middle esophagus between GERD and non-GERD. |
 |
Figure 4 Correlation between mucosal admittance at distal esophagus and AET. |
 |
Figure 5 Correlation between mucosal admittance at distal esophagus and DeMeester score. |
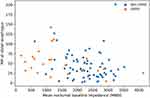 |
Figure 6 Correlation between mucosal admittance at distal esophagus and MNBI. |
Accuracy in GERD Diagnosis
We developed a prediction model for GERD diagnosis using logistic regression, including some factors possibly related to GERD, including sex, BMI, esophagitis on histopathology, and mucosal admittance at the distal esophagus (Table 2). In this model, higher mucosal admittance was associated with higher odds of GERD, but the association was insignificant. The received-operating characteristic curve of this model had an area under the curve of 0.704, suggesting a moderate accuracy in predicting GERD using these factors (Figure S1).
 |
Table 2 Logistic Regression Model for GERD Diagnosis |
Discussion
In this study, we explored the role of the esophageal MA in GERD diagnosis. Although the median MA tended to be higher in GERD patients, the difference was not remarkable for the MA to be of diagnostic value. MA was not correlated to pH-impedance parameters used to diagnose GERD (AET, DeMeester score, and MNBI).
In GERD patients, the median MA at the distal esophagus tended to be higher than at the middle esophagus. This result was also consistent with the findings in another study, which shows that the mucosa was more impaired at the distal esophagus.24 Studies using mucosal impedance also showed that impedance gradually decreased along the esophagus, with lower impedance observed in the distal esophagus, especially in the eroded regions.25,26 However, this mechanistic explanation alone could not justify the variations in esophageal MA. Although the esophageal MA in the GERD group was higher than in the non-GERD group, it markedly varied in both groups and at both positions. A previous study by Matsumura et al also suggested this variability.27 It might be due to the variability between endoscopists (measurement techniques) and patients (esophageal mucosa condition) or the esophageal MA’s natural variability.
Other studies also demonstrated differences in the esophagus’s mucosal permeability between GERD and non-GERD patients.25–27 Matsumura et al used the same TCM catheter as in our study and reported that the median MA at the distal esophagus was significantly higher in patients with erosive esophagitis than in non-erosive reflux disease patients and healthy volunteers.27 The authors also found a cutoff (13.14) to distinguish between GERD and functional heartburn, but the cutoff had modest sensitivity and specificity. Other groups used an endoscopic-guided mucosal impedance catheter and reported that impedance measured at 2cm above the Z line was significantly lower in GERD patients.25,26 The cutoffs for mucosal impedance in these studies had better specificity in diagnosing GERD. The difference could be due to the different definitions of GERD. In the MI studies, patients with EE and patients with no EE but with abnormal AET were considered to have GERD, and patients with EE did not undergo 24-hour pH monitoring. In our study, all patients had to undergo 24-hour pH monitoring, and the diagnosis of GERD followed the 2018 Lyon consensus, including abnormal AET or high-grade EE. Using this definition of GERD, even though most of our patients had EE (80.4%), only 27.0% of patients with EE were diagnosed with GERD. Another explanation could be that although MA is theoretically the inverse of MI, MI catheters and MA catheters evaluated the integrity or permeability of injured mucosa differently. Studies on both techniques have shown the correlation between the mucosal impedance/admittance measured during endoscopy and the dilated intercellular spaces and AET on 24-hour pH monitoring.25,27,28 The most important difference between MI and MA techniques is the structure of each catheter. The TCM catheter measures the mucosal MA only at the contact point of the catheter, while the Sandhill catheter in Yuksel et al measured the MI of the area between the two censor rings.14 In addition, the TCM catheter is considered to be able to evaluate the full thickness of the esophageal mucosa, while the Sandhill catheter evaluates the MI of the surface layer. Since most histopathology changes in GERD patients, such as diluted intercellular space, basal cell hyperplasia, and altered tight junction, occur in the epithelial layer of the mucosa, the MI catheter might be more effective than the MA catheter in evaluating esophageal mucosal integrity.29,30
We did not observe any correlation between MA and pH-impedance parameters used for diagnosing GERD. While AET and DeMeester scores focus on the amount of acid reflux, they might not correlate with the degree of esophageal mucosal injury. The extent and degree of injury might vary among people who have the same amount of time exposed to acid reflux. Also, in addition to acid reflux, other types of refluxates (such as alkaline reflux) can also damage the esophageal mucosa.31
Esophagitis on histopathology was not correlated with the diagnosis of GERD in our study. According to the Lyon consensus or current ACG 2022 guideline, histopathology is not considered a confirmatory diagnostic tool.4,5 It might be due to the heterogeneity in evaluating microscopic injuries in GERD. However, the additional information that histopathology provides could be used as supportive evidence for GERD diagnosis. The Esohisto guideline helps to unify and assess the severity of esophageal mucosa.9,10 However, the biopsy location in the Esohisto guideline was 2cm above the Z line, while our study took the biopsies at the measurement site of the distal esophagus, which was 5cm above the Z line. This difference could explain the low correlations.
The strength of this study was the use of 24-hour pH-impedance monitoring as the “gold” standard for GERD diagnosis. However, our study did not collect data on patients’ responses to PPI therapy; therefore, we could not evaluate MA in PPI-refractory patients.
Conclusion
Esophageal MA was not different between GERD and non-GERD patients. The diagnostic model integrating MA did not have high accuracy in diagnosing GERD.
Acknowledgments
We deeply thank Hoang Long Clinic (Hanoi, Vietnam) for the agreement to collaborate in this study and their administrative support.
Funding
This study was part of the national project “Evaluation of motility and secretion disorders in some upper gastrointestinal diseases” (No. 1846, approved on 27 June 2019) funded by Vietnam’s Ministry of Science and Technology and was also supported by the Fogarty International Center of the US National Institutes of Health under award number D43 TW011394-01.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Eusebi LH, Ratnakumaran R, Yuan Y, Solaymani-Dodaran M, Bazzoli F, Ford AC. Global prevalence of, and risk factors for, gastro-oesophageal reflux symptoms: a meta-analysis. Gut. 2018;67(3):430–440. doi:10.1136/gutjnl-2016-313589
2. Goh KL. Gastroesophageal reflux disease in Asia: a historical perspective and present challenges. J Gastroenterol Hepatol. 2011;26(Suppl 1):2–10. doi:10.1111/j.1440-1746.2010.06534.x
3. Qumseya B, Sultan S, Bain P, et al. ASGE guideline on screening and surveillance of Barrett’s esophagus. Gastrointest Endosc. 2019;90(3):335–359.e2. doi:10.1016/j.gie.2019.05.012
4. Gyawali CP, Kahrilas PJ, Savarino E, et al. Modern diagnosis of GERD: the Lyon consensus. Gut. 2018;67(7):1351–1362. doi:10.1136/gutjnl-2017-314722
5. Katz PO, Dunbar KB, Schnoll-Sussman FH, et al. Guideline for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2022;117(1):27–56. doi:10.14309/ajg.0000000000001538
6. Kim BJ, Cheon WS, Oh HC, Kim JW, Park JD, Kim JG. Prevalence and risk factor of erosive esophagitis observed in Korean National cancer screening program. J Korean Med Sci. 2011;26(5):642–646. doi:10.3346/jkms.2011.26.5.642
7. Hershcovici T, Fass R. Nonerosive Reflux Disease (NERD) - an update. J Neurogastroenterol Motil. 2010;16(1):8–21. doi:10.5056/jnm.2010.16.1.8
8. Roman S, Gyawali CP, Savarino E, et al. Ambulatory reflux monitoring for diagnosis of gastroesophageal reflux disease: update of the Porto consensus and recommendations from an international consensus group. Neurogastroenterol Motil. 2017;29(10):1–15. doi:10.1111/nmo.13067
9. Fiocca R, Mastracci L, Riddell R, et al. Development of consensus guidelines for the histologic recognition of microscopic esophagitis in patients with gastroesophageal reflux disease: the Esohisto project. Hum Pathol. 2010;41(2):223–231. doi:10.1016/j.humpath.2009.07.016
10. Yerian L, Fiocca R, Mastracci L, et al. Refinement and reproducibility of histologic criteria for the assessment of microscopic lesions in patients with gastroesophageal reflux disease: the esohisto project. Dig Dis Sci. 2011;56(9):2656–2665. doi:10.1007/s10620-011-1624-z
11. Kessing BF, Bredenoord AJ, Weijenborg PW, Hemmink GJM, Loots CM, Smout AJPM. Esophageal acid exposure decreases intraluminal baseline impedance levels. Am J Gastroenterol. 2011;106(12):2093–2097. doi:10.1038/ajg.2011.276
12. Zhong C, Duan L, Wang K, et al. Esophageal intraluminal baseline impedance is associated with severity of acid reflux and epithelial structural abnormalities in patients with gastroesophageal reflux disease. J Gastroenterol. 2013;48(5):601–610. doi:10.1007/s00535-012-0689-6
13. Ustaoglu A, Nguyen A, Spechler S, Sifrim D, Souza R, Woodland P. Mucosal pathogenesis in gastroesophageal reflux disease. Neurogastroenterol Motil. 2020;32:12. doi:10.1111/nmo.14022
14. Matsumura T, Arai M, Ishigami H, et al. Evaluation of esophageal mucosal integrity in patients with gastroesophageal reflux disease. Digestion. 2018;97(1):31–37. doi:10.1159/000484106
15. Suzuki H, Matsuzaki J, Okada S, Hirata K, Fukuhara S, Hibi T. Validation of the GerdQ questionnaire for the management of gastro-oesophageal reflux disease in Japan. United Eur Gastroenterol J. 2013;1(3):175–183. doi:10.1177/2050640613485238
16. WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–163. doi:10.1016/S0140-6736(03)15268-3
17. Kusano M, Shimoyama Y, Sugimoto S, et al. Development and evaluation of FSSG: frequency scale for the symptoms of GERD. J Gastroenterol. 2004;39(9):888–891. doi:10.1007/s00535-004-1417-7
18. Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45(2):172–180. doi:10.1136/gut.45.2.172
19. Cho YK. How to interpret esophageal impedance pH monitoring. J Neurogastroenterol Motil. 2010;16(3):327–330. doi:10.5056/jnm.2010.16.3.327
20. Hoshikawa Y, Sawada A, Sonmez S, et al. Measurement of esophageal nocturnal baseline impedance: a simplified method. J Neurogastroenterol Motil. 2020;26(2):241–247. doi:10.5056/jnm19183
21. Kleiman DA, Sporn MJ, Beninato T, et al. Early referral for 24-hour esophageal pH monitoring may prevent unnecessary treatment with acid-reducing medications. Surg Endosc. 2013;27(4):1302–1309. doi:10.1007/s00464-012-2602-z
22. Pollard TJ, Johnson AEW, Raffa JD, Mark RG. Table one: an open source python package for producing summary statistics for research papers. JAMIA Open. 2018;1(1):26–31. doi:10.1093/jamiaopen/ooy012
23. Seabold S, Perktold J. Statsmodels: econometric and statistical modeling with python. In:
24. van Hoeij FB, Weijenborg PW, van den Bergh Weerman MA, et al. Mucosal integrity and sensitivity to acid in the proximal esophagus in patients with gastroesophageal reflux disease. Am J Physiol Gastrointest Liver Physiol. 2016;311(1):G117–G122. doi:10.1152/ajpgi.00134.2016
25. Saritas Yuksel E, Higginbotham T, Slaughter JC, et al. Use of direct, endoscopic-guided measurements of mucosal impedance in diagnosis of gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2012;10(10):1110–1116. doi:10.1016/j.cgh.2012.05.018
26. Ates F, Yuksel ES, Higginbotham T, et al. Mucosal impedance discriminates GERD from non-GERD conditions. Gastroenterology. 2015;148(2):334–343. doi:10.1053/j.gastro.2014.10.010
27. Matsumura T, Ishigami H, Fujie M, et al. Endoscopic-guided measurement of mucosal admittance can discriminate gastroesophageal reflux disease from functional heartburn. Clin Transl Gastroenterol. 2017;8(6):e94. doi:10.1038/ctg.2017.22
28. Weijenborg PW, Rohof WOA, Akkermans LMA, Verheij J, Smout AJPM, Bredenoord AJ. Electrical tissue impedance spectroscopy: a novel device to measure esophageal mucosal integrity changes during endoscopy. Neurogastroenterol Motil. 2013;25(7):574–e458. doi:10.1111/nmo.12106
29. Zentilin P, Savarino V, Mastracci L, et al. Reassessment of the diagnostic value of histology in patients with GERD, using multiple biopsy sites and an appropriate control group. Am J Gastroenterol. 2005;100(10):2299–2306. doi:10.1111/j.1572-0241.2005.50209.x
30. Björkman EVC, Edebo A, Oltean M, Casselbrant A. Esophageal barrier function and tight junction expression in healthy subjects and patients with gastroesophageal reflux disease: functionality of esophageal mucosa exposed to bile salt and trypsin in vitro. Scand J Gastroenterol. 2013;48(10):1118–1126. doi:10.3109/00365521.2013.828772
31. Lin KM, Ueda RK, Hinder RA, Stein HJ, DeMeester TR. Etiology and importance of alkaline esophageal reflux. Am J Surg. 1991;162(6):553–557. doi:10.1016/0002-9610(91)90107-o
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
