Back to Journals » Drug Design, Development and Therapy » Volume 13
Erythropoietin enhances osteogenic differentiation of human periodontal ligament stem cells via Wnt/β-catenin signaling pathway
Authors Zheng DH, Wang XX, Ma D, Zhang LN, Qiao QF, Zhang J
Received 1 May 2019
Accepted for publication 1 July 2019
Published 26 July 2019 Volume 2019:13 Pages 2543—2552
DOI https://doi.org/10.2147/DDDT.S214116
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Qiongyu Guo
De-Hua Zheng,1 Xu-Xia Wang,2 Dan Ma,1 Li-Na Zhang,3 Qing-Fang Qiao,1 Jun Zhang4
1Shandong Provincial Key Laboratory of Oral Tissue Regeneration, School of Stomatology, Shandong University, Jinan, Shandong Province, People’s Republic of China; 2Department of Oral and Maxillofacial Surgery, School of Dentistry, Shandong University, Jinan, Shandong Province, People’s Republic of China; 3Department of Orthodontics, Liaocheng People’s Hospital, Liaocheng, Shandong Province, People’s Republic of China; 4Department of Orthodontics, School of Stomatology, Shandong University, Jinan, Shandong Province, People’s Republic of China
Objectives: The aim of this study is to examine the roles of erythropoietin (EPO) in regulating proliferation and osteogenic differentiation of periodontal ligament stem cells (PDLSCs) and analyze the underlying signaling of these processes.
Materials and methods: PDLSCs were isolated and characterized. The PDLSCs were transfected with β-catenin shRNA. qRT-PCR and Western blot analysis were used to examine the osteogenic effects of EPO on the expression of osteogenic-related genes and protein (Runx2, OCN and Osterix) in PDLSCs. Alizarin Red-S staining was used to detect mineralized nodule formation. In addition, the relationship between the Wnt/β-catenin pathway and the effect of EPO on the osteogenesis of PDLSCs was investigated.
Results: The results suggested that EPO exerts positive osteogenic effects on PDLSCs. The results showed that EPO decreased the growth of PDLSCs slightly and increased alkaline phosphatase activity and calcium deposition in a dose-dependent manner. The expression of Runx2, Osterix and OCN was increased after EPO administration. EPO increases β-catenin and Cyclin D1 in PDLSCs. After transfected with β-catenin shRNA, the osteogenic effect of EPO on PDLSCs was attenuated.
Conclusion: EPO promotes osteogenic differentiation of PDLSCs. The underlying mechanism may be activating Wnt/β-catenin signaling pathway.
Keywords: erythropoietin, periodontal ligament stem cell, osteogenesis, Wnt/β-catenin
Introduction
Periodontitis is characterized by destruction of periodontium due to chronic inflammation. It is the most common disease and affects approximately 90% of the worldwide population.1–3 Periodontitis involves the progressive destruction of periodontal tissue, loosening of teeth, toothache, swollen gums, deep periodontal pocket and ultimately, tooth loss.4,5 The ideal therapy of periodontitis is to reverse the microenvironmental inflammation of periodontium, achieve successful periodontal regeneration of cementum-PDL complexes and alveolar bone. Over the past several decades, conventional approaches such as periodontal scaling, guided tissue regeneration and root planing have been attempted to control periodontal inflammation and regenerate periodontal tissues; however, complete biological regeneration of lost periodontal structures has not been achieved.6–8 Periodontal ligament stem cells (PDLSCs) are the main functional cells which are widely known in the regeneration and repair of periodontium. PDLSCs can differentiate into osteoblasts, adipocytes and cementoblasts, which is essential for physiological healing of cementum-PDL complexes and alveolar bone.9 Because of osteogenic differentiation capacity of PDLSCs, it can be used as a promising method for the functional restoration of periodontal tissue damaged by periodontitis.10,11
Erythropoietin (EPO) is a glycoprotein cytokine, which is commonly identified by its role in increasing the circulating erythrocyte mass.12 Despite being widely recognized for its erythropoietic activity, EPO has been largely known for its multiple biological functions outside the hematopoietic system. Nonhematopoietic functions of EPO have been reported to include angiogenic,12 cardioprotective,13 neuroprotective,14 neurotrophic,15 anti-inflammatory,16 anti-apoptosis,17 antioxidative,18 effects in different cells such as brain capillary endothelial cells,19 vascular smooth muscle cells,20 myocardial cells,21 neurons22 and astrocytes.23 Recent studies have found that EPO can enhance osteoclastogenesis by activating the release of VEGF and BMP-2 in cellular microenvironment.24 In addition, EPO has been shown to stimulate mesenchymal stromal cell (MSC) differentiation toward osteoblasts in vitro25,26 as well as to increase bone formation and the number of osteoblasts in vivo, especially if ephrinB2/EphB4 signaling in concomitantly activated.27 However, whether EPO can affect osteogenic differentiation of PDLSCs remains uncertain. Therefore, what role does EPO play in osteogenic differentiation of PDLSCs still needs to be explored.
Lots of researches showed that EPO could regulate the versatile function of stem cells through multi-pathway. Shiozawa et al28 suggests that EPO has the capacity of stimulating osteoblastic differentiation BMSCs (bone marrow mesenchymal stem cells) by EPO-EPOR signaling pathway. Activation of the canonical Wnt pathway was detected upon EPO treatment in MSC during neuronal differentiation.29 In addition, investigators who explored the effect of EPO on osteoblastic differentiation in hematopoietic stem cells observed a direct correlation between the JAK/STAT pathway and bone morphogenetic proteins.30 However, few studies have investigated whether EPO influences the osteogenic differentiation of PDLSCs. Moreover, the molecular mechanisms of EPO in stimulating osteogenic differentiation of PDLSCs have not been yet explored. Therefore, the aim of the present study was to examine the positive effect of EPO in osteogenic differentiation of PDLSCs and its correlation with Wnt/β-catenin signaling.
Materials and methods
PDLSCs isolation, cultivation and characterization
Normal human first premolars (n=20) were collected from malocclusion patients (14–18 years) who visit the department of orthodontics after approval from the Shandong University’s research Ethical Committee. Written informed consent was obtained from a parent of patients. According to previous methods,31 PDLSCs were isolated and cultured in a complete medium containing α-MEM and 10% FBS (BI, USA) at 37°C under a humidified atmosphere containing 5% CO2 . PDLSCs from P3–P5 were used for subsequent experiments. To characterize their stem cell properties, fluorescein isothiocyanate (FITC) labeled CD34, CD90 and CD105 coupled with flow cytometer (Beckman Coulter, Franklin Lakes, NJ, USA) were used to flow cytometric analysis.
Osteogenic and adipogenic induction
For osteogenic differentiation, PDLSCs were plated into 6-well dishes at a density of 1.5×105 cell/well with complete medium. After reaching 90% confluence, osteogenic induction (Cyagen Biosciences, Guangzhou, People's Republic of China) was initiated and maintained for 3 weeks with medium changed every 3 days. Different concentrations of EPO (10, 20 and 50 U/mL) were added at every medium change. When osteogenic induction finished, PDLSCs were fixed with 4% paraformaldehyde and then stained with Alizarin Red-S (Cyagen Biosciences, ). For adipogenesis, PDLSCs were cultured in adipogenic medium (α-MEM supplemented with 500 mM isobutyl-methylxanthine, 0.5 μM hydrocortisone, 60 mM indomethacin, 10 mM insulin and 10% FBS) for 3 weeks. After finishing adipogenesis, PDLSCs were fixed with 4% paraformaldehyde and then stained with Oil Red O. All experiments were repeated three times.
Cell proliferation assay
The effect of EPO on cell proliferation was examined by Cell Counting kit-8 (CCK8, Dojindo Laboratories) assay. PDLSCs were placed in 96-well plates at a density of 5×103 cell/dish in normal culture medium. After overnight incubation, PDLSCs were incubated in growth medium supplemented with different concentrations of EPO (0, 5, 10, 20, 50 U/mL) for various times (0, 12, 24, 48 hrs). The absorbance of samples was measured at 450 nm with a microplate reader (SPECTRAstar, Nano, BMG Labtech, Germany). The assays were repeated three times.
Alkaline phosphatase activity assay
After 7 and 14 days of induction, the alkaline phosphatase (ALP) test kit (Jian Cheng Biotech, Nanjing, People's Republic of China) was used for quantitative analysis of ALP activity. The absorbance at a wavelength of 520 nm was measured with a microplate reader. Protein concentration was detected with a BCA protein assay kit (Solarbio Beijing, People's Republic of China). Values were normalized and expressed as U/mg protein.
RNA isolation and real-time PCR
After culturing for 7 and 14 days, total RNA was extracted by Trizol reagent (Takara, Kusatsu, Japan) and cDNA was prepared using PrimeScript® RTase (Takara). SYBR® Premix Ex Taq™ ІІ (Takara) was used for qRT-PCR. qRT-PCR performed on a Roche Light Cycler® 480 Sequence Detection System (Roche Diagnostics GmbH, Mannheim, Baden-Württemberg, Germany). The primer sequences in the present study are listed in Table 1. The values were analyzed using comparative 2−ΔΔCt method. All examinations were performed in triplicate and the data were averaged.
 |
Table 1 qRT-PCR primers used in this study |
Western blotting
After culturing for 14 days, the whole cell lysates were prepared using RIPA Lysis Buffer (Beyotime, Shanghai, People's Republic of China) and 1% PMSF (Beyotime). The whole protein concentrations were measured by bicinchoninic acid (BCA) protein assay kit (Beyotime). Total cell lysates were separated on 10% SDS-PAGE and transferred onto polyvinylidene fluoridemembranes. After blocking with 5% nonfat dried milk in PBST (PBS+0.1% Tween-20), blots were probed with primary antibodies to Runx2, Osterix, OCN, Cyclin D1 (Abcam, Cambridge, MA, USA) β-catenin and β-actin (Cell Signalling Technology, USA) overnight at 4°C and secondary antibody (Absin, Shanghai, People's Republic of China) incubation at 37°C for 1 hr according to standard protocols. Immunoreactive proteins were visualized using a electrochemiluminescence system (Millipore).
Immunofluorescence staining
After culturing for 14 days, cells were fixed and treated with β-catenin antibody (1:100, Cell Signaling Technology) overnight at 4°C. After washed twice with PBS, the samples incubated with an IgG-FITC antibody (Absin) at 1:100 dilution in TBS with 1% BSA for 1 hr. DAPI solution was used to visualize fluorescence-stained biomedical nuclei. The images were captured with a fluorescence microscope (Olympus, Japan)
Lentivirus vector package and transfection
Lentivirus vector consisting of shRNA silencing β-catenin and negative control (the scrambled sequence) was constructed and packaged by School of Pharmaceutical Sciences, Shandong University. The sequences of β-catenin shRNA32 were 5ʹ-AAACTACTGTGGACCACAAGCCCTGTCTC-3ʹ and 5ʹ-AAGCTTGTGGTCCACAGTAGTCCTGTCTC-3ʹ. Successful transfection of PDLSCs was identified by green fluorescence. In total, >70–80% cells flashed with green fluorescence under a fluorescence microscope indicated stable transfection with lentivirus-mediated shRNA.
Statistical analysis
All data were expressed as mean (SD), and all experiments performed three replicates. Statistical calculations were analyzed using the Student's t-test, or one-way ANOVA and a P-value <0.05 was considered a statistically significant difference (SPSS16.0, Inc., Chicago, IL, USA).
Results
Cell culture and characterization of PDLSCs
As detected by flow cytometry, the positive expression of surface markers in PDLSCs was CD90 and CD105, and thenegative expression of hematopoietic marker was CD34 (Figure 1). The osteogenic and adipogenic potentials of PDLSCs were proved by the formation of Alizarin Red-positive nodules and Oil Red O-positive lipid droplets (Figure 2).
 |
Figure 1 Characterization of Peridontal ligament stem cells (PDLSCs) by flow cytometric analysis. Flow cytometric analysis of PDLSCs reve aled expression of CD34 (A, B), CD90 (C, D) and CD105 (E, F). |
The proliferation of PDLSCs
As compared to control group (0 U/mL EPO), 5, 10, 20 and 50 U/mL of EPO exhibited stimulative effect on the proliferation of PDLSCs at 24 and 48 hrs. It is obvious that the stimulative effect of EPO on proliferation peaked at 20 U/mL. As compared to the concentration of 20 U/mL EPO cultures, 50 U/mL EPO group possessed a reduced proliferative rate at 24 and 48 hrs. (Figure 3).
 |
Figure 3 Effect of Erythropoietin (EPO) on the proliferation of Periodontal ligament stem cells (PDLSCs).Note:*P<0.05. |
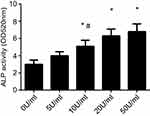
ALP activity
To asssess the effect of 10 and 20 U/mL EPO, ALP activity of PDLSCs were significantly promoted and were higher than that in control group. Compared to 10 U/mL EPO, the concentration of 50 U/mL EPO obviously stimulates the ALP activity of PDLSCs. Although the ALP activity of PDLSCs cultured with 50 U/mL EPO was increased, there was no significant difference at the concentration of 20 U/mL EPO and 50 U/mL EPO. (Figure 4).
EPO promoted the osteogenic-related gene and protein expression of PDLSCs
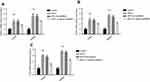
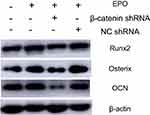
The osteogenic differentiation of PDLSCs cultured with 20 U/mL EPO was assessed by RT-qPCR to measure mRNA expression of Osterix, Runx2 and OCN. The expression of Runx2, OCN and Osterix increased significantly both in mRNA and protein levels 7 and 14 day(s) during EPO treatment (Figure 5A–C). It is obvious that EPO-induced increase in Runx2, OCN and Osterix was attenuated by knocking-down of β-catenin (Figure 6). Furthermore, Alizarin Red staining for mineralized deposition was consistent with Western blot analysis (Figure 7).
EPO enhances mineralized nodule formation in PDLSCs
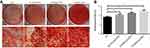
To detect the mineralized nodules formation during the differentiation process, the CPC colorimetric method was used to quantify the mineralized nodule formation of PDLSCs. Compared to osteogenic inductive medium, the quantitation of calcium of PDLSCs cultured with 10, 20 and 50 U/mL EPO showed an increase of calcium deposits in a dose-dependent manner (Figure 8A and B).
EPO promotes osteogenic differentiation of PDLSCs through Wnt/β-catenin signaling
To further study the underlying signaling mechanism, Wnt/β-catenin signaling in osteogenic differentiation of PDLSCs (by Western blot) was evaluated. As shown in Figure 9, EPO can upregulate the expression of β-catenin and CyclinD1 in the process of osteogenesis in a concentration-dependent manner at the protein level. The results showed that the expression of β-catenin and CyclinD1 was significantly promoted by 20 U/mL EPO treatment. Following transfected with β-catenin-specific shRNA, EPO-induced β-catenin and CyclinD1 was significantly inhibited (Figure 10 and 11). Subsequently, we confirmed that EPO induced the expression of β-catenin in osteogenic differentiation of PDLSCs using immunofluorescence staining (Figure 11). Moreover, mineralization-related gene and protein expression of Osterix, Runx2 and OCN induced by EPO were also significantly decreased after shRNA transfection. These results suggesting EPO promote osteogenic differentiation of PDLSCs through Wnt/β-catenin signaling.
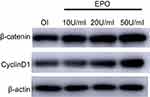 |
Figure 9 EPO can upregulate the expression of β-catenin and CyclinD1 in the process of osteogenesis in a concentration-dependent manner. |
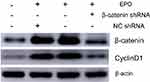 |
Figure 10 The protein level of β-catenin and CyclinD1 was assessed by Western blot analysis. (Osteogenic induction group, EPO group, EPO+NCshRNA group and EPO+β-catenin shRNA group). |
Discussion
PDLSCs are pluripotent cells, which can differentiate into chondrocyte, osteoblasts, cementoblast, fibroblasts, lipoblasts and other cell phenotypes. PDLSCs also play an important role in the restoration of periodontal tissues, including periodontal ligament, alveolar bone and cementum. Therefore, medicament which could promote osteogenic differentiation of PDLSCs may act as a promising candidate to restore the function of PDLSCs in periodontitis. Despite EPO possesses multiple biological functions besides the regulation of red blood cell generation, the effects of EPO on osteogenic differentiation and periodontal regeneration remain controversial. Hence, we investigate the relationship between EPO and osteogenic differentiation of PDLSCs. Additionally, the underlying mechanism of this process was also explored.
Open flap debridement with guided tissue regeneration technology represents an alternative strategy against periodontal tissue damage that occurs in many periodontal diseases, such as periodontitis. Although tissue engineering and regenerative medicine couple with PDLSCs can promote restoration of periodontal tissue to a certain degree, the methods were inefficient for complete biological and functional regeneration of lost periodontal structures. PDLSCs were the most promising source to achieve the regeneration of cementum-PDL complexes and alveolar bone by their multipotent differentiation ability. Although current applications with tissue engineering have already made certain progress, it would be more meaningful to explore effective factors and formulate their mechanisms. In this study, we investigate whether the use of EPO could be able to promote osteogenic differentiation of PDLSCs. For this purpose, detailed research is firstly given on the optimal concentration of EPO for cell proliferation and osteogenic differentiation. Because the optimal condition for periodontal tissue restoration requires minimal inhibition of cell proliferation with the maximal stimulation of osteogenic differentiation, we proceeded to demonstrate the ALP activity of PDLSCs after different concentration of EPO treatment. According to our results, the concentration of 20and 50 U/mL EPO both increased ALP activity in a dose- and time-dependent manner. Based on these results, EPO at 20 U/mL exhibited both positive effects on proliferation and osteogenic differentiation of PDLSCs.
The molecular mechanism of bone formation involves three major phases: 1) proliferation, 2) extracellular matrix maturation and 3) mineralization, which are orchestrated by various key molecules that regulate this phase transfer. Runx2 is proved to be the most important transcription factors necessary for the process of osteogenesis and is responsible for the activation of osteoblast differentiation marker genes.33,34 OCN was involved in controlling the mineralization process, appeared at a late stage of osteogenic differentiation and was characterized by mature cells of the osteoblastic lineage.35,36 Furthermore, osterix (also known as Sp7) is a novel zinc finger-containing osteoblast-specific transcription factor that is essential for osteoblast differentiation, proliferation and bone formation. It regulates the expression of many osteogenic factors, including Runx2, osteonectin, osteopontin, osteocalcin and (ALP.37–39 Runx2, OCN and Osterix are closely related to multiple steps of osteogenic differentiation of PDLSCs. To confirm the relationship between EPO and osteogenic differentiation of PDLSCs, we verified the expression of Runx2, OCN and Osterix after 14 day(s) treated with EPO. The results indicate that Runx2, OCN and Osterix are upregulated in the EPO group both at gene and protein level, Alizarin Red staining for mineralized deposition was also significant in EPO treated group.
Several studies have shown that Wnt/β-catenin signaling pathway is involved in osteogenic differentiation of PDLSCs,40–42 which motivated us to explore whether this signaling pathway plays a role in EPO-induced expression of osteogenic markers in PDLSCs. The Wnt/β-catenin signaling pathway is linked to osteogenesis via the activation of β-catenin and upregulate the expression of its target gene Cyclin D1. In our study, the increased expression of β-catenin induced by EPO significantly upregulated the protein levels of Cyclin D1 and Runx2, knock-down of β-catenin downregulated these two proteins. Moreover, knock-down of β-catenin can also depress the formation of mineralization. These findings revealed that EPO stimulated the expression of osteogenic markers through the activation of β-catenin and Cyclin D1.
In conclusion, our study proves that EPO (20 U/mL of concentration) promotes proliferation and osteogenic differentiation of PDLSCs. The underlying mechanism may be activating Wnt/β-catenin signaling pathway.
Abbreviation list
EPO, erythropoietin; PDLSCs, periodontal ligament stem cells; MSC, mesenchymal stromal cell; HSCs, hematopoietic stem cells; ARS, Alizarin Red-S staining; GTR, guided tissue regeneration.
Acknowledgment
This study was supported by the National Natural Science Foundation of China (Grant No. 81371180).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chen FM, Sun HH, Lu H, Yu Q. Stem cell-delivery therapeutics for periodontal tissue regeneration. Biomaterials. 2012;33:6320–6644. doi:10.1016/j.biomaterials.2012.05.048
2. Park JY, Jeon SH, Choung PH. Efficacy of periodontal stem cell transplantation in the treatment of advanced periodontitis. Cell Transplant. 2011;20:271–285. doi:10.3727/096368910X519292
3. Schulze U, Turner R, Wang Y, et al. Relationship of bone metabolism biomarkers and periodontal disease: the osteoporotic fractures in men (MrOS) study. J Clin Endocrinol Metab. 2015;100(6):2425–2433. doi:10.1210/jc.2014-4180
4. Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nature Rev Microbiol. 2010;8(7):481. doi:10.1038/nrmicro2337
5. Nanci A, Bosshardt DD. Structure of periodontal tissues in health and disease. Periodontol. 2006;40:11–28. doi:10.1111/j.1600-0757.2005.00141.x
6. Chen FM, Zhang J, Zhang M, An Y, Chen F, Wu ZF. A review on endogenous regenerative technology in periodontal regenerative medicine. Biomaterials. 2010;31:7892–7927. doi:10.1016/j.biomaterials.2010.07.019
7. Venezia E, Goldstein M, Boyan BD, et al. The use of enamel matrix derivative in the treatment of periodontal defects: a literature review and meta-analysis. Crit Rev Oral Biol Med Off Publ Am Assoc Oral Biologists. 2004;15(6):382–402. doi:10.1177/154411130401500605
8. Kaigler D, Cirelli JA, Giannobile WV. Growth factor delivery for oral and periodontal tissue engineering. Expert Opin Drug Deliv. 2006;3(5):647–662. doi:10.1517/17425247.3.5.647
9. Washio K, Iwata T, Mizutani M, et al. Assessment of cell sheets derived from human periodontal ligament cells: a pre-clinical study. Cell Tissue Res. 2010;341(3):397–404. doi:10.1007/s00441-010-1009-1
10. Kato T, Hattori K, Deguchi T, et al. Osteogenic potential of rat stromal cells derived from periodontal ligament. J Tissue Eng Regener Med. 2011;5(10):798–805. doi:10.1002/term.379
11. Gault P, Black A, Romette JL, et al. Tissue‐engineered ligament: implant constructs for tooth replacement. J Pharm Sci Res. 2010;37(9):750–758.
12. Kertesz N, Wu J, Chen HP, et al. The role of erythropoietin in regulating angiogenesis. Dev Biol. 2004;276(1):101–110. doi:10.1016/j.ydbio.2004.08.025
13. Cho GW, Koh SH, Kim MH, et al. The neuroprotective effect of erythropoietin-transduced human mesenchymal stromal cells in an animal model of ischemic stroke. Brain Res. 2010;1353(2):1–13.16. doi:10.1016/j.brainres.2010.06.013
14. cKumral A, Tüzün F, Oner MG, et al. Erythropoietin in neonatal brain protection: the past, the present and the future. Brain Dev. 2011;33(8):632–643.
15. Tsai PT, Ohab JJ, Kertesz N, et al. A critical role of erythropoietin receptor in neurogenesis and post-stroke recovery. J Neurosci. 2006;26(4):1269–1274. doi:10.1523/JNEUROSCI.4480-05.2006
16. Arcasoy MO. The non‐haematopoietic biological effects of erythropoietin. Br J Haematol. 2010;141(1):14–31. doi:10.1111/j.1365-2141.2008.07014.x
17. Zhi-kun SUN, Sheng-di CHEN. Erythropoietin and neuron apoptosis: signalpathway. Chin Pharmacol Bulletin. 2006;22(12):1429–1432.
18. Chateauvieux S, Grigorakaki C, Morceau F, Dicato M, Diederich M. Erythropoietin, erythropoiesis and beyond. Biochem Pharmacol. 2011;82(10):1291–1303. doi:10.1016/j.bcp.2011.06.045
19. Acheson A, Richards JB, De WH. Effects of sleep deprivation on impulsive behaviors in men and women. Physiol Behav. 2007;91(5):579–587. doi:10.1016/j.physbeh.2007.03.020
20. Cianferotti L, Brandi ML. Muscle–bone interactions: basic and clinical aspects. Endocrine. 2014;45(2):165–177. doi:10.1007/s12020-013-0026-8
21. Wright GL, Hanlon P, Amin K, et al. Erythropoietin receptor expression in adult rat cardiomyocytes is associated with an acute cardioprotective effect for recombinant erythropoietin during ischemia-reperfusion injury. Faseb J Off Publ Fed Am Soc Exp Biol. 2004;18(9):1031. doi:10.1096/fj.03-0678fje
22. Bernaudin M, Marti HH, Roussel S, et al. A potential role for erythropoietin in focal permanent cerebral ischemia in mice. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 1999;19(6):643. doi:10.1097/00004647-199906000-00007
23. Sugawa M, Sakurai Y, Ishikawaieda Y, et al. Effects of erythropoietin on glial cell development; oligodendrocyte maturation and astrocyte proliferation. Neurosci Res. 2002;44(4):391–403.
24. Holstein JH, Orth M, Scheuer C, et al. Erythropoietin stimulates bone formation, cell proliferation, and angiogenesis in a femoral segmental defect model in mice. Bone. 2011;49(5):1037–1045. doi:10.1016/j.bone.2011.08.004
25. Rölfing JH, Bendtsen M, Jensen J, et al. Erythropoietin augments bone formation in a rabbit posterolateral spinal fusion model. J Orthop Res. 2012;30(7):1083–1088. doi:10.1002/jor.22027
26. Sun H, Jung Y, Shiozawa Y, et al. Erythropoietin modulates the structure of bone morphogenetic protein 2-engineered cranial bone. Tissue Eng Part A. 2012;18(19–20):2095–2105. doi:10.1089/ten.TEA.2011.0742
27. Li C, Shi C, Kim J, et al. Erythropoietin promotes bone formation through EphrinB2/EphB4 signaling. J Dent Res. 2015;94(3):455–463. doi:10.1177/0022034514566431
28. Shiozawa Y, Jung Y, Ziegler AM, et al. Erythropoietin couples hematopoiesis with bone formation. PLoS One. 2010;5(5):10853. doi:10.1371/journal.pone.0010853
29. Balaian E, Wobus M, Weidner H, et al. Erythropoietin inhibits osteoblast function in myelodysplastic syndromes via the canonical Wnt pathway. Haematologica. 2017;103(1):61. doi:10.3324/haematol.2017.172726
30. Wan L, Zhang F, He Q, et al. EPO promotes bone repair through enhanced cartilaginous callus formation and angiogenesis. PLoS One. 2014;9(7):102010. doi:10.1371/journal.pone.0102010
31. Wang X, Wang Y, Dai X, et al. Effects of intermittent administration of parathyroid hormone (1-34) on bone differentiation in stromal precursor antigen-1 positive human periodontal ligament stem cells. Stem Cells Int. 2016;2016:1–9. doi:10.1155/2016/4058656
32. Verma UN, Surabhi RM, Schmaltieg A, et al. Small interfering RNAs directed against b-catenin inhibit the in vitro and in vivo growth of coloncancer cells. Clinical Cancer Res. 2003;9:1291–1300.
33. Vimalraj S, Arumugam B, Miranda PJ, et al. Runx2: structure, function, and phosphorylation in osteoblast differentiation. Int J Biol Macromol. 2015;78:202–208. doi:10.1016/j.ijbiomac.2015.04.008
34. Chuang LS, Ito K, Ito Y. RUNX family: regulation and diversification of roles through interacting proteins. Int J Cancer. 2013;132(6):1260–1271. doi:10.1002/ijc.27964
35. Sun H, Feng K, Hu J, et al. Osteogenic differentiation of human amniotic fluid–derived stem cells induced by bone morphogenetic protein-7 and enhanced by nanofibrous scaffolds. Biomaterials. 2010;31(6):1133. doi:10.1016/j.biomaterials.2010.01.042
36. Beck GR, Zerler B, Moran E. Phosphate is a specific signal for induction of osteopontin gene expression. Proc Natl Acad Sci U S A. 2000;97(15):8352–8357. doi:10.1073/pnas.140021997
37. Fu H, Doll B, Mcnelis T, et al. Osteoblast differentiation in vitro, and in vivo, promoted by Osterix. J Biomed Mater Res Part A. 2007;83(3):770–778. doi:10.1002/jbm.a.31356
38. Kim YJ, Kim HN, Park EK, et al. The bone-related Zn finger transcription factor Osterix promotes proliferation of mesenchymal cells. Gene. 2005;366(1):145–151. doi:10.1016/j.gene.2005.08.021
39. Hatta M, Yoshimura Y, Deyama Y, et al. Molecular characterization of the zinc finger transcription factor, Osterix. Int J Mol Med. 2006;17(3):425–430.
40. Rossini M, Gatti D, Adami S. Involvement of WNT/β-catenin signaling in the treatment of osteoporosis. Calcif Tissue Int. 2013;93(2):121–132. doi:10.1007/s00223-013-9749-z
41. Saidak Z, Le Henaff C, Azzi S, et al. Wnt/β-catenin signaling mediates osteoblast differentiation triggered by peptide-induced α5β1 integrin priming in mesenchymal skeletal cells. J Biol Chem. 2015;290(11):6903–6912. doi:10.1074/jbc.M114.621219
42. Chen LJ, Hu BB, Shi XL, et al. Baicalein enhances the osteogenic differentiation of human periodontal ligament cells by activating the Wnt/β-catenin signaling pathway. Arch Oral Biol. 2017;78:100–108. doi:10.1016/j.archoralbio.2017.01.019
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.