Back to Journals » Cancer Management and Research » Volume 12
Esophageal Microenvironment: From Precursor Microenvironment to Premetastatic Niche
Authors Han P , Cao P, Hu S, Kong K, Deng Y, Zhao B , Li F
Received 24 April 2020
Accepted for publication 29 June 2020
Published 16 July 2020 Volume 2020:12 Pages 5857—5879
DOI https://doi.org/10.2147/CMAR.S258215
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Peng Han, Peng Cao, Shan Hu, Kangle Kong, Yu Deng, Bo Zhao, Fan Li
Department of Thoracic Surgery, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, People’s Republic of China
Correspondence: Fan Li Email [email protected]
Abstract: Esophageal cancer (EC) is the sixth most deadly cancer, and its incidence is still increasing year by year. Although the researches on the molecular mechanisms of EC have been widely carried out and incremental progress has been made, its overall survival rate is still low. There is cumulative evidence showing that the esophageal microenvironment plays a vital role in the development of EC. In precancerous lesions of the esophagus, high-risk environmental factors can promote the development of precancerous lesions by inducing the production of inflammatory factors and the recruitment of immune cells. In the tumor microenvironment, tumor-promoting cells can inhibit anti-tumor immunity and promote tumor progression through a variety of pathways, such as bone marrow-derived suppressor cells (MDSCs), tumor-associated fibroblasts (CAFs), and regulatory T cells (Tregs). The formation of extracellular hypoxia and acidic microenvironment and the change of extracellular matrix stiffness are also important factors affecting tumor progression and metastasis. Simultaneously, primary tumor-derived cytokines and bone marrow-derived immune cells can also promote the formation of pre-metastasis niche of EC lymph nodes, which are beneficial to EC lymph node metastasis. Further research on the specific mechanism of these processes in the occurrence, development, and metastasis of each EC subtype will support us to grasp the overall pre-cancerous prevention, targeted treatment, and metastatic assessment of EC.
Keywords: esophageal cancer, tumor precursor microenvironment, tumor microenvironment, premetastatic niche
Introduction
Esophageal cancer (EC) is the sixth leading cause of cancer-related deaths and one of the eight most common malignancies. According to different tissue subtypes, it can be mainly divided into esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC). The incidence rate of ESCC is increasing globally, but it is rapidly rising in some European countries for EAC and has even exceeded incidence rate of ESCC in some regions,1,2 which may be related to different lifestyles in different countries. Although the diagnosis and treatment of EC have been gradually improved, the five-year survival rate (19%) for EC is the same as that for lung cancer (19%) and is second only to liver cancer (18%) and pancreatic cancer (9%)3 according to the latest statistical analysis. This is mainly due to the late diagnosis and metastatic tendency.4 The current standard treatments of EC mainly include chemoradiotherapy, surgery, and endoscopic resection.5 Although the Food and Drug Administration (FDA) has approved some targeted drugs such as programmed cell death protein 1 (PD-1) inhibitors, human epidermal growth factor receptor-2 (HER2), and anti-vascular endothelial growth factor (VEGF) monoclonal antibodies for the treatment of EC, the survival rate of most patients with advanced EC is still low,6 which requires us to conduct a more systematic evaluation of the exact molecular pathogenesis of EC in order to develop novel biomarkers and therapeutic targets.
According to previous reports, the dynamic process of cancer immunity includes three steps: elimination, equilibrium, and escape.7 These steps can be corresponding to the occurrence, development, and invasion of cancer.8 Similarly, we assume that the EC dynamic microenvironment could be roughly divided into three stages: tumor precursor microenvironment, tumor microenvironment (TME), and pre-metastatic niche. TME is a vital space for the metabolism of tumor cells. Nowadays, numerous studies have shown that TME could mainly promote the growth, proliferation, migration, and metastasis of EC. The main components of TME are a variety of adaptive and innate immune cells, fibroblasts, adipocytes, endothelial cells, and extracellular matrix (ECM) components, which have been extensively studied in a variety of tumors.9–12 TME is not a fixed tumor survival environment, but a dynamic environment that is constantly remodeled by tumors and tumor-related cells to adapt to the survival of tumor cells. Therefore, the tumor precursor microenvironment and pre-metastasis niche should also be taken seriously as the microenvironment before tumorigenesis and pre-metastasis, respectively. A comprehensive and systematic study of this dynamic process will not only enable us to better understand the occurrence and development of EC but also provide us with a variety of therapeutic targets and biomarkers, and guidance for future diagnostic and therapeutic directions. In this review, we summarized the main research advances in EC tumor precursor microenvironment, TME, and premetastatic niche.
Tumor Precursor Microenvironment
Barrett’s Esophagus (BE) Microenvironment
BE is a well-known precancerous lesion of EAC, and high-risk environmental factors are the promoters of this process. We also found that TME-like changes have partially occurred in the BE microenvironment (Figure 1) as follows: the production of various inflammatory factors, immunity cell infiltration, remodeling of ECM, etc.
The tumor hypoxia microenvironment may be formed in BE previously. Studies have shown that esophageal ulcers and ischemia caused by reflux can result in hypoxia in injured esophageal tissues and increase the expression of hypoxia-induced factor 1α (HIF-1α), which regulates the expression of VEGR and promotes angiogenesis and ulcer healing in turn.13 However, we are not sure whether HIF-1α is friend or foe in BE. For example, in other studies, it has been demonstrated that HIF-1α could highly express in metaplastic esophageal epithelial cells, nevertheless, the expression of HIF-1α did not increase further with the progress of BE epithelial dysplasia and was not associated with histopathological parameters or survival rate.14 It is also worth mentioning that the level of glycolytic metabolism is more significantly increased in advanced BE cell lines than in early BE cell lines.15 However, HIF-1α is well known as the main regulator of tumor glycolysis metabolism. This process of BE cells continuously adapting to changes in the microenvironment by regulating energy metabolism pathways may be the origin of tumor aerobic glycolysis, but more evidence would be needed to verify this hypothesis.
The production of inflammatory factors has played an important role in the progress of BE. For example, gastroesophageal reflux can induce the up-regulation of inflammatory factors such as IL1B, IL4, and IL6. These inflammatory factors can activate cardia stem cells by inflammatory response and promote columnar epithelial metaplasia in the distal esophagus.16,17 In addition, the inflammatory factor IL-6 can significantly increase the anti-apoptotic rate of Barrett epithelial cells in vitro through the IL-6/STAT3 pathway.18,19 More than that, obesity, which is a high-risk factor for BE, can also up-regulate some inflammatory factors. Hypertrophic fat cells can lead to tissue hypoxia, which ultimately induces the up-expression of IL-6 and tumor necrosis factor α (TNF-α),20 which can not only mediate the up-regulation of IL-8 expression, promote angiogenesis and migration of ECM by activating endothelial cells21,22 but also increase the expression of β-catenin-mediated oncogene c-myc in BE epithelial cells.23 It is noteworthy that TNF-α expression increases with the progression of metaplastic-proliferative-adenocarcinoma sequences.
Changes in the composition of immune cells are an important feature of BE microenvironment, and the formation of immunosuppressive BE microenvironment may be a major factor in the progress of BE. In a flow cytometric analysis, it was found that the proportion of immune cells in the BE microenvironment had decreased overall, and the immune environment had changed from a T-cell-based microenvironment to a B-cell-based microenvironment.24 Similar to EAC, the ratio of Th1/Th2 is significantly reduced in EC, and the Th2-based immune performance was shown in the BE microenvironment, which may promote tumorigenesis.25,26 In addition, some studies have reported that the expression of forkhead box P3 (FOXP3) +, the major regulator of Tregs immunosuppressive function, was significantly up-regulated in BE tissues.27 However, in the relatively early metaplasia stage of BE, acids and bile salts can stabilize the expression of HIF-2α in esophageal epithelial cells, thereby upregulating pro-inflammatory cytokines and recruiting T lymphocytes and other lymphocytes to damage the esophagus through the Nuclear factor-kappaB (NF-κB) pathway.28,29 In summary, the composition of immune cells in the BE microenvironment is relatively complex and constantly changing. Of course, more researches will be needed to elucidate the development process and mechanism from early inflammatory infiltration injury to final immunosuppression.
The transformation of the ECM is also a major feature of changes in the BE microenvironment. Matrix metalloproteinases (MMPs) are enzymes involved in inflammation, ECM remodeling, and tumor metastasis. Previous studies have shown that MMP-7 in BE epithelial cells could stimulate stromal cell migration and invasion and remodel the microenvironment under the regulation of PI3-K kinases.30 In addition, MMP9 and MMP13 are also up-regulated in BE.31 It is noteworthy that MMP13 is more highly expressed in BE, while MMP-9 is more highly expressed in EAC. Once BE is converted to EAC, the expression ratio of MMP13/MMP9 in epithelial cells is significantly decreased. The study on the transition mechanism may be helpful in exploring novel therapeutic targets.
The BE microenvironment is also a complex inflammatory microenvironment. In the process of exploring its mechanism of inflammation microenvironment, the viewpoint is put forward of anti-inflammatory treatment of BE,22,27 but the exact effect and specific mechanism need to be further clarified. The above discussions show that BE, as a precancerous lesion of EAC, is inextricably linked with EAC in the microenvironment. In-depth exploration and application of BE microenvironment in different stages will be a powerful method to prevent EAC.
Esophageal Squamous Epithelium Dysplasia (ESD) Microenvironment
In a multi-region whole-exome sequencing, it was found that atypical proliferative epithelial cells have polyclonality, significant heterogeneity, and severe mutations similar to ESCC cells,32 and a large amount of evidence indicates that ESD is a precursor lesion of ESCC.33 Smoking, drinking, and eating very hot food or beverages are high-risk environmental factors for ESCC.34 Although there are few studies on ESD microenvironment, we can still find some valuable information about that. For example, it was reported that glucose transporter 1 (GLUT-1), an important glycolytic pathway enzyme, is highly expressed not only in ESCC tumor cells but also in tumor precursor cells.35 Similar to the above-mentioned BE, this may indicate that ESD cells also continuously adapt to changes in the microenvironment by transforming energy metabolism pathways. The occurrence of glycolysis and local inflammatory reactions will promote the oxygen consumption of the precursor microenvironment and the accumulation of acidic metabolites.36 Under acidic conditions, mutations and heterogeneity of ESD cell may increase again, and further lead to the accumulation of carcinogenic mutations.37 With the accumulation of carcinogenic mutations, the cell polyclonality also evolves, which increases the oxygen energy supply burden in the interstitial space, leads to a vicious cycle of hypoxia and acidic microenvironment, and eventually results in the occurrence of cancer and the formation of TME. The cumulative process of carcinogenic mutations is extremely long, which may also be the reason for the relatively long period of precancerous lesions.38 However, more evidence will be needed to prove the evolution of this cycle. Finally, the occurrence of EC is the result of the interaction between the precancerous microenvironment and ESD. At present, there are many studies on the high-risk factors of ESCC, ESD itself and post-cancer, and we also should pay attention to the research on ESD microenvironment. This will be a powerful weapon for our cancer prevention.
In short, the occurrence of cancer as a continuous and uninterrupted process, we should pay attention to all stages of its occurrence and development, and prevention as the “biblical” of humans against disease should receive more attention. At this time, the abundant signal molecules produced by the tumor precursor microenvironment and precursor cell interactions, as well as cell composition and function changes, have become our favorable biomarkers and targets for preventive treatment.
Tumor Microenvironment
As the oncogenic mutation of tumor precursor cells continues to accumulate, the precancerous lesions further develop into cancer. Cancer tissues continue to deteriorate by interacting with TME and eventually lead to the occurrence of metastasis. In the following, we will review the research progression on the interaction between the main components of TME and EC tissue (Figure 2).
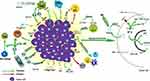 |
Figure 2 Immune landscape of ESCC tumor microenvironment. |
Hypoxia, Acidosis, and Nutrient Depletion are the Basic Characteristics of TME
Hypoxia and acidosis are common phenomenon in TME and can cause a series of metabolic changes in tumors. When we talk about the hypoxic microenvironment, the famous Warburg effect and HIF-1 should be mentioned. Under hypoxic conditions, prolyl-hydroxylase 2 (PHD-2) cannot hydroxylate HIF-1α, which blocks the degradation of HIF-1α.39,40 The Warburg effect means that even in a microenvironment with sufficient oxygen, tumor cells obtain energy mainly through glycolysis rather than oxidative phosphorylation.41 This metabolic transformation is caused by the HIF-1 transcriptional regulatory factors, which up-regulate GLUTs, hexokinase isoform 2 (HK2), pyruvate kinase isoform M (PKM) and other key factors of glycolytic metabolism, so as to transform the cancer metabolism into a mainly aerobic glycolytic metabolic pathway.40–42 The theory is also applicable to EC. For instance, GLUT-1 highly expressed in ESCC tumor cells and pre-cancerous tissues of dysplasia,35 in order to improve the efficiency of glucose transport in response to hypoxia microenvironment. PKM2, an important enzyme that converts phosphoenolpyruvate to pyruvate during glycolysis, can promote the aerobic glycolysis metabolism of ESCC under the regulation of the mTOR pathway.43
Another consequence brought about by glycolysis is the accumulation of its metabolite lactic acid in cells.44 In order to prevent intracellular pH imbalance, tumor cells trigger the proton transport mechanism to maintain the intracellular environment.45 Carbonic anhydrase IX (CAIX) is a target gene of HIF-1α, which mainly maintains the pH value of cells under hypoxic conditions. It is found that its expression increased in ESCC, and CAIX can also promote the migration and metastasis of ESCC cells while maintaining intracellular pH stability.46 Elevated CAIX expression is predictive of greater aggressiveness and shorter survival of EC.47,48 In addition, a recent EC study found that the vacuolar H + -adenosine triphosphatase (ATPase) subunit V0C (ATP6V0C), whose main function is to maintain a constant pH and induce acidification of organelles, can interact with PKM2 to upregulate the expression of glycolytic enzymes and increase extracellular acidification rate.49 Finally, the accumulation of metabolites and the depletion of interstitial nutrients pose great challenges for immune cells to play an anti-tumor role in TME, but cancer cells themselves are not affected by microenvironment selection.40
Importantly, as a crucial intermediate molecule in the regulation of tumor metabolism, HIF-1α also plays tumor-promoting functions in many different types of tumors, including EC, such as inducing and regulating angiogenesis,50,51 promoting tumor growth, proliferation, recurrence and metastasis and remodeling ECM. First, the regulation of HIF-1α on angiogenesis in esophageal tissues has been reported in the BE microenvironment.13 In EC, HIF-1α protein expression is also significantly correlated with VEGF protein expression and related to poor prognosis of EC.52–54 Secondly, as a key transcription factor in tumor cells, HIF-1 can specifically bind to various other promoters to regulate the progress of EC. For example, it can bind to the SP1 promoter and regulate its transcription program in order to enhance the ability of ESCC cells to migrate and invade and promote tumor recurrence and metastasis.55 Other studies have mentioned that it also could bind to the hypoxic response element, which located at the upstream of the Insulin-like growth factor binding protein 3 (IGFBP3) transcription initiation site, and then induce continuous translation of IGFBP3 mRNA44 and promote ESCC cell growth and proliferation through the insulin-like growth factor-independent pathway.56 Finally, it can also directly bind to the promoter-specific hypoxic response element of ECM metalloproteinase inducer (EMMPRIN), and further promote EC cell migration and epithelial–mesenchymal transition (EMT).57 In addition, another effect of HIF-1α is to inhibit E-cadherin and increase the expression of MMP-2 to stimulate EC infiltration and metastasis,58 and its mechanism may be related to the upstream regulation of Nuclear factor erythroid-2-related factor 2 (an important cytoprotective factor).59 The consistency of these results shows that HIF-1α also has a regulatory effect on ECM components in EC TME. In summary, overexpression of HIF-1 can be produced for EC through a variety of regulatory mechanisms and is closely related to a variety of adverse prognostic factors such as tumor invasion depth, lymph node metastasis, and resistance to chemoradiation.60,61 However, some studies have shown that HIF-1α has different effects on the prognosis of ESCC and EAC, respectively.62 This suggested that more detailed and precise researches were required on different cell and molecular subtypes.
In recent years, researches have particularly focused on extracellular vesicles (EVs). The EVs in tumor TME play an important role in the information transmission and material exchange of tumor cells and mediating the local or remote mutual regulation of cancer cells. Studies have shown that tumor cells can release more EVs in TME under hypoxic conditions.63 According to the latest ESCC miRNA expression profiling analysis, the miRNAs in EVs secreted by ESCC cells would change significantly under hypoxic conditions. A total of 10,810 miRNAs were detected in EVs. Fifty miRNA was up-regulated and 34 miRNA was down-regulated significantly in hypoxic-treated cell lines, and these differentially expressed miRNAs are mainly involved in cancer-related and phospholipase D signaling pathways.64 Base on the studies of kidney cancer and breast cancer cells, it was speculated that the enhanced phospholipase D activity was necessary for stable expression of HIF-1 and HIF-2 and aerobic glycolysis of cells.65–67 Exosomes are special types of EVs with a diameter of 50–200nm. In previous studies, ESCC cell exosomes cultured under hypoxic conditions could promote the proliferation and invasion of endothelial cells in vivo and in vitro, and show significant angiogenic effects compared with non-hypoxic conditions.68 ESCC-derived exosomes can also promote the expansion of PD-1+ TAM and the expression of CD206 to promote the progress of ESCC.69 In addition to the study of the number and general function of exosomes, we are also committed to the study of the contents of exosomes such as miRNA, DNA, or protein molecules. For example, high expression of exosomal microRNA-21 in ESCC can significantly reduce the sensitivity of cisplatin to chemotherapy70 and promote tumor cell migration and invasion.71,72 Exosome microRNA-212 can promote the movement and invasion of ESCC cells by promoting EMT and degrading ECM.73 Studies of exosomes in EC have been reported in detail in several reviews.74–76 Although many studies have proved that exosomes have great potential as markers for early diagnosis, treatment, or assessing prognosis in EC, there is no standard method for the isolation and purification of exosomes, and the functional mechanism of their inclusion bodies is not fully understood. In summary, anoxic microenvironment is the basis of important mechanisms of action in many tumors. Many targeted studies on HIF and its regulatory pathways, key enzymes of glycolysis, proton transport-related pathways, or exosomes have been actively carried out,77–79 but there is still a huge gap in this field (Table 1).
 |
Table 1 The Role of Hypoxia and Acidosis in the TME of Esophageal Carcinoma |
Myeloid Cell Line is an Important Regulatory Cell Population for TME
Table 2 MDSCs are immature bone marrow cells produced under persistent inflammatory conditions such as cancer, chronic disease, or persistent infection.80 MDSC can be mainly divided into two subgroups: polymorphonuclear (PMN)-MDSC and monocyte (M)-MDSC.81 The morphological and phenotypic characteristics of PMN-MDSC are similar to neutrophils, while M-MDSC is similar to monocytes.82,83 Numerous studies have proven that MDSCs have a powerful inhibitory effect on tumor immunity, including inhibition of T cell activity84 and NK cell function,85 induction of Tregs activation,86 promotion of fibroblasts to CAFs,87 and tumor resistance. High infiltration of MDSCs is a poor prognostic marker for many types of tumors.81,88,89
 |
Table 2 The Role of Myeloid Cells in the TME of Esophageal Carcinoma |
On the one hand, the interaction of cytokines with MDSCs plays a key role in the progress of EC. These cytokines mainly include IL-4, IL-6, IL-8, IL-13, and so on. For example, tumor-associated gene MAEL is over-expressed in EC tissues and up-regulates the inflammatory factor IL-8 to promote the recruitment of PMN-MDSCs in tumor tissues for tumor-promoting, while the up-regulated PMN-MDSCs can promote MAEL expression through the TGF-β/Smad pathway.90 In addition, Th2-derived cytokines IL-4, IL-6, and IL-13 can significantly enhance the immunosuppressive function of MDSCs in EC by inducing the expression of arginase-1 (ARG1).81,89,91 On the other hand, the heterogeneity of MDSCs is also paid special attention. A study found that MDSCs with high CD38 expression in EC had stronger activated T cell immunosuppressive and tumor-promoting functions, and the anti-CD38 monoclonal antibody Daratumumab could inhibit esophageal tumor cell growth in vitro and in vivo.92 At present, Daratumumab has been clinically used to treat multiple myeloma with acceptable safety and efficacy.93 Therefore, the application of this drug in EC may be a promising treatment direction. The functional research of MDSCs in different subgroups may become a novel development direction.
Macrophages can differentiate into two cell types with completely different functions: tumor suppressor macrophages (M1) and tumor-promoting macrophages (M2). M1 macrophages play a role in tumor rejection as “classic” activated macrophages, while M2 macrophages can promote tumor progression. M2 macrophages can be activated and promoted by tumor cell Th-2 cytokines such as IL-4 and IL-13.94,95 TAMs are M2-like macrophages, and some of them overexpress CD163, CD206, and CD204. The progression of EC can be promoted by TAMs through inducing tumor-associated lymphangiogenesis,96 tumor cell proliferation and invasion,97 and EC immune escape.95 It has been also demonstrated that TAMs infiltration in EC TME was a predictor of poor prognosis.98,99
The complexity of the interaction between TAMs and cancer cells also reveals its importance to cancer cells, and the expression of various cytokines is an important way for TAMs to participate in tumor regulation. For instance, several studies by Naoki et al have shown that in vitro cultured ESCC cell lines, Growth Differentiation Factor 15 (GDF15) derived from TAMs and tumor cells could indirectly activate TGF-βRII receptors in cancer cells, thereby inducing PI3K/Akt and MEK/Erk pathways and increase ESCC cell proliferation and migration.100,101 In addition, TAM-like peripheral-blood monocyte (PBMo)-derived macrophages were found in co-culture to express IL-8 and phosphorylate the Akt and Erk1/2 pathways by binding to CXCR1, CXCR2 expressed in ESCC cell lines and to promote tumor migration and invasion.102 It is also noteworthy that CD204 + subtype TAM shows a significant tumor-promoting effect in EC. In previous report, CD204 + macrophages in ESCC tissues were closely related to microvessel density, clinical stage, vascular invasion, depth of tumor invasion, lymph node metastasis, and disease-free survival.103 Simultaneously, Cyr61, an angiogenesis inducer of the CCN protein family,104 can be expressed in ESCC cells and TAMs, which can not only promote macrophage migration through the MEK/ERK pathway but also promote CD204 expression in macrophages.105 CD204 + TAM can also increase the expression of tumor suppressor protein annexin A10 (ANXA10) in cancer cells, and promote the proliferation of ESCC cells through phosphorylation of Akt and Erk1/2 pathways.106 The high expression of ANXA10 in cancer cells has been found to promote cancer cell growth and proliferation in a variety of tumors including lung cancer,107 ovarian cancer,108 and prostate cancer.109 Indeed, various secreted factors such as Cyr61,105 GDF15,100,101,110 TGF-βRII,100,111,112 and ANXA10106 have been reported to be associated with poor prognosis of ESCC. This series of studies revealed that TAMs could promote ESCC progression through strong secretory functions, and demonstrated the huge potential of TAMs-related pathways and molecules as novel therapeutic targets and biomarkers. Meanwhile, CD204 + subtypes of TAMs show more obvious tumor-promoting effects, which requires further studies on the specific mechanism of the interaction between TAMs of different functional subgroups and tumor cells. In addition, TAMs of different subtypes have been reported to have different tumor-promoting effects on EAC, but the specific mechanism remains to be studied in the future.113
DCs can not only secrete cytokines to regulate immune function in TME but also induce adaptive immunity through its powerful antigen-presenting ability, exerting immune-stimulating effect further. DCs present in esophageal tissue belong to Langerhans cells, which are bone marrow-derived dendritic cells.114 When DCs capture antigens and mature, lysosomal-associated membrane glycoprotein 3 (LAMP-3) is significantly up-regulated. LAMP-3 can transiently express and participate in the loading and transport of MHC class II peptides to the cell surface.115,116 Therefore, it can be regarded as a specific marker of mature dendritic cells. In an analysis of specimens from 80 postoperative patients with EC, it was found that CD8 + T cells clustered around LAMP-3 DC and formed LAMP-3 DC-CD8 + T cell clusters. In the same pathological stage, the 5-year survival rate of patients with high infiltration of LAMP-3 was higher than with low infiltration of LAMP-3.117 However, the expression of LAMP-3 in non-DC cells has different clinical results, and many reports have shown that the expression of LAMP-3 in non-DC (including EC) is closely related to poor prognosis.118–121 This may be related to the activation of its downstream signaling pathway.
Antigen presentation function is one of the major functions of DCs cells, and the application of vaccines based on DCs antigen presentation function in cancer has been widely studied. However, these studies are only performed primarily in patients with prostate cancer, melanoma, renal cell carcinoma, and glioblastoma nowadays.122 For example, Sipuleucel-T is the first antigen-presenting cell-based therapeutic cancer vaccine approved by the FDA and has been used clinically for prostate cancer treatment.123 The DCs vaccine in EC has also been partially reported.124,125 It was shown that the strategy of using DCs loaded with autologous tumor RNA to improve the cytotoxic response was effective in the treatment of EC.126 Another in vitro experiment confirmed that DC vaccine could activate SART1 peptide-specific CTLs,127 nevertheless, exact clinical efficacy and survival benefits have not been observed in clinical trials. However, in a Phase I clinical trial of 40 EC patients, the 1-year (82.1% vs 50.0%, P=0.04) and 2-year (67.8% vs 33.3%, P=0.04) survival rates of DC vaccine combined with radiotherapy were significantly improved compared to patients not receiving the DC vaccine therapy.128 This result confirms its reliability and therapeutic potential from a clinical perspective. These contradictory results may suggest that it was needed to develop individualized treatment standards for patients and prepare higher quality DC vaccines. The role of DC vaccine in the treatment of EC needs to be determined by further researches.
Tumor-Infiltrating Lymphocytes (TILs) are Important Components of Anti-Tumor Immunity
TILs are the crucial components of tumor antagonism in TME, including T lymphocytes, B lymphocytes, and NK cells. Most of TILs are associated with good prognosis in a variety of solid tumors such as EC,129–132 melanoma,133 breast cancer,134 and gastric cancer.135 In the following, we will explain the main types of TILs in EC and their research progression (Table 3).
 |
Table 3 The Role of Lymphocytes in the Tumor Microenvironment of Esophageal Carcinoma |
CD8 + T lymphocytes are essential immune components which exert anti-tumor cell immunity. The primary cells differentiated and matured into cytotoxic T lymphocytes after interacting with DCs, CD4 + T cells, and NK cells, and mediated their cytotoxic response through the perforin-granzyme pathway and FASL pathway. CD8 + T cells have also been proven to be positive predictors of both ESCC136 and EAC,137 and T cell suppression is an important mechanism for tumor immune escape. Previous studies have shown that the expression of indoleamine 2,3-dioxygenase (IDO) in ESCC was associated with decreased the number of CD8 + TILs infiltration and poor prognosis.138,139 This may be due to IDO-mediated tryptophan catabolism, thus suppressing T lymphocyte proliferation.140 In addition, it has been reported that CAFs and microvascular endothelial cells were the major IDO expressing cells in the ESCC interstitial.141 This finding indicates that IDO and its pathway may become new therapeutic targets and prognostic markers.
The exploration of immune checkpoints has always been the focus of our research. PD-1 is mainly expressed on the surface of activated T cells and can bind to programmed death ligand-1 (PD-L1) expressed in tumor cells to make tumor cells escape from the cytotoxicity of CTL.142 In ESCC, multiple studies including a meta-analysis have shown that the increased expression of PD-L1 was associated with poor prognosis,143–146 while a study of EAC seemed to suggest that upregulation of PD-L1 was conversely a favorable prognostic factor.147 Certainly, more studies are required to confirm the exact effect of PD-L1 expression in EAC, and it is also essential to conduct more research on PD-1/PD-L1 inhibitors in ESCC and EAC. So far PD-1/PD-L1 inhibitors have been applied clinically in more than 10 tumors. In a recent Phase III clinical trial148 and previous Phase II clinical trials,149 the results consistently showed that pembrolizumab (anti-PD-1 antibody) can improve OS in patients with advanced ESCC. Based on these results, the clinical application of pembrolizumab in patients with relapsed metastasis or locally advanced ESCC has been approved by the FDA. Simultaneously, several phase III clinical trials exploring the application of such drug in other indications of EC are also ongoing.150 Other immunotherapy strategies based on CD8 + T cells in EC are also promising. For example, the cytotoxicity of T lymphocytes can be enhanced by an exogenous pathway, such as the aforementioned DCs vaccine. Or develop other immune checkpoint inhibitors, such as PD-L2 and CTLA-4.151 This will be the direction of researches for EC treatment.
Th17 lymphocyte is a branch of CD4 + helper T cells, and its main effector molecule is IL-17. However, the role of Th17 lymphocytes in antitumor immunity remains controversial. A meta-analysis showed that IL-17A overexpression was significantly associated with poor prognosis in liver cancer and non-small cell lung cancer, but IL-17A expression was associated with a significant improvement in overall survival (OS) in patients with ESCC.152 Similarly, we found in chemotaxis analysis that IL-17A expressed by Th17 cells can induce ESCC cells to produce chemokines such as CCL20, CXCL-9, CXCL-10, and CXCL13, thereby chemotactic effector T cells, DCs, B cells, and NK cells migrate to ESCC tissue and then exert antitumor effect.153,154 In addition, Chen et al showed that the expression of chemokines such as CCL17, CCL20, and CCL22 in EC TME is up-regulated and can bind to CCR4 and CCR6 expressed by Th17 lymphocytes to induce Th17 lymphocytes infiltration.155 In other studies, cytokines and chemokines can also promote the differentiation and expansion of Th17 lymphocytes in ESCC TME, and the increase of Th17 lymphocytes is positively correlated with more lymph node metastasis and later clinical stages.156 However, studies in another EAC have found that IL-17A could activate MMP-2 and MMP-9 through the ROS/NF-κB signaling pathway. MMPs can catalyze the degradation of ECM and promote the migration and metastasis of cancer cells.157 The above results indicate that the role of Th17 lymphocytes is still controversial. Therefore, we need further research to clarify the role of Th17 lymphocytes, especially the role of Th17 lymphocytes in different pathological types of EC.
Tregs are a subset of CD4 + helper T cells, which are distinguished from other lymphocytes mainly by the expression of CD25 and Foxp3. Tregs can promote tumor progression by inhibiting the maturation of antigen-presenting cells (APC), reducing the secretion of cytokines and killing effect of CTL cells, and promoting angiogenesis.158–160 Many studies have reported that Foxp3 expression was increased in EC and closely related to poor prognosis.161–164 One of the specific mechanisms may be that FOXP3 directly inhibited the IL-2 gene and promoted the expression of CTLA-4 and CD25.158 It is also reported that IL-33 is highly expressed in ESCC tumor tissues, and IL-33 can promote CCL2 expression through the NF-κB pathway, thereby recruiting Tregs to promote tumor progression.165,166 The current therapeutic strategies targeting Tregs signaling pathways are quite promising. Researches on candidate target inhibitors such as CD25, CTLA-4, CCR4, and IDO-1 are underway,158 but their complex interactions in EC need further exploration.
Most TILs-related studies currently focus on T lymphocytes, while relatively little attention has been paid to the tumor immune function of B lymphocytes. B lymphocytes are another major component of TILs. They mainly exert antitumor immunity by secreting specific antibodies, presenting antigens to T cells, or directly killing cancer cells. Studies have shown that B lymphocytes can exert the above-mentioned tumor immune effects through the CCL19, 21/CCR7 axis, and CXCL13/CXCR5 axis,167 but their specific effects in different cancer types and different stages have yet to be explored. In EC, studies have shown that infiltration of B cells and T cells in tumor tissues indicated a better prognosis.131 However, specific subgroup analysis showed that the regulatory B cell (Bregs) subgroup in peripheral blood can promote the growth and proliferation of EAC tumors.168 Bregs are a subset of lymphocytes that can be activated in TME and interact with immune cells such as Tregs, MDSCs, and TAMs to promote tumor growth.169 There is no doubt that the study of this subgroup is a promising future direction. The targeted therapy designed for B cells is mainly concentrated in the field of B cell malignancies. Ibrutinib is a BTK (part of B-cell receptor signaling pathway) inhibitor approved for the treatment of B-cell malignancies.170 In addition, this drug can also increase the antitumor effect by inhibiting IL-2-inducible T-cell kinase (an important enzyme in Th2 cells), thereby changing the ratio between Th1 and Th2 cells.171 Importantly, Ibrutinib showed a significant increase in antitumor efficacy when combined with PD-1/PD-L1 inhibitors. However, the biological function of B lymphocytes in TME of EC is relatively insufficient. The related mechanism needs to be further explored, which will become a new direction for the treatment of EC.
NK cells are very important cells, which are involved in anti-tumor immunity and related to the good prognosis of EC.131,172 Impaired NK cell activity is one of the dominating mechanisms for tumor immune escape. Studies have shown that in EC TME, the expression of co-suppressor receptor Tim-3173 in NK cells was up-regulated, and NK cells overexpressing Tim-3 appeared to be dysfunctional and suggested poor prognosis.174 The increase of Tim-3 is related to the increase of the inflammatory factor TNF-α induced by the NF-κB pathway,174 but the related mechanism remains to be further studied. Tim-3 related pathway inhibitors based on NK cell immune activity may be a promising therapeutic direction for EC. In addition, it is shown in previous studies that NK cells expanded and co-cultured with special cells could achieve high proliferation and high cytotoxicity. These NK cells have a stronger cytotoxic effect on ESCC cells expressing NK cell activation receptor NKG2D,175 which provides a basis for the clinical application of NK cells. Of course, more biological functions of NK cells need further research.
Tumor Stromal Cells are Imperative Supporters for TME
Tumor mesenchymal cells mainly include three types of mesenchymal stem cells (MSCs):176 normal mesenchymal stem cells, tumor-associated mesenchymal stem cells, and CAFs. The role of MSCs in tumors is still controversial, but their supportive role in tumor progression is dominant.177,178 The results of two studies in EC also show that MSCs promote progression of tumor in vivo,179,180 which is a promising therapeutic direction, but the research on its specific mechanism and related effects need to be further studied.
CAFs, as the major components of TME interstitial cells, play a vital role in the process of remodeling TME and promoting the progression of many types of tumors.181–183 Similarly, CAFs can remodel TME through various mechanisms such as promoting angiogenesis, secreting cytokines, inducing EMT, recombining matrix components, and recruiting inflammatory cells to promote EC progression.184,185 In the EC field, multiple studies have consistently shown that CAFs were predictors of poor prognosis.186–189 Common effector molecules expressed by CAFs, including platelet-derived growth factor receptor (PDGFRα), PDGFRβ, smooth muscle actin (SMA), fibroblast activating protein (FAP), fibroblast-specific protein-1 (FSP1), etc.190 More specifically, SMA is associated with ESCC T stage progression, lymph node metastasis, and poor prognosis; FSP1 is associated with lower survival rates; PDGFRβ expression is associated with poorly differentiated tumors; FAP expression is associated with increased frequency of deaths.186 In addition, IL-6 cytokines secreted by FAP + CAFs not only promote ESCC cell growth and migration, EMT, and the occurrence of drug resistance191,192 but also recruit FoxP3 + T cells193 and induce M2 polarization of TAMs194 to promote tumor immunosuppression. The tumor-promoting effect of IL-6 has been detected in the BE microenvironment mentioned above,18,19 and it is a vital cytokine in multiple progression processes of EC. In addition, other signaling pathways have also been studied. The urokinase plasminogen activator (uPA) secreted by CAFs promotes the progression of ESCC through the PI3K/AKT and ERK pathways.195 The high expression of the transcription factor Twist1 in CAFs promotes the secretion of CXCL12 itself and the EMT process of EC cells through the ERK/AKT pathway.196,197 Two other important signaling molecules are hepatocyte growth factor HGF and transforming growth factor β (TGF-β), both of which are closely related to tumor cell invasion and metastasis. It has been confirmed that CAFs could express HGF and TGF-β1, and they promoted the progression and metastasis of EC through the HGF/Met198 and TGFβ1/Smad pathways,199,200 which were associated with poor prognosis of EC.201 Moreover, the mechanism by which TGFβ1 and HGF play a role is related to the angiogenic effect of EGFR.202,203 Due to the extensive expression of HGF and TGFβ in tumor stroma, targeted therapy for corresponding pathways has been widely studied.204–206 The treatment direction of EC has a guiding role. In addition, other therapeutic strategies against CAFs, such as infrared photoimmunotherapy targeted therapy strategies for FAP + CAFs, have also been proven to be effective.207 Infrared photoimmunotherapy is a combination of a specific monoclonal antibody and a photosensitizer and can produce great cytotoxicity on cells expressing specific antigens.208 Of course, the current research is still inadequate. CAFs show a strong tumor-promoting effect. The researches on the target of CAFs expression molecules and their corresponding pathways will be our new study direction (Table 4).
 |
Table 4 The Role of CAFs and ECM in the Tumor Microenvironment of Esophageal Cancer |
ECM is a Complex and Integral Part of TME
A tumor cell population is dynamically changing that continuously reshapes TME to adapt to its own progression. Matrix reprogramming is an important part of TME reconstruction,209 and the role of CAFs is essential for ECM reconstruction.181,210 As the original “soil” for the survival of tumor and other cells, a variety of ECM proteins contained in ECM, such as type I collagen, Fibronectin (FN),211 and Tenascin-C (TNC), can be up-regulated in tumor.212 Type I collagen is the most abundant protein of ECM and its role in tumors is still controversial,213 but it is increasingly believed that it played a major role in promoting tumor progression.214 In a recent ESCC study, cancer cells can produce cancer-derived type I collagen and inhibit tumor growth in contrast to previous studies which discovered that CAFs-derived collagen fibers could promote tumor growth,215 and these two type I collagens appear to have different molecular weights.216 This result gives us new insights and treatment directions for EC. Fibronectin (FN) is a high-molecular-weight glycoprotein component of the ECM. It has been reported that the high-matrix FN environment could promote the movement and migration of ESCC cells through a series of signaling pathways.217 TNC is an ECM protein secreted by tumor cells and myofibroblasts. It mainly promotes the movement and invasion of cancer cells by inhibiting cell adhesion.218 Studies have shown that the expression of TNC in ESCC was significantly correlated with the expression of C4.4A, which was a glycolipid-anchored membrane protein associated with poor prognosis in many human malignancies.218 In addition, TNC expressed in ESCC can also improve cancer stem cell characteristics and promote EMT-like changes through the Akt/HIF-1α axis.219 It is a predictor of poor prognosis in ESCC and a possible target for treatment.190 In addition, the high expression of the gene ADAM12-L in ESCC can promote the activation of focal adhesion kinase (FAK), and then promote cancer cell metastasis and migration through the FAK/JNK/c-Jun axis.220 Lysyl oxidase (LOX) also plays an important role in ECM reconstruction. The LOX protein family includes LOX and LOX-like 1–4 (LOXL1–4), whose main function is to maintain the homeostasis of the ECM by catalyzing the oxidative deamination of lysine in matrix proteins.221,222 Current studies in ESCC have shown that expression of LOX,223,224 LOXL4,225 and 5-LOX226,227 was elevated and predicted shorter survival and poor prognosis for patients. Its specific mechanism is not clear. The unique mechanism of action of different LOX proteins is our future research direction.
MMPs play an important role in tumor ECM remodeling, which can promote tumor spread and metastasis mainly by regulating the degradation and remodeling of matrix protein networks. MMPs include matrix proteases, collagenases (MMP-1, -8, and -13), and gelatinases (MMP-2 and MMP-9) according to their structural and functional specificity.228,229 Studies have found that the expression of apurinic/apyrimidinic endonuclease (APE1) was abnormally increased in EAC cell lines and induced ARF6 activity in a redox-dependent manner to up-regulate MMP-14 (also known as membrane-type matrix metalloproteinase MT1-MMP).230 The increased expression of MMP-14 can activate MMP-2 and then mediate ECM degradation,231 thereby promoting tumor migration. In addition, a number of other studies have shown that MMP-2,232,233 MMP-3,234 MMP-7,235 MMP-9,232,236 MMP-12,237 MT2-MMP,238 and MMMP-16 (namely MT3-MMP)239 all play negative regulatory roles in the process of EC through their different mechanisms. Among the nearly 30 different subtypes of MMPs, MMP-2 and MMP-9 are most closely related to EC. The MAPK pathway seems to be the core mechanism regulating MMP-2 and MMP-9 expression.209,240 In short, ECM is a space for information exchange and growth between tumor cell subgroups in tumors. It contains a large number of signal pathways and involves hundreds of signal molecules. Our current research is still the tip of the iceberg (Table 4).
The Interaction Between Immune Cells is an Indirect Promoter of Tumor Progression
In this complex network mechanism of tumor microenvironment, there is also important information exchange between each immune cell apart from the interaction between various immune cells and esophageal cancer cells, which indirectly promotes the progress of esophageal cancer (Figure 3). For example, a variety of cytokines (IL-4, IL-6, IL-13) derived from Th-2 can promote the recruitment of MDSCs in the tumor microenvironment of EC81,89,91 and the polarization of macrophages into M2 macrophages.94,95 In addition, as an important component of TME, CAFs can also promote the development of ESCC by secreting cytokines such as IL-6 to recruit Tregs193 and promote the polarization of M2 macrophages.194 Moreover, MDSCs, especially MDSCs with high expression of CD38, can strongly inhibit the cytotoxic effect of activated T cells in EC.92 The activity of NK cells is also inhibited by MDSCs through inhibiting NK cell perforin, instead of granular enzyme through direct contact-dependent pathway.85 Other functions of MDSCs include inducing the activation of Tregs,86 promoting the differentiation of CAFs,87 etc. The activated Tregs can further inhibit the antigen-presenting cells and reduce the secretion of cytokines, thereby indirectly reducing the cytotoxic effect of CTL.158–160 Additionally, the activation of the immune system mediated by both the familiar specific and non-specific immune systems plays a crucial part in antitumor immunity. For example, as the main cellular component of antitumor immunity, CTL can be activated by DCs, CD4+T cells, B cells, or NK cells and other immune cells, so as to further exert its cytotoxic effect. The infiltration of B cells, NK cells or DCs has also been reported to predict a good prognosis in EC.117,131,167,172 Therefore, it is not hard to draw the conclusion that there exists a balance and antagonism between anti-tumor immunity and pro-tumor immunity. With the progress of tumor, the balance of tumor immunity gradually shifted to the direction of anti-tumor immunity and finally became completely unbalanced. Therefore, the enhancement of anti-tumor immunity and the weakening of anti-tumor immunity will be a promising therapeutic development direction. However, for esophageal cancer, especially for different subtypes of EC (ESCC and EAC), the specific mechanism and degree of these interactions at different stages of the tumor remain to be explored.
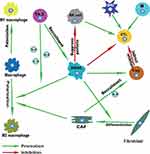 |
Figure 3 Immune landscape of immune cell interactions. |
Premetastatic Niche
Metastasis is the leading cause of cancer death and the final stage of cancer development. Metastasis is a step in which circulating tumor cells colonize other tissues or organs and become diffuse tumor cells. However, only 0.01% of circulating tumor cells were reported to successfully colonize and develop into spreading tumor cells.241,242 The main reason is that circulating tumor cells are severely affected by the local microenvironment. The well-known “seed and soil” hypothesis can help us understand this tumor metastasis process: tumor cells in situ (“seeds”) tend to settle on specific target organs (“soil”). The target organ microenvironment is formed before tumor cell metastasis and facilitates the colonization and metastasis of tumor cells, which is the pre-metastatic niche. The formation mechanism of the niche before metastasis mainly includes the following points: 1) Primary tumor secretion factors: tumor-derived secretion factors,243 EVs,244 etc. 2) Bone marrow-derived cell infiltration: MDSCs,245 Tregs or Th cells,246 TAMs,247 CAFs,248 etc. 3) Remodeling of the matrix microenvironment.249 Although research on the ecology before metastasis has been gradually paid attention to, its relationship with EC is still insufficiently understood.
It is well known that lymph nodes are the most common metastatic organs of EC, and lymph node metastasis is the most important prognostic factor of EC.250 Several studies have shown that this is closely related to the dense lymphatics and special lymphatic drainage directions in the anatomy of the esophagus.251,252 However, EC lymph node metastasis is not simply a process of direct cancer cell migration. Otto et al253 reported that the lymph node niche has changed significantly before EC lymph node metastasis. And another previous study found that ESCC could inhibit the cytotoxicity of lymphocytes in lymph nodes through factors other than metastasis.254 Both of these results have hinted at the existence of the lymph node pre-metastasis niche in EC. Otto et al253 also showed that the immune status of lymph nodes in patients with pN0 and pN1 was completely different. The lymph nodes in pN0 group showed obvious immune activation, while the non-metastatic lymph nodes in pN1 showed reduced immune response. A clear pattern of immune response activation was observed in the pN0 group. In contrast, lymph nodes in the pN1 group exhibited significant suppression patterns such as reduced immune response, decreased proliferation, and increased apoptosis. This finding is of great significance. It is assumed that in the relatively early stage of EC, tumor antigen drainage to lymph nodes led to the development of anti-tumor immunity. However, with the immune regulation of tumor secretion factors and the recruitment of immunosuppressive cells, the immune status of the lymph nodes can be transformed from an anti-tumor response to a tumor-promoting mode until the first colonization of the tumor cells, the formation of micro-metastases. It has also been found in previous studies that melanoma cells injected directly into lymph nodes can induce CD8 + cytotoxic T cell responses and exert anti-tumor immunity.255 Similarly, the formation of advanced micro-metastasis also implies the formation of an immunosuppressive microenvironment in lymph nodes.256,257 In summary, the formation of the microenvironment before lymph node metastasis is the only precondition for lymph node metastasis. The study of its mechanism about action can not only provide us with new therapeutic targets to prevent cancer metastasis but also find biomarkers before lymph node metastasis. Guide the scope of lymph node dissection during surgery, which will be one of the development directions of our treatment.
Conclusion
In this review, we divide the dynamic evolution of the microenvironment into three stages: tumor precursor microenvironment, TME, and premetastatic niche. The related research progression in EC is reviewed separately. Under high-risk environmental factors, such as gastroesophageal reflux, drinking, smoking and eating overheated food, metaplasia, and dysplasia can occur in normal esophageal epithelium. At the same time, environmental factors have also led to a partial remodeling of the epithelial microenvironment. Mild atypical epithelial hyperplasia can continuously accumulate malignant mutations and form the tumor precursor microenvironment under the induction of high-risk environmental factors. When it reaches a certain stage (with the removal of atypical hyperplasia in patients with high-risk environmental factors still irreversible), the tumor precursor cells and microenvironment can form a vicious cycle. For example, ESD can increase epithelial cell mutation and heterogeneity in an acidic microenvironment, and the increase in mutations promotes the formation of a highly proliferative state of cells and can produce more acidic metabolites, thereby forming a vicious cycle and eventually leading to the appearance of cancer. Therefore, the formation of malignant microenvironment changes dynamically with the change of precancerous cells in the microenvironment, which has also been confirmed in a small amount of relevant literature on breast ductal carcinoma in situ microenvironment.258,259 After tumorigenesis, tumors and tumor-related cells can remodel the distal metastatic environment by remote regulation such as secretion of cytokines or exosomes to prepare for metastasis while tumor cells continue to reshape TME. In EC metastasis, we mainly explored the microenvironment of lymph node metastasis and demonstrated that the pre-metastatic ecology of lymph nodes occurred before tumor cell colonization. All in all, understanding the entire dynamic evolution of the microenvironment is important for tumorigenesis and development. Of course, research on TME is helpful for targeted treatment of cancer. However, the study of the early tumor precursor microenvironment will become an important direction for cancer prevention, and the study of the pre-metastasis niche will become an important method of re-evaluating treatment strategies and preventing metastasis. Research at these stages is equally significant.
Some aspects need to be pointed out that may guide the future development of the EC field. 1). Regarding the EC precursor microenvironment, most of the current studies focus on the microenvironment of EAC precancerous lesion (BE), while little is known about the microenvironment of ESCC precancerous lesion. 2). We mainly discussed the associations between primary but not secondary EC microenvironment and tumor. The significance of secondary EC for this study lies in: whether there are some differences between the microenvironment of secondary EC and that of primary EC. More importantly, when other tumor cells colonize the esophageal tissue, whether the premetastatic niche of the esophagus has formed and plays a role in the process of secondary metastasis. 3). With respect to TME, most of the current studies focus on ESCC or all types of EC. However, it is essential to separate studies on ESCC and EAC, and more attention should be paid to the research on EAC. In general, TME is a relatively complex, wide-ranging, and promising field. However, there are still a lot of gaps in the field of EC. More research and exploration in this area is essential and critical for us to further elucidate the occurrence and development of EC.
Disclosure
The authors have no conflicts of interest to disclose.
References
1. Haiyu Z, Xiaofeng P, Xiangqiong M, et al. Incidence and survival changes in patients with esophageal adenocarcinoma during 1984–2013. Biomed Res Int. 2019;2019:7431850. doi:10.1155/2019/7431850
2. Gupta B, Kumar N. Worldwide incidence, mortality and time trends for cancer of the oesophagus. Eur J Cancer Prev. 2017;26(2):107–118. doi:10.1097/CEJ.0000000000000249
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi:10.3322/caac.21551
4. Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381(9864):400–412. doi:10.1016/S0140-6736(12)60643-6
5. Kim JA, Shah PM. Screening and prevention strategies and endoscopic management of early esophageal cancer. Chin Clin Oncol. 2017;6(5):50. doi:10.21037/cco.2017.09.05
6. Barsouk A, Rawla P, Hadjinicolaou AV, Aluru JS, Barsouk A. Targeted therapies and immunotherapies in the treatment of esophageal cancers. Med Sci (Basel). 2019;7(10):100.
7. Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22(1):329–360. doi:10.1146/annurev.immunol.22.012703.104803
8. Wang M, Zhao J, Zhang L, et al. Role of tumor microenvironment in tumorigenesis. J Cancer. 2017;8(5):761–773. doi:10.7150/jca.17648
9. Altorki NK, Markowitz GJ, Gao D, et al. The lung microenvironment: an important regulator of tumour growth and metastasis. Nat Rev Cancer. 2019;19(1):9–31. doi:10.1038/s41568-018-0081-9
10. Rojas A, Araya P, Gonzalez I, Morales E. Gastric tumor microenvironment. Adv Exp Med Biol. 2020;1226:23–35.
11. Perus LJM, Walsh LA. Microenvironmental heterogeneity in brain malignancies. Front Immunol. 2019;10:2294. doi:10.3389/fimmu.2019.02294
12. Höpken UE, Rehm A. Targeting the tumor microenvironment of leukemia and lymphoma. Trends Cancer. 2019;5(6):351–364. doi:10.1016/j.trecan.2019.05.001
13. Baatar D, Jones MK, Tsugawa K, et al. Esophageal ulceration triggers expression of hypoxia-inducible factor-1 alpha and activates vascular endothelial growth factor gene: implications for angiogenesis and ulcer healing. Am J Pathol. 2002;161(4):1449–1457. doi:10.1016/S0002-9440(10)64420-3
14. Ling FC, Khochfar J, Baldus SE, et al. HIF-1alpha protein expression is associated with the environmental inflammatory reaction in Barrett’s metaplasia. Dis Esophagus. 2009;22(8):694–699. doi:10.1111/j.1442-2050.2009.00957.x
15. Suchorolski MT, Paulson TG, Sanchez CA, Hockenbery D, Reid BJ. Warburg and crabtree effects in premalignant Barrett’s esophagus cell lines with active mitochondria. PLoS One. 2013;8(2):e56884. doi:10.1371/journal.pone.0056884
16. Quante M, Graham TA, Jansen M. Insights into the pathophysiology of esophageal adenocarcinoma. Gastroenterology. 2018;154(2):406–420. doi:10.1053/j.gastro.2017.09.046
17. Stairs DB, Kong J, Lynch JP. Cdx genes, inflammation, and the pathogenesis of intestinal metaplasia. Prog Mol Biol Transl Sci. 2010;96:231–270.
18. Zhang HY, Zhang Q, Zhang X, et al. Cancer-related inflammation and Barrett’s carcinogenesis: interleukin-6 and STAT3 mediate apoptotic resistance in transformed Barrett’s cells. Am J Physiol Gastrointest Liver Physiol. 2011;300(3):G454–60. doi:10.1152/ajpgi.00458.2010
19. Yu C, Zhang Q, Zhang HY, et al. Targeting the intrinsic inflammatory pathway: honokiol exerts proapoptotic effects through STAT3 inhibition in transformed Barrett’s cells. Am J Physiol Gastrointest Liver Physiol. 2012;303(5):G561–9. doi:10.1152/ajpgi.00033.2012
20. Park HS, Park JY, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Res Clin Pract. 2005;69(1):29–35. doi:10.1016/j.diabres.2004.11.007
21. Chemnitzer O, Götzel K, Maurer L, et al. Response to TNF-α is increasing along with the progression in Barrett’s esophagus. Dig Dis Sci. 2017;62(12):3391–3401. doi:10.1007/s10620-017-4821-6
22. O’Sullivan KE, Phelan JJ, O’Hanlon C, Lysaght J, O’Sullivan JN, Reynolds JV. The role of inflammation in cancer of the esophagus. Expert Rev Gastroenterol Hepatol. 2014;8(7):749–760. doi:10.1586/17474124.2014.913478
23. Tselepis C, Perry I, Dawson C, et al. Tumour necrosis factor-alpha in Barrett’s oesophagus: a potential novel mechanism of action. Oncogene. 2002;21(39):6071–6081. doi:10.1038/sj.onc.1205731
24. Sen M, Hahn F, Black TA, et al. Flow based single cell analysis of the immune landscape distinguishes Barrett’s esophagus from adjacent normal tissue. Oncotarget. 2019;10(38):3592–3604. doi:10.18632/oncotarget.26911
25. Kavanagh ME, Conroy MJ, Clarke NE, et al. Impact of the inflammatory microenvironment on T-cell phenotype in the progression from reflux oesophagitis to Barrett oesophagus and oesophageal adenocarcinoma. Cancer Lett. 2016;370(1):117–124. doi:10.1016/j.canlet.2015.10.019
26. Lind A, Siersema PD, Kusters JG, Van der Linden JA, Knol EF, Koenderman L. The immune cell composition in Barrett’s metaplastic tissue resembles that in normal duodenal tissue. PLoS One. 2012;7(4):e33899. doi:10.1371/journal.pone.0033899
27. Lind A, Siersema PD, Kusters JG, Konijn T, Mebius RE, Koenderman L. The microenvironment in Barrett’s esophagus tissue is characterized by high FOXP3 and RALDH2 levels. Front Immunol. 2018;9:1375. doi:10.3389/fimmu.2018.01375
28. Souza RF. Reflux esophagitis and its role in the pathogenesis of Barrett’s metaplasia. J Gastroenterol. 2017;52(7):767–776. doi:10.1007/s00535-017-1342-1
29. Souza RF, Bayeh L, Spechler SJ, Tambar UK, Bruick RK. A new paradigm for GERD pathogenesis. Not acid injury, but cytokine-mediated inflammation driven by HIF-2α: a potential role for targeting HIF-2α to prevent and treat reflux esophagitis. Curr Opin Pharmacol. 2017;37:93–99. doi:10.1016/j.coph.2017.10.004
30. Garalla HM, Lertkowit N, Tiszlavicz L, et al. Matrix metalloproteinase (MMP)-7 in Barrett’s esophagus and esophageal adenocarcinoma: expression, metabolism, and functional significance. Physiol Rep. 2018;6(10):e13683. doi:10.14814/phy2.13683
31. Davelaar AL, Straub D, Buttar NS, Fockens P, Krishnadath KK. Active matrix metalloproteases are expressed early on and are high during the Barrett’s esophagus malignancy sequence. Scand J Gastroenterol. 2015;50(3):321–332. doi:10.3109/00365521.2014.940379
32. !!! INVALID CITATION !!! 32.
33. Taylor PR, Abnet CC, Dawsey SM. Squamous dysplasia—the precursor lesion for esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2013;22(4):540–552. doi:10.1158/1055-9965.EPI-12-1347
34. Auld M, Srinath H, Jeyarajan E. Oesophageal squamous dysplasia. J Gastrointest Cancer. 2018;49(3):385–388. doi:10.1007/s12029-018-0122-3
35. de Andrade Barreto E, de Souza Santos PT, Bergmann A, et al. Alterations in glucose metabolism proteins responsible for the Warburg effect in esophageal squamous cell carcinoma. Exp Mol Pathol. 2016;101(1):66–73. doi:10.1016/j.yexmp.2016.05.014
36. Punnia-Moorthy A. Evaluation of pH changes in inflammation of the subcutaneous air pouch lining in the rat, induced by carrageenan, dextran and Staphylococcus aureus. J Oral Pathol. 1987;16(1):36–44. doi:10.1111/j.1600-0714.1987.tb00674.x
37. Boedtkjer E, Pedersen SF. The acidic tumor microenvironment as a driver of cancer. Annu Rev Physiol. 2020;82(1):103–126. doi:10.1146/annurev-physiol-021119-034627
38. Pedersen SF, Novak I, Alves F, Schwab A, Pardo LA. Alternating pH landscapes shape epithelial cancer initiation and progression: focus on pancreatic cancer. Bioessays. 2017;39(6).
39. Masoud GN, Li W. HIF-1α pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B. 2015;5(5):378–389. doi:10.1016/j.apsb.2015.05.007
40. Sormendi S, Wielockx B. Hypoxia pathway proteins as central mediators of metabolism in the tumor cells and their microenvironment. Front Immunol. 2018;9:40. doi:10.3389/fimmu.2018.00040
41. Kato Y, Maeda T, Suzuki A, Baba Y. Cancer metabolism: new insights into classic characteristics. Jpn Dent Sci Rev. 2018;54(1):8–21. doi:10.1016/j.jdsr.2017.08.003
42. Marín-Hernández A, Gallardo-Pérez JC, Ralph SJ, Rodríguez-Enríquez S, Moreno-Sánchez R. HIF-1alpha modulates energy metabolism in cancer cells by inducing over-expression of specific glycolytic isoforms. Mini Rev Med Chem. 2009;9(9):1084–1101. doi:10.2174/138955709788922610
43. Xiaoyu H, Yiru Y, Shuisheng S, et al. The mTOR pathway regulates PKM2 to affect glycolysis in esophageal squamous cell carcinoma. Technol Cancer Res Treat. 2018;17:1533033818780063. doi:10.1177/1533033818780063
44. Zeng L, Zhou HY, Tang NN, et al. Wortmannin influences hypoxia-inducible factor-1 alpha expression and glycolysis in esophageal carcinoma cells. World J Gastroenterol. 2016;22(20):4868–4880. doi:10.3748/wjg.v22.i20.4868
45. Kolosenko I, Avnet S, Baldini N, Viklund J, De Milito A. Therapeutic implications of tumor interstitial acidification. Semin Cancer Biol. 2017;43:119–133. doi:10.1016/j.semcancer.2017.01.008
46. Drenckhan A, Freytag M, Supuran CT, Sauter G, Izbicki JR, Gros SJ. CAIX furthers tumour progression in the hypoxic tumour microenvironment of esophageal carcinoma and is a possible therapeutic target. J Enzyme Inhib Med Chem. 2018;33(1):1024–1033. doi:10.1080/14756366.2018.1475369
47. Jomrich G, Jesch B, Birner P, et al. Stromal expression of carbonic anhydrase IX in esophageal cancer. Clin Transl Oncol. 2014;16(11):966–972. doi:10.1007/s12094-014-1180-z
48. Driessen A, Landuyt W, Pastorekova S, et al. Expression of carbonic anhydrase IX (CA IX), a hypoxia-related protein, rather than vascular-endothelial growth factor (VEGF), a pro-angiogenic factor, correlates with an extremely poor prognosis in esophageal and gastric adenocarcinomas. Ann Surg. 2006;243(3):334–340. doi:10.1097/01.sla.0000201452.09591.f3
49. Son SW, Chau GC, Kim ST, Um SH. Vacuolar H+-ATPase subunit V0C regulates aerobic glycolysis of esophageal cancer cells via pkm2 signaling. Cells. 2019;8(10):1137.
50. Li Y, Fu L, Li JB, et al. Increased expression of EIF5A2, via hypoxia or gene amplification, contributes to metastasis and angiogenesis of esophageal squamous cell carcinoma. Gastroenterology. 2014;146(7):1701–13.e9. doi:10.1053/j.gastro.2014.02.029
51. Ahluwalia A, Tarnawski AS. Critical role of hypoxia sensor – HIF-1 in VEGF gene activation. Implications for angiogenesis and tissue injury healing. Curr Med Chem. 2012;19(1):90–97. doi:10.2174/092986712803413944
52. Matsuyama T, Nakanishi K, Hayashi T, et al. Expression of hypoxia‐inducible factor‐1α in esophageal squamous cell carcinoma. Cancer Sci. 2005;96(3):176–182. doi:10.1111/j.1349-7006.2005.00025.x
53. Takala H, Saarnio J, Wiik H, Ohtonen P, Soini Y. HIF-1a and VEGF are associated with disease progression in esophageal carcinoma. J Surg Res. 2011;167(1):41–48. doi:10.1016/j.jss.2009.11.725
54. Tzao C, Lee SC, Tung HJ, et al. Expression of hypoxia-inducible factor (HIF)-1α and vascular endothelial growth factor (VEGF)-D as outcome predictors in resected esophageal squamous cell carcinoma. Dis Markers. 2008;25(3):141–148. doi:10.1155/2008/468323
55. Hu X, Lin J, Jiang M, et al. HIF-1α promotes the metastasis of esophageal squamous cell carcinoma by targeting SP1. J Cancer. 2020;11(1):229–240. doi:10.7150/jca.35537
56. Natsuizaka M, Kinugasa H, Kagawa S, et al. IGFBP3 promotes esophageal cancer growth by suppressing oxidative stress in hypoxic tumor microenvironment. Am J Cancer Res. 2014;4(1):29–41.
57. Wu X, Qiao B, Liu Q, Zhang W. Upregulation of extracellular matrix metalloproteinase inducer promotes hypoxia-induced epithelial-mesenchymal transition in esophageal cancer. Mol Med Rep. 2015;12(5):7419–7424. doi:10.3892/mmr.2015.4410
58. Jing SW, Wang YD, Kuroda M, et al. HIF-1α contributes to hypoxia-induced invasion and metastasis of esophageal carcinoma via inhibiting E-cadherin and promoting MMP-2 expression. Acta Med Okayama. 2012;66(5):399–407. doi:10.18926/AMO/48964
59. Shen H, Yang Y, Xia S, Rao B, Zhang J, Wang J. Blockage of Nrf2 suppresses the migration and invasion of esophageal squamous cell carcinoma cells in hypoxic microenvironment. Dis Esophagus. 2014;27(7):685–692. doi:10.1111/dote.12124
60. Ping W, Sun W, Zu Y, Chen W, Fu X. Clinicopathological and prognostic significance of hypoxia-inducible factor-1α in esophageal squamous cell carcinoma: a meta-analysis. Tumour Biol. 2014;35(5):4401–4409. doi:10.1007/s13277-013-1579-0
61. Jing SW, Wang J, Xu Q. Expression of hypoxia inducible factor 1 alpha and its clinical significance in esophageal carcinoma: a meta-analysis. Tumour Biol. 2017;39(7):1010428317717983. doi:10.1177/1010428317717983
62. Goscinski MA, Nesland JM, Giercksky KE, Dhakal HP. Primary tumor vascularity in esophagus cancer. CD34 and HIF1-α expression correlate with tumor progression. Histol Histopathol. 2013;28(10):1361–1368. doi:10.14670/HH-28.1361
63. Shao C, Yang F, Miao S, et al. Role of hypoxia-induced exosomes in tumor biology. Mol Cancer. 2018;17(1):120. doi:10.1186/s12943-018-0869-y
64. Chen F, Chu L, Li J, et al. Hypoxia induced changes in miRNAs and their target mRNAs in extracellular vesicles of esophageal squamous cancer cells. Thorac Cancer. 2020;11(3):570–580. doi:10.1111/1759-7714.13295
65. Toschi A, Edelstein J, Rockwell P, Ohh M, Foster DA. HIF alpha expression in VHL-deficient renal cancer cells is dependent on phospholipase D. Oncogene. 2008;27(19):2746–2753. doi:10.1038/sj.onc.1210927
66. Toschi A, Lee E, Thompson S, et al. Phospholipase D-mTOR requirement for the Warburg effect in human cancer cells. Cancer Lett. 2010;299(1):72–79. doi:10.1016/j.canlet.2010.08.006
67. Zheng Y, Rodrik V, Toschi A, et al. Phospholipase D couples survival and migration signals in stress response of human cancer cells. J Biol Chem. 2006;281(23):15862–15868. doi:10.1074/jbc.M600660200
68. Mao Y, Wang Y, Dong L, et al. Hypoxic exosomes facilitate angiogenesis and metastasis in esophageal squamous cell carcinoma through altering the phenotype and transcriptome of endothelial cells. J Exp Clin Cancer Res. 2019;38(1):389. doi:10.1186/s13046-019-1384-8
69. Li B, Song T-N, Wang F-R, et al. Tumor-derived exosomal HMGB1 promotes esophageal squamous cell carcinoma progression through inducing PD1+ TAM expansion. Oncogenesis. 2019;8(3):17. doi:10.1038/s41389-019-0126-2
70. Yang YC, Liu GJ, Yuan DF, Li CQ, Xue M, Chen LJ. Influence of exosome-derived miR-21 on chemotherapy resistance of esophageal cancer. Eur Rev Med Pharmacol Sci. 2019;23(4):1513–1519. doi:10.26355/eurrev_201902_17109
71. Liao J, Liu R, Shi YJ, Yin LH, Pu YP. Exosome-shuttling microRNA-21 promotes cell migration and invasion-targeting PDCD4 in esophageal cancer. Int J Oncol. 2016;48(6):2567–2579. doi:10.3892/ijo.2016.3453
72. Tanaka Y, Kamohara H, Kinoshita K, et al. Clinical impact of serum exosomal microRNA-21 as a clinical biomarker in human esophageal squamous cell carcinoma. Cancer. 2013;119(6):1159–1167. doi:10.1002/cncr.27895
73. Chen Z, Liu Y, Qi B, et al. MicroRNA‑212 facilitates the motility and invasiveness of esophageal squamous carcinoma cells. Mol Med Rep. 2019;20(4):3633–3641. doi:10.3892/mmr.2019.10647
74. Su LL, Chang XJ, Zhou HD, Hou LB, Xue XY. Exosomes in esophageal cancer: a review on tumorigenesis, diagnosis and therapeutic potential. World J Clin Cases. 2019;7(8):908–916. doi:10.12998/wjcc.v7.i8.908
75. Lin M, Zhou C, He S, et al. The research advances of exosomes in esophageal cancer. Biomark Med. 2019;13(8):685–695. doi:10.2217/bmm-2018-0314
76. Chen Y, Liu L, Li J, Du Y, Wang J, Liu J. Effects of long noncoding RNA (linc-VLDLR) existing in extracellular vesicles on the occurrence and multidrug resistance of esophageal cancer cells. Pathol Res Pract. 2019;215(3):470–477. doi:10.1016/j.prp.2018.12.033
77. Yu T, Tang B, Sun X. Development of inhibitors targeting hypoxia-inducible factor 1 and 2 for cancer therapy. Yonsei Med J. 2017;58(3):489–496. doi:10.3349/ymj.2017.58.3.489
78. Roma-Rodrigues C, Mendes R, Baptista PV, Fernandes AR. Targeting tumor microenvironment for cancer therapy. Int J Mol Sci. 2019;20(4):840. doi:10.3390/ijms20040840
79. Zhu Y, Zang Y, Zhao F, et al. Inhibition of HIF-1α by PX-478 suppresses tumor growth of esophageal squamous cell cancer in vitro and in vivo. Am J Cancer Res. 2017;7(5):1198–1212.
80. Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol. 2018;19(2):108–119. doi:10.1038/s41590-017-0022-x
81. Chen M-F, Kuan F-C, Yen T-C, et al. IL-6-stimulated CD11b+ CD14+ HLA-DR− myeloid-derived suppressor cells, are associated with progression and poor prognosis in squamous cell carcinoma of the esophagus. Oncotarget. 2014;5(18):8716–8728. doi:10.18632/oncotarget.2368
82. Gabrilovich DI. MDSC. Cancer Immunol Res. 2017;5(1):3–8. doi:10.1158/2326-6066.CIR-16-0297
83. Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi:10.1038/nri2506
84. Mazzoni A, Bronte V, Visintin A, et al. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J Immunol. 2002;168(2):689–695. doi:10.4049/jimmunol.168.2.689
85. Liu C, Yu S, Kappes J, et al. Expansion of spleen myeloid suppressor cells represses NK cell cytotoxicity in tumor-bearing host. Blood. 2007;109(10):4336–4342. doi:10.1182/blood-2006-09-046201
86. Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid derived suppressor cells promote cross-tolerance in B cell lymphoma by expanding regulatory T cells. Cancer Res. 2008;68(13):5439–5449. doi:10.1158/0008-5472.CAN-07-6621
87. Zhou J, Nefedova Y, Lei A, Gabrilovich D. Neutrophils and PMN-MDSC: their biological role and interaction with stromal cells. Semin Immunol. 2018;35:19–28. doi:10.1016/j.smim.2017.12.004
88. Karakasheva TA, Dominguez GA, Hashimoto A, et al. CD38+ M-MDSC expansion characterizes a subset of advanced colorectal cancer patients. JCI Insight. 2018;3(6):e97022. doi:10.1172/jci.insight.97022
89. Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother. 2011;60(10):1419–1430. doi:10.1007/s00262-011-1028-0
90. Li P, Chen X, Qin G, et al. Maelstrom directs myeloid-derived suppressor cells to promote esophageal squamous cell carcinoma progression via activation of the Akt1/RelA/IL8 signaling pathway. Cancer Immunol Res. 2018;6(10):1246–1259. doi:10.1158/2326-6066.CIR-17-0415
91. Li J, Zhang B-Z, Qin Y-R, et al. CD68 and interleukin 13, prospective immune markers for esophageal squamous cell carcinoma prognosis prediction. Oncotarget. 2016;7(13):15525–15538. doi:10.18632/oncotarget.6900
92. Karakasheva TA, Waldron TJ, Eruslanov E, et al. CD38-expressing myeloid-derived suppressor cells promote tumor growth in a murine model of esophageal cancer. Cancer Res. 2015;75(19):4074–4085. doi:10.1158/0008-5472.CAN-14-3639
93. Touzeau C, Moreau P. Daratumumab for the treatment of multiple myeloma. Expert Opin Biol Ther. 2017;17(7):887–893. doi:10.1080/14712598.2017.1322578
94. Van Dyken SJ, Locksley RM. Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: roles in homeostasis and disease. Annu Rev Immunol. 2013;31(1):317–343. doi:10.1146/annurev-immunol-032712-095906
95. Gao J, Wu Y, Su Z, et al. Infiltration of alternatively activated macrophages in cancer tissue is associated with MDSC and Th2 polarization in patients with esophageal cancer. PLoS One. 2014;9(8):e104453–e. doi:10.1371/journal.pone.0104453
96. Sun -M-M, He -L-L, Zhang H-X, et al. The synergistic effect of esophageal squamous cell carcinoma KYSE150 cells and M2 macrophages on lymphatic endothelial cells. Am J Transl Res. 2017;9(11):5105–5115.
97. Wang P, Xu L-J, Qin -J-J, Zhang L, Zhuang G-H. MicroRNA-155 inversely correlates with esophageal cancer progression through regulating tumor-associated macrophage FGF2 expression. Biochem Biophys Res Commun. 2018;503(2):452–458. doi:10.1016/j.bbrc.2018.04.094
98. Li J, Xie Y, Wang X, et al. Prognostic impact of tumor-associated macrophage infiltration in esophageal cancer: a meta-analysis. Future Oncol. 2019;15(19):2303–2317. doi:10.2217/fon-2018-0669
99. Miyashita T, Tajima H, Shah FA, et al. Impact of inflammation–metaplasia–adenocarcinoma sequence and inflammatory microenvironment in esophageal carcinogenesis using surgical rat models. Ann Surg Oncol. 2014;21(6):2012–2019. doi:10.1245/s10434-014-3537-5
100. Okamoto M, Koma Y-I, Kodama T, Nishio M, Shigeoka M, Yokozaki H. Growth differentiation factor 15 promotes progression of esophageal squamous cell carcinoma via TGF-β type II receptor activation. Pathobiology. 2020;87(2):1–14.
101. Urakawa N, Utsunomiya S, Nishio M, et al. GDF15 derived from both tumor-associated macrophages and esophageal squamous cell carcinomas contributes to tumor progression via Akt and Erk pathways. Lab Invest. 2015;95(5):491–503. doi:10.1038/labinvest.2015.36
102. Hosono M, Koma Y-I, Takase N, et al. CXCL8 derived from tumor-associated macrophages and esophageal squamous cell carcinomas contributes to tumor progression by promoting migration and invasion of cancer cells. Oncotarget. 2017;8(62):106071–106088. doi:10.18632/oncotarget.22526
103. Shigeoka M, Urakawa N, Nakamura T, et al. Tumor associated macrophage expressing CD204 is associated with tumor aggressiveness of esophageal squamous cell carcinoma. Cancer Sci. 2013;104(8):1112–1119. doi:10.1111/cas.12188
104. Chen Y, Du X-Y. Functional properties and intracellular signaling of CCN1/Cyr61. J Cell Biochem. 2007;100(6):1337–1345. doi:10.1002/jcb.21194
105. Shigeoka M, Urakawa N, Nishio M, et al. Cyr61 promotes CD204 expression and the migration of macrophages via MEK/ERK pathway in esophageal squamous cell carcinoma. Cancer Med. 2015;4(3):437–446. doi:10.1002/cam4.401
106. Kodaira H, Koma Y-I, Hosono M, et al. ANXA10 induction by interaction with tumor-associated macrophages promotes the growth of esophageal squamous cell carcinoma. Pathol Int. 2019;69(3):135–147. doi:10.1111/pin.12771
107. Hung M-S, Chen Y-C, Lin P, et al. Cul4A modulates invasion and metastasis of lung cancer through regulation of ANXA10. Cancers (Basel). 2019;11(5):618. doi:10.3390/cancers11050618
108. Wang J, Zhao S, Wang F, Wang J, Zhang Y. Prognostic significance of increased expression of annexin A10 (ANXA10) in serous epithelial ovarian cancer. Med Sci Monit. 2019;25:5666–5673. doi:10.12659/MSM.915911
109. Miyazawa Y, Sekine Y, Kato H, Furuya Y, Koike H, Suzuki K. Simvastatin up-regulates annexin A10 that can inhibit the proliferation, migration, and invasion in androgen-independent human prostate cancer cells. Prostate. 2017;77(4):337–349. doi:10.1002/pros.23273
110. Yang L, Chang -C-C, Sun Z, et al. GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nat Med. 2017;23(10):1158–1166. doi:10.1038/nm.4394
111. Fukai Y, Fukuchi M, Masuda N, et al. Reduced expression of transforming growth factor-beta receptors is an unfavorable prognostic factor in human esophageal squamous cell carcinoma. Int J Cancer. 2003;104(2):161–166. doi:10.1002/ijc.10929
112. Cheng H, Chen C, Liu LU, Zhan NA, Li B. Expression of Smad4, TGF-βRII, and p21waf1 in esophageal squamous cell carcinoma tissue. Oncol Lett. 2015;9(6):2847–2853. doi:10.3892/ol.2015.3146
113. Calpe S, Hoefnagel S, Bouchmal S, Del Sancho-Serra C, Straub D, Krishnadath KK. Tumor-associated macrophages as novel targets for immunotherapy in esophageal adenocarcinoma. United European Gastroenterol J. 2017;5(5 Supplement 1):A127–A8.
114. Yang W, Yu J. Immunologic function of dendritic cells in esophageal cancer. Dig Dis Sci. 2008;53(7):1739–1746. doi:10.1007/s10620-007-0095-8
115. Barois N, de Saint-vis B, Lebecque S, Geuze HJ, Kleijmeer MJ. MHC class II compartments in human dendritic cells undergo profound structural changes upon activation. Traffic. 2002;3(12):894–905. doi:10.1034/j.1600-0854.2002.31205.x
116. de Saint-vis B, Vincent J, Vandenabeele S, et al. A novel lysosome-associated membrane glycoprotein, DC-LAMP, induced upon DC maturation, is transiently expressed in MHC class II compartment. Immunity. 1998;9(3):325–336. doi:10.1016/S1074-7613(00)80615-9
117. Nishimura J, Tanaka H, Yamakoshi Y, et al. Impact of tumor‑infiltrating LAMP‑3 dendritic cells on the prognosis of esophageal squamous cell carcinoma. Esophagus. 2019;16(4):333–344. doi:10.1007/s10388-019-00669-w
118. Li Y, Du W, Han J, Ge J. LAMP3 promotes the invasion of osteosarcoma cells via SPP1 signaling. Mol Med Rep. 2017;16(5):5947–5953. doi:10.3892/mmr.2017.7349
119. Liu S, Yue J, Du W, Han J, Zhang W. LAMP3 plays an oncogenic role in osteosarcoma cells partially by inhibiting TP53. Cell Mol Biol Lett. 2018;23:33. doi:10.1186/s11658-018-0099-8
120. Liao X, Chen Y, Liu D, Li F, Li X, Jia W. High expression of LAMP3 is a novel biomarker of poor prognosis in patients with esophageal squamous cell carcinoma. Int J Mol Sci. 2015;16(8):17655–17667. doi:10.3390/ijms160817655
121. Lu J, Ma H, Lian S, et al. Clinical significance and prognostic value of the expression of LAMP3 in oral squamous cell carcinoma. Dis Markers. 2017;2017:1218254. doi:10.1155/2017/1218254
122. Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol. 2020;20(1):7–24. doi:10.1038/s41577-019-0210-z
123. Cheever MA, Higano CS. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin Cancer Res. 2011;17(11):3520–3526. doi:10.1158/1078-0432.CCR-10-3126
124. Milano F, Krishnadath KK. Novel therapeutic strategies for treating esophageal adenocarcinoma: the potential of dendritic cell immunotherapy and combinatorial regimens. Hum Immunol. 2008;69(10):614–624. doi:10.1016/j.humimm.2008.07.006
125. Guo G-H, Chen S-Z, Yu J, et al. In vivo anti-tumor effect of hybrid vaccine of dendritic cells and esophageal carcinoma cells on esophageal carcinoma cell line 109 in mice with severe combined immune deficiency. World J Gastroenterol. 2008;14(8):1167–1174. doi:10.3748/wjg.14.1167
126. Milano F, Rygiel AM, Buttar N, et al. An ex vivo readout for evaluation of dendritic cell-induced autologous cytotoxic T lymphocyte responses against esophageal cancer. Cancer Immunol Immunother. 2007;56(12):1967–1977. doi:10.1007/s00262-007-0341-0
127. Narita M, Kanda T, Abe T, et al. Immune responses in patients with esophageal cancer treated with SART1 peptide‑pulsed dendritic cell vaccine. Int J Oncol. 2015;46(4):1699–1709. doi:10.3892/ijo.2015.2846
128. Wang C, Pu J, Yu H, et al. A dendritic cell vaccine combined with radiotherapy activates the specific immune response in patients with esophageal cancer. J Immunother. 2017;40(2):71–76. doi:10.1097/CJI.0000000000000155
129. Jiang D, Liu Y, Wang H, et al. Tumour infiltrating lymphocytes correlate with improved survival in patients with esophageal squamous cell carcinoma. Sci Rep. 2017;7:44823. doi:10.1038/srep44823
130. Sudo T, Nishida R, Kawahara A, et al. Clinical impact of tumor-infiltrating lymphocytes in esophageal squamous cell carcinoma. Ann Surg Oncol. 2017;24(12):3763–3770. doi:10.1245/s10434-017-5796-4
131. Svensson MC, Warfvinge CF, Fristedt R, et al. The integrative clinical impact of tumor-infiltrating T lymphocytes and NK cells in relation to B lymphocyte and plasma cell density in esophageal and gastric adenocarcinoma. Oncotarget. 2017;8(42):72108–72126. doi:10.18632/oncotarget.19437
132. Yagi T, Baba Y, Ishimoto T, et al. PD-L1 expression, tumor-infiltrating lymphocytes, and clinical outcome in patients with surgically resected esophageal cancer. Ann Surg. 2019;269(3):471–478. doi:10.1097/SLA.0000000000002616
133. Lee N, Zakka LR, Mihm MC
134. Stanton SE, Disis ML. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J Immunother Cancer. 2016;4:59. doi:10.1186/s40425-016-0165-6
135. Zhang D, He W, Wu C, et al. Scoring system for tumor-infiltrating lymphocytes and its prognostic value for gastric cancer. Front Immunol. 2019;10:71. doi:10.3389/fimmu.2019.00071
136. Zhu Y, Li M, Mu D, et al. CD8+/FOXP3+ ratio and PD-L1 expression associated with survival in pT3N0M0 stage esophageal squamous cell cancer. Oncotarget. 2016;7(44):71455–71465. doi:10.18632/oncotarget.12213
137. Rauser S, Langer R, Tschernitz S, et al. High number of CD45RO+ tumor infiltrating lymphocytes is an independent prognostic factor in non-metastasized (stage I-IIA) esophageal adenocarcinoma. BMC Cancer. 2010;10(1):608. doi:10.1186/1471-2407-10-608
138. Zhang G, Liu W-L, Zhang L, et al. Involvement of indoleamine 2,3-dioxygenase in impairing tumor-infiltrating CD8+ T-cell functions in esophageal squamous cell carcinoma. Clin Dev Immunol. 2011;2011:384726. doi:10.1155/2011/384726
139. Kiyozumi Y, Baba Y, Okadome K, et al. IDO1 expression is associated with immune tolerance and poor prognosis in patients with surgically resected esophageal cancer. Ann Surg. 2019;269(6):1101–1108. doi:10.1097/SLA.0000000000002754
140. Sucher R, Kurz K, Weiss G, Margreiter R, Fuchs D, Brandacher G. IDO-mediated tryptophan degradation in the pathogenesis of malignant tumor disease. Int J Tryptophan Res. 2010;3:113–120. doi:10.4137/IJTR.S4157
141. Cui G, Li C, Xu G, et al. Tumor-associated fibroblasts and microvessels contribute to the expression of immunosuppressive factor indoleamine 2, 3-dioxygenase in human esophageal cancers. Pathol Oncol Res. 2018;24(2):269–275. doi:10.1007/s12253-017-0244-0
142. Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest. 2015;125(9):3384–3391. doi:10.1172/JCI80011
143. Wang Q, Feng F, Wang F, et al. PD-L1 expression on tumor cells was associated with unfavorable prognosis in esophageal squamous cell carcinoma. J Cancer. 2018;9(12):2224–2231. doi:10.7150/jca.24493
144. Hsieh -C-C, Hsu H-S, Li AF-Y, Chen Y-J. Clinical relevance of PD-L1 and PD-L2 overexpression in patients with esophageal squamous cell carcinoma. J Thorac Dis. 2018;10(7):4433–4444. doi:10.21037/jtd.2018.06.167
145. Jiang Y, Lo AWI, Wong A, et al. Prognostic significance of tumor-infiltrating immune cells and PD-L1 expression in esophageal squamous cell carcinoma. Oncotarget. 2017;8(18):30175–30189. doi:10.18632/oncotarget.15621
146. Guo W, Wang P, Li N, et al. Prognostic value of PD-L1 in esophageal squamous cell carcinoma: a meta-analysis. Oncotarget. 2017;9(17):13920–13933. doi:10.18632/oncotarget.23810
147. Kollmann D, Ignatova D, Jedamzik J, et al. PD-L1 expression is an independent predictor of favorable outcome in patients with localized esophageal adenocarcinoma. Oncoimmunology. 2018;7(6):e1435226. doi:10.1080/2162402X.2018.1435226
148. Metges J, François E, Shah M, et al. The Phase 3 KEYNOTE-181 study: pembrolizumab versus chemotherapy as second-line therapy for advanced esophageal cancer. Ann Oncol. 2019;30(Suppl 4):iv130–iv. doi:10.1093/annonc/mdz154.011
149. Shah MA, Kojima T, Hochhauser D, et al. Efficacy and safety of pembrolizumab for heavily pretreated patients with advanced, metastatic adenocarcinoma or squamous cell carcinoma of the esophagus: the phase 2 KEYNOTE-180 study. JAMA Oncol. 2019;5(4):546–550. doi:10.1001/jamaoncol.2018.5441
150. Kuo H-Y, Guo J-C, Hsu C-H. Anti-PD-1 immunotherapy in advanced esophageal squamous cell carcinoma: a long-awaited breakthrough finally arrives. J Formos Med Assoc. 2020;119(2):565–568. doi:10.1016/j.jfma.2019.10.010
151. Wang W, Chen D, Zhao Y, et al. Characterization of LAG-3, CTLA-4, and CD8+ TIL density and their joint influence on the prognosis of patients with esophageal squamous cell carcinoma. Ann Transl Med. 2019;7(23):776. doi:10.21037/atm.2019.11.38
152. Wang S, Li Z, Hu G. Prognostic role of intratumoral, zhi’an li2 and guoming hu3 IL-17A expression by immunohistochemistry in solid tumors: a meta-analysis. Oncotarget. 2017;8(39):66382–66391. doi:10.18632/oncotarget.18807
153. Lu L, Pan K, Zheng H-X, et al. IL-17A promotes immune cell recruitment in human esophageal cancers and the infiltrating dendritic cells represent a positive prognostic marker for patient survival. J Immunother. 2013;36(8):451–458. doi:10.1097/CJI.0b013e3182a802cf
154. Lu L, Weng C, Mao H, et al. IL-17A promotes migration and tumor killing capability of B cells in esophageal squamous cell carcinoma. Oncotarget. 2016;7(16):21853–21864. doi:10.18632/oncotarget.7869
155. Chen D, Jiang R, Mao C, et al. Chemokine/chemokine receptor interactions contribute to the accumulation of Th17 cells in patients with esophageal squamous cell carcinoma. Hum Immunol. 2012;73(11):1068–1072. doi:10.1016/j.humimm.2012.07.333
156. Chen D, Hu Q, Mao C, et al. Increased IL-17-producing CD4+ T cells in patients with esophageal cancer. Cell Immunol. 2012;272(2):166–174. doi:10.1016/j.cellimm.2011.10.015
157. Liu D, Zhang R, Wu J, et al. Interleukin-17A promotes esophageal adenocarcinoma cell invasiveness through ROS-dependent, NF-κB-mediated MMP-2/9 activation. Oncol Rep. 2017;37(3):1779–1785. doi:10.3892/or.2017.5426
158. Shitara K, Nishikawa H. Regulatory T cells: a potential target in cancer immunotherapy. Ann N Y Acad Sci. 2018;1417(1):104–115. doi:10.1111/nyas.13625
159. Facciabene A, Motz GT, Coukos G. T-regulatory cells: key players in tumor immune escape and angiogenesis. Cancer Res. 2012;72(9):2162–2171. doi:10.1158/0008-5472.CAN-11-3687
160. Paluskievicz CM, Cao X, Abdi R, Zheng P, Liu Y, Bromberg JS. T regulatory cells and priming the suppressive tumor microenvironment. Front Immunol. 2019;10:2453. doi:10.3389/fimmu.2019.02453
161. Wang G, Liu G, Liu Y, Li X, Su Z. FOXP3 expression in esophageal cancer cells is associated with poor prognosis in esophageal cancer. Hepatogastroenterology. 2012;59(119):2186–2191. doi:10.5754/hge11961
162. Huang C, Fu Z-X. Localization of IL-17+Foxp3+ T cells in esophageal cancer. Immunol Invest. 2011;40(4):400–412. doi:10.3109/08820139.2011.555489
163. Karstens K-F, Kempski J, Giannou AD, et al. Anti‑inflammatory microenvironment of esophageal adenocarcinomas negatively impacts survival. Cancer Immunol Immunother. 2020. doi:10.1007/s00262-020-2517-8
164. Xue L, Lu HQ, He J, et al. Expression of FOXP3 in esophageal squamous cell carcinoma relating to the clinical data. Dis Esophagus. 2010;23(4):340–346. doi:10.1111/j.1442-2050.2009.01013.x
165. Yue Y, Lian J, Wang T, et al. Interleukin-33-nuclear factor-κB-CCL2 signaling pathway promotes progression of esophageal squamous cell carcinoma by directing regulatory T cells. Cancer Sci. 2020;111(3):795–806. doi:10.1111/cas.14293
166. Cui G, Li Z, Ren J, Yuan A. IL-33 in the tumor microenvironment is associated with the accumulation of FoxP3-positive regulatory T cells in human esophageal carcinomas. Virchows Arch. 2019;475(5):579–586. doi:10.1007/s00428-019-02579-9
167. Tokunaga R, Naseem M, Lo JH, et al. B cell and B cell-related pathways for novel cancer treatments. Cancer Treat Rev. 2019;73:10–19. doi:10.1016/j.ctrv.2018.12.001
168. Qian L, Bian G-R, Zhou Y, et al. Clinical significance of regulatory B cells in the peripheral blood of patients with oesophageal cancer. Cent Eur J Immunol. 2015;40(2):263–265. doi:10.5114/ceji.2015.52840
169. Schwartz M, Zhang Y, Rosenblatt JD. B cell regulation of the anti-tumor response and role in carcinogenesis. J Immunother Cancer. 2016;4(1):40. doi:10.1186/s40425-016-0145-x
170. Bond DA, Woyach JA. Targeting BTK in CLL: beyond Ibrutinib. Curr Hematol Malig Rep. 2019;14(3):197–205. doi:10.1007/s11899-019-00512-0
171. Sagiv-Barfi I, Kohrt HEK, Czerwinski DK, Ng PP, Chang BY, Levy R. Therapeutic antitumor immunity by checkpoint blockade is enhanced by ibrutinib, an inhibitor of both BTK and ITK. Proc Natl Acad Sci U S A. 2015;112(9):E966–E72. doi:10.1073/pnas.1500712112
172. Xu B, Chen L, Li J, et al. Prognostic value of tumor infiltrating NK cells and macrophages in stage II+III esophageal cancer patients. Oncotarget. 2016;7(46):74904–74916. doi:10.18632/oncotarget.12484
173. Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity. 2016;44(5):989–1004. doi:10.1016/j.immuni.2016.05.001
174. Zheng Y, Li Y, Lian J, et al. TNF-α-induced Tim-3 expression marks the dysfunction of infiltrating natural killer cells in human esophageal cancer. J Transl Med. 2019;17(1):165. doi:10.1186/s12967-019-1917-0
175. Lim KS, Mimura K, Kua L-F, Shiraishi K, Kono K. Implication of highly cytotoxic natural killer cells for esophageal squamous cell carcinoma treatment. J Immunother. 2018;41(6):261–273. doi:10.1097/CJI.0000000000000227
176. Trivanović D, Krstić J, Djordjević IO, et al. The roles of mesenchymal stromal/stem cells in tumor microenvironment associated with inflammation. Mediators Inflamm. 2016;2016:7314016. doi:10.1155/2016/7314016
177. Atiya H, Frisbie L, Pressimone C, Coffman L. Mesenchymal stem cells in the tumor microenvironment. Adv Exp Med Biol. 2020;1234:31–42.
178. Rahmatizadeh F, Gholizadeh-Ghaleh Aziz S, Khodadadi K, et al. Bidirectional and opposite effects of naïve mesenchymal stem cells on tumor growth and progression. Adv Pharm Bull. 2019;9(4):539–558. doi:10.15171/apb.2019.063
179. Tian LLH, Yue W, Zhu F, Li S, Li W. Human mesenchymal stem cells play a dual role on tumor cell growth in vitro and in vivo. J Cell Physiol. 2011;226(7):1860–1867. doi:10.1002/jcp.22511
180. Yang X, Li Z, Ma Y, et al. Human umbilical cord mesenchymal stem cells promote carcinoma growth and lymph node metastasis when co-injected with esophageal carcinoma cells in nude mice. Cancer Cell Int. 2014;14(1):93. doi:10.1186/s12935-014-0093-9
181. Liu T, Zhou L, Li D, Andl T, Zhang Y. Cancer-associated fibroblasts build and secure the tumor. Front Cell Dev Biol. 2019;7:60. doi:10.3389/fcell.2019.00060
182. Truffi M, Mazzucchelli S, Bonizzi A, et al. Nano-strategies to target breast cancer-associated fibroblasts: rearranging the tumor microenvironment to achieve antitumor efficacy. Int J Mol Sci. 2019;20(6):1263. doi:10.3390/ijms20061263
183. Pereira BA, Vennin C, Papanicolaou M, et al. CAF subpopulations: a new reservoir of stromal targets in pancreatic cancer. Trends Cancer. 2019;5(11):724–741. doi:10.1016/j.trecan.2019.09.010
184. Marsh T, Pietras K, McAllister SS. Fibroblasts as architects of cancer pathogenesis. Biochim Biophys Acta. 2013;1832(7):1070–1078. doi:10.1016/j.bbadis.2012.10.013
185. Mishra PJ, Mishra PJ, Glod JW, Banerjee D. Mesenchymal stem cells: flip side of the coin. Cancer Res. 2009;69(4):1255–1258. doi:10.1158/0008-5472.CAN-08-3562
186. Ha SY, Yeo S-Y, Xuan Y-H, Kim S-H, Hoheisel JD. The prognostic significance of cancer-associated fibroblasts in esophageal squamous cell carcinoma. PLoS One. 2014;9(6):e99955–e. doi:10.1371/journal.pone.0099955
187. Cheng Y, Wang K, Ma W, et al. Cancer-associated fibroblasts are associated with poor prognosis in esophageal squamous cell carcinoma after surgery. Int J Clin Exp Med. 2015;8(2):1896–1903.
188. Wang J, Zhang G, Wang J, Wang L, Huang X, Cheng Y. The role of cancer-associated fibroblasts in esophageal cancer. J Transl Med. 2016;14(1):30. doi:10.1186/s12967-016-0788-x
189. Kashima H, Noma K, Ohara T, et al. Cancer-associated fibroblasts (CAFs) promote the lymph node metastasis of esophageal squamous cell carcinoma. Int J Cancer. 2019;144(4):828–840. doi:10.1002/ijc.31953
190. Yang Z-T, Yeo S-Y, Yin Y-X, et al. Tenascin-C, a prognostic determinant of esophageal squamous cell carcinoma. PLoS One. 2016;11(1):e0145807–e. doi:10.1371/journal.pone.0145807
191. Ebbing EA, van der Zalm AP, Steins A, et al. Stromal-derived interleukin 6 drives epithelial-to mesenchymal transition and therapy resistance in esophageal adenocarcinoma. Proc Natl Acad Sci U S A. 2019;116(6):2237–2242. doi:10.1073/pnas.1820459116
192. Qiao Y, Zhang C, Li A, et al. IL6 derived from cancer-associated fibroblasts promotes chemoresistance via CXCR7 in esophageal squamous cell carcinoma. Oncogene. 2018;37(7):873–883. doi:10.1038/onc.2017.387
193. Kato T, Noma K, Ohara T, et al. Cancer-associated fibroblasts affect intratumoral CD8þ and FoxP3þ T cells via IL6 in the tumor microenvironment. Clin Cancer Res. 2018;24(19):4820–4833. doi:10.1158/1078-0432.CCR-18-0205
194. Higashino N, Koma Y-I, Hosono M, et al. Fibroblast activation protein-positive fibroblasts promote tumor progression through secretion of CCL2 and interleukin-6 in esophageal squamous cell carcinoma. Lab Invest. 2019;99(6):777–792. doi:10.1038/s41374-018-0185-6
195. Tian B, Chen X, Zhang H, et al. Urokinase plasminogen activator secreted by cancer-associated fibroblasts induces tumor progression via PI3K/AKT and ERK signaling in esophageal squamous cell carcinoma. Oncotarget. 2017;8(26):42300–42313. doi:10.18632/oncotarget.15857
196. Yeo S-Y, Ha S-Y, Lee K-W, et al. Twist1 is highly expressed in cancer-associated fibroblasts of esophageal squamous cell carcinoma with a prognostic significance. Oncotarget. 2017;8(39):65265–65280. doi:10.18632/oncotarget.17941
197. Zhu X, Han S, Wu S, Bai Y, Zhang N, Wei L. Dual role of twist1 in cancer-associated fibroblasts and tumor cells promoted epithelial-mesenchymal transition of esophageal cancer. Exp Cell Res. 2019;375(2):41–50. doi:10.1016/j.yexcr.2019.01.002
198. Grugan KD, Miller CG, Yao Y, et al. Fibroblast-secreted hepatocyte growth factor plays a functional role in esophageal squamous cell carcinoma invasion. Proc Natl Acad Sci U S A. 2010;107(24):11026–11031. doi:10.1073/pnas.0914295107
199. Xu Z, Wang S, Wu M, Zeng W, Wang X, Dong Z. TGFβ1 and HGF protein secretion by esophageal squamous epithelial cells and stromal fibroblasts in oesophageal carcinogenesis. Oncol Lett. 2013;6(2):401–406. doi:10.3892/ol.2013.1409
200. Ozawa Y, Nakamura Y, Fujishima F, et al. c-Met in esophageal squamous cell carcinoma: an independent prognostic factor and potential therapeutic target. BMC Cancer. 2015;15(1):451. doi:10.1186/s12885-015-1450-3
201. Ren Y, Cao B, Law S, et al. Hepatocyte growth factor promotes cancer cell migration and angiogenic factors expression: a prognostic marker of human esophageal squamous cell carcinomas. Clin Cancer Res. 2005;11(17):6190–6197. doi:10.1158/1078-0432.CCR-04-2553
202. Gholamin M, Moaven O, Memar B, et al. Overexpression and interactions of interleukin-10, transforming growth factor beta, and vascular endothelial growth factor in esophageal squamous cell carcinoma. World J Surg. 2009;33(7):1439–1445. doi:10.1007/s00268-009-0070-y
203. Wang H, Jiang D, Song Q, et al. Prognostic impact and potential interaction of EGFR and c-Met in the progression of esophageal squamous cell carcinoma. Tumour Biol. 2016;37(7):9771–9779. doi:10.1007/s13277-015-4692-4
204. De Silva DM, Roy A, Kato T, et al. Targeting the hepatocyte growth factor/Met pathway in cancer. Biochem Soc Trans. 2017;45(4):855–870. doi:10.1042/BST20160132
205. Cheng F, Guo D. MET in glioma: signaling pathways and targeted therapies. J Exp Clin Cancer Res. 2019;38(1):270. doi:10.1186/s13046-019-1269-x
206. Mazzocca A, Fransvea E, Lavezzari G, Antonaci S, Giannelli G. Inhibition of transforming growth factor beta receptor I kinase blocks hepatocellular carcinoma growth through neo-angiogenesis regulation. Hepatology. 2009;50(4):1140–1151. doi:10.1002/hep.23118
207. Watanabe S, Noma K, Ohara T, et al. Photoimmunotherapy for cancer-associated fibroblasts targeting fibroblast activation protein in human esophageal squamous cell carcinoma. Cancer Biol Ther. 2019;20(9):1234–1248. doi:10.1080/15384047.2019.1617566
208. Mitsunaga M, Ogawa M, Kosaka N, Rosenblum LT, Choyke PL, Kobayashi H. Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat Med. 2011;17(12):1685–1691. doi:10.1038/nm.2554
209. Palumbo A
210. Najafi M, Mortezaee K, Majidpoor J. Stromal reprogramming: a target for tumor therapy. Life Sci. 2019;239:117049. doi:10.1016/j.lfs.2019.117049
211. Leppänen J, Bogdanoff S, Lehenkari PP, et al. Tenascin-C and fibronectin in normal esophageal mucosa, Barrett’s esophagus, dysplasia and adenocarcinoma. Oncotarget. 2017;8(40):66865–66877. doi:10.18632/oncotarget.19196
212. Senthebane DA, Jonker T, Rowe A, et al. The role of tumor microenvironment in chemoresistance: 3D extracellular matrices as accomplices. Int J Mol Sci. 2018;19(10):2861. doi:10.3390/ijms19102861
213. Fang M, Yuan J, Peng C, Li Y. Collagen as a double-edged sword in tumor progression. Tumour Biol. 2014;35(4):2871–2882. doi:10.1007/s13277-013-1511-7
214. Paszek MJ, Zahir N, Johnson KR, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8(3):241–254. doi:10.1016/j.ccr.2005.08.010
215. Hanley CJ, Noble F, Ward M, et al. A subset of myofibroblastic cancer-associated fibroblasts regulate collagen fiber elongation, which is prognostic in multiple cancers. Oncotarget. 2016;7(5):6159–6174. doi:10.18632/oncotarget.6740
216. Fang S, Dai Y, Mei Y, et al. Clinical significance and biological role of cancer-derived type I collagen in lung and esophageal cancers. Thorac Cancer. 2019;10(2):277–288. doi:10.1111/1759-7714.12947
217. Xiao J, Yang W, Xu B, et al. Expression of fibronectin in esophageal squamous cell carcinoma and its role in migration. BMC Cancer. 2018;18(1):976. doi:10.1186/s12885-018-4850-3
218. Orend G, Chiquet-Ehrismann R. Adhesion modulation by antiadhesive molecules of the extracellular matrix. Exp Cell Res. 2000;261(1):104–110. doi:10.1006/excr.2000.5041
219. Yang Z, Zhang C, Feng Y, Qi W, Cui Y, Xuan Y. Tenascin-C is involved in promotion of cancer stemness via the Akt/HIF1ɑ axis in esophageal squamous cell carcinoma. Exp Mol Pathol. 2019;109:104239. doi:10.1016/j.yexmp.2019.03.007
220. Luo M-L, Zhou Z, Sun L, et al. An ADAM12 and FAK positive feedback loop amplifies the interaction signal of tumor cells with extracellular matrix to promote esophageal cancer metastasis. Cancer Lett. 2018;422:118–128. doi:10.1016/j.canlet.2018.02.031
221. Mäki JM, Tikkanen H, Kivirikko KI. Cloning and characterization of a fifth human lysyl oxidase isoenzyme: the third member of the lysyl oxidase-related subfamily with four scavenger receptor cysteine-rich domains. Matrix Biol. 2001;20(7):493–496. doi:10.1016/S0945-053X(01)00157-3
222. Wang SX, Mure M, Medzihradszky KF, et al. A crosslinked cofactor in lysyl oxidase: redox function for amino acid side chains. Science. 1996;273(5278):1078–1084. doi:10.1126/science.273.5278.1078
223. Kalikawe R, Baba Y, Nomoto D, et al. Lysyl oxidase impacts disease outcomes and correlates with global DNA hypomethylation in esophageal cancer. Cancer Sci. 2019;110(12):3727–3737. doi:10.1111/cas.14214
224. Sakai M, Kato H, Sano A, et al. Expression of lysyl oxidase is correlated with lymph node metastasis and poor prognosis in esophageal squamous cell carcinoma. Ann Surg Oncol. 2009;16(9):2494–2501. doi:10.1245/s10434-009-0559-5
225. Xie W, Huang P, Wu B, et al. Clinical significance of LOXL4 expression and features of LOXL4‑associated protein–protein interaction network in esophageal squamous cell carcinoma. Amino Acids. 2019;51(5):813–828. doi:10.1007/s00726-019-02723-4
226. Hoque A, Lippman SM, Wu -T-T, et al. Increased 5-lipoxygenase expression and induction of apoptosis by its inhibitors in esophageal cancer: a potential target for prevention. Carcinogenesis. 2005;26(4):785–791. doi:10.1093/carcin/bgi026
227. Shi H-Y, Lv F-J, Zhu S-T, Wang Q-G, Zhang S-T. Dual inhibition of 5-LOX and COX-2 suppresses esophageal squamous cell carcinoma. Cancer Lett. 2011;309(1):19–26. doi:10.1016/j.canlet.2011.05.010
228. Curran S, Murray GI. Matrix metalloproteinases in tumour invasion and metastasis. J Pathol. 1999;189(3):300–308. doi:10.1002/(SICI)1096-9896(199911)189:3<300::AID-PATH456>3.0.CO;2-C
229. Stamenkovic I. Matrix metalloproteinases in tumor invasion and metastasis. Semin Cancer Biol. 2000;10(6):415–433. doi:10.1006/scbi.2000.0379
230. Lu H, Bhat AA, Peng D, et al. APE1 upregulates MMP-14 via redox-sensitive ARF6-mediated recycling to promote cell invasion of esophageal. Cancer Res. 2019;79(17):4426–4438. doi:10.1158/0008-5472.CAN-19-0237
231. Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6(4):389–395. doi:10.1038/74651
232. Groblewska M, Siewko M, Mroczko B, Szmitkowski M. The role of matrix metalloproteinases (MMPs) and their inhibitors (TIMPs) in the development of esophageal cancer. Folia Histochem Cytobiol. 2012;50(1):12–19. doi:10.5603/FHC.2012.0002
233. Li Y, Ma J, Guo Q, et al. Overexpression of MMP-2 and MMP-9 in esophageal squamous cell carcinoma. Dis Esophagus. 2009;22(8):664–667. doi:10.1111/j.1442-2050.2008.00928.x
234. Zhang J, Jin X, Fang S, et al. The functional SNP in the matrix metalloproteinase-3 promoter modifies susceptibility and lymphatic metastasis in esophageal squamous cell carcinoma but not in gastric cardiac adenocarcinoma. Carcinogenesis. 2004;25(12):2519–2524. doi:10.1093/carcin/bgh269
235. Miao S, Zhou S-Y, Han C-S, Zhang L-N, Sun H-B, Yang B. Clinicopathological significance of matrix metalloproteinase-7 protein expression in esophageal cancer: a meta-analysis. Drug Des Devel Ther. 2015;9:3729–3740. doi:10.2147/DDDT.S85987
236. Wu J, Zhang L, Luo H, Zhu Z, Zhang C, Hou Y. Association of matrix metalloproteinases-9 gene polymorphisms with genetic susceptibility to esophageal squamous cell carcinoma. DNA Cell Biol. 2008;27(10):553–557. doi:10.1089/dna.2008.0732
237. Han F, Zhang S, Zhang L, Hao Q. The overexpression and predictive significance of MMP-12 in esophageal squamous cell carcinoma. Pathol Res Pract. 2017;213(12):1519–1522. doi:10.1016/j.prp.2017.09.023
238. Chen L, Di D, Luo G, et al. Immunochemical staining of MT2-MMP correlates positively to angiogenesis of human esophageal cancer. Anticancer Res. 2010;30(10):4363–4368.
239. Xue Z, Wu X, Chen X, Luo Q. MT3-MMP down- regulation promotes tumorigenesis and correlates to poor prognosis in esophageal squamous cell carcinoma. Cancer Med. 2016;5(9):2459–2468. doi:10.1002/cam4.790
240. Zhu Y-H, Fu L, Chen L, et al. Downregulation of the novel tumor suppressor DIRAS1 predicts poor prognosis in esophageal squamous cell carcinoma. Cancer Res. 2013;73(7):2298–2309. doi:10.1158/0008-5472.CAN-12-2663
241. Cheung KJ, Ewald AJ. A collective route to metastasis: seeding by tumor cell clusters. Science. 2016;352(6282):167–169. doi:10.1126/science.aaf6546
242. Doglioni G, Parik S, Fendt SM. Interactions in the (pre)metastatic niche support metastasis formation. Front Oncol. 2019;9:219. doi:10.3389/fonc.2019.00219
243. Krüger A. Premetastatic niche formation in the liver: emerging mechanisms and mouse models. J Mol Med (Berl). 2015;93(11):1193–1201. doi:10.1007/s00109-015-1342-7
244. Wortzel I, Dror S, Kenific CM, Lyden D. Exosome-mediated metastasis: communication from a distance. Dev Cell. 2019;49(3):347–360. doi:10.1016/j.devcel.2019.04.011
245. Wang Y, Ding Y, Guo N, Wang S. MDSCs: key criminals of tumor pre-metastatic niche formation. Front Immunol. 2019;10:172. doi:10.3389/fimmu.2019.00172
246. Seebauer CT, Brunner S, Glockzin G, et al. Peritoneal carcinomatosis of colorectal cancer is characterized by structural and functional reorganization of the tumor microenvironment inducing senescence and proliferation arrest in cancer cells. Oncoimmunology. 2016;5(12):e1242543. doi:10.1080/2162402X.2016.1242543
247. Chen XW, Yu TJ, Zhang J, et al. CYP4A in tumor-associated macrophages promotes pre-metastatic niche formation and metastasis. Oncogene. 2017;36(35):5045–5057. doi:10.1038/onc.2017.118
248. Kong J, Tian H, Zhang F, et al. Extracellular vesicles of carcinoma-associated fibroblasts creates a pre-metastatic niche in the lung through activating fibroblasts. Mol Cancer. 2019;18(1):175. doi:10.1186/s12943-019-1101-4
249. Wu S, Zheng Q, Xing X, et al. Matrix stiffness-upregulated LOXL2 promotes fibronectin production, MMP9 and CXCL12 expression and BMDCs recruitment to assist pre-metastatic niche formation. J Exp Clin Cancer Res. 2018;37(1):99. doi:10.1186/s13046-018-0761-z
250. Roder JD, Busch R, Stein HJ, Fink U, Siewert JR. Ratio of invaded to removed lymph nodes as a predictor of survival in squamous cell carcinoma of the oesophagus. Br J Surg. 1994;81(3):410–413. doi:10.1002/bjs.1800810330
251. Shibata T, Takita K, Inomata M. Observation of the cytoarchitecture of the human esophageal mucosa with special attention to the lamina muscularis mucosae and the distribution of lymphatic vessels. Esophagus. 2019;16(1):44–51. doi:10.1007/s10388-018-0632-x
252. Wang Y, Zhu L, Xia W, Wang F. Anatomy of lymphatic drainage of the esophagus and lymph node metastasis of thoracic esophageal cancer. Cancer Manag Res. 2018;10:6295–6303. doi:10.2147/CMAR.S182436
253. Otto B, Koenig AM, Tolstonog GV, et al. Molecular changes in pre-metastatic lymph nodes of esophageal cancer patients. PLoS One. 2014;9(7):e102552. doi:10.1371/journal.pone.0102552
254. O’Sullivan GC, Corbett AR, Shanahan F, Collins JK. Regional immunosuppression in esophageal squamous cancer: evidence from functional studies with matched lymph nodes. J Immunol. 1996;157(10):4717–4720.
255. Preynat-Seauve O, Contassot E, Schuler P, Piguet V, French LE, Huard B. Extralymphatic tumors prepare draining lymph nodes to invasion via a T-cell cross-tolerance process. Cancer Res. 2007;67(10):5009–5016. doi:10.1158/0008-5472.CAN-06-4494
256. Cochran AJ, Huang RR, Lee J, Itakura E, Leong SP, Essner R. Tumour-induced immune modulation of sentinel lymph nodes. Nat Rev Immunol. 2006;6(9):659–670. doi:10.1038/nri1919
257. Kim R, Emi M, Tanabe K, Arihiro K. Immunobiology of the sentinel lymph node and its potential role for antitumour immunity. Lancet Oncol. 2006;7(12):1006–1016. doi:10.1016/S1470-2045(06)70975-5
258. Campbell MJ, Baehner F, O’Meara T, et al. Characterizing the immune microenvironment in high-risk ductal carcinoma in situ of the breast. Breast Cancer Res Treat. 2017;161(1):17–28. doi:10.1007/s10549-016-4036-0
259. Gorringe KL, Fox SB. Ductal carcinoma in situ biology, biomarkers, and diagnosis. Front Oncol. 2017;7:248. doi:10.3389/fonc.2017.00248
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.