Back to Journals » International Journal of Women's Health » Volume 7
Eribulin for the treatment of metastatic breast cancer: an update on its safety and efficacy
Received 17 September 2014
Accepted for publication 20 November 2014
Published 6 January 2015 Volume 2015:7 Pages 47—58
DOI https://doi.org/10.2147/IJWH.S74462
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Elie Al-Chaer
Mark K Doherty, Patrick G Morris
Department of Medical Oncology, Beaumont Hospital, Dublin, Ireland
Abstract: Breast cancer remains a leading cause of cancer-related death internationally. Treatment approaches for metastatic breast cancer have evolved in recent years; however chemotherapy remains a core component for the majority of patients. Agents such as anthracyclines and taxanes have been extensively studied and form standard treatment. Eribulin mesylate is a novel synthetic microtubule-directed chemotherapy, based on a naturally-occurring compound. Through phase I studies, eribulin was found to be tolerable and activity was seen in patients with metastatic breast cancer. Phase II studies in metastatic breast cancer further demonstrated its efficacy, with responses and survival which compare favorably with other studied chemotherapy agents. The phase III EMBRACE study showed superior survival for patients treated with eribulin compared with those who received a physician’s choice control. This led to its approval for use in many countries in this setting. Its toxicity profile is well established and manageable for the most part, with the commonest reported toxicities being alopecia, fatigue, neutropenia and peripheral neuropathy. A second reported phase III study comparing eribulin to capecitabine failed to show an improvement in survival in pretreated patients. This article reviews the clinical pharmacology and mechanism of action of eribulin, and summarizes the results of the major preclinical and clinical studies of eribulin in metastatic breast cancer.
Keywords: eribulin, breast cancer, metastatic breast cancer, review, new treatments, chemotherapy
Corrigendum has been published
Introduction
Breast cancer is the commonest cancer in women, affecting almost one in eight women worldwide,1 and the second most common cause of cancer deaths in women. Despite advances in the treatment of breast cancer, 4%–10% of women will present with metastatic or incurable disease.2 Furthermore, up to 30% of patients with early-stage tumors will develop distant metastases after primary treatment. For these women, no curative treatment approach exists at present. In that setting, the goals of treatment are to prolong survival and maximize quality of life by optimizing disease control. A range of effective therapies are available, which are increasingly tailored to an individual patient’s tumor biology. As most breast cancers express the estrogen receptor, an important aspect of treatment is the use of endocrine therapy. However, chemotherapy plays an important part in the management of patients with tumors that develop resistance to these treatments. Another validated therapeutic target is human epidermal growth factor receptor 2 (HER2). For tumors that overexpress HER2, the natural history of this disease has been significantly altered by the advent of effective, well tolerated targeted therapies. Despite this progress, the most effective treatment strategies have generally combined anti-HER2 agents with cytotoxic chemotherapy. Finally, for patients with tumors that lack expression of the estrogen receptor, HER2, and progesterone receptor, chemotherapy plays a critical role in systemic therapy. In fact, these so-called “triple” negative tumors are generally among the most sensitive to chemotherapy. Hence, despite significant biological heterogeneity, chemotherapy continues to play a major role in the treatment of metastatic breast cancer, particularly for women with severe symptoms or extensive visceral metastases, in whom early tumor response is most critical.
Chemotherapy
Breast cancer is, in general, a chemosensitive disease, and a range of active agents are available. Anthracyclines and taxanes are considered to be among the most active treatments for breast cancer. These agents are prescribed frequently as initial treatment for patients with early-stage cancers as well as for those with metastatic disease. However, there are two major limitations with their use for patients with metastatic breast cancer (MBC). First, prior exposure often leads to tumor resistance. Second, there is a risk of cumulative toxicities, including cardiac damage and peripheral neuropathy. Therefore, novel approaches are needed. For patients whose disease has progressed on these agents, or who are unable to tolerate them, there is no universally accepted standard therapy. Agents such as capecitabine,3 gemcitabine,4 vinorelbine,5 and ixabepilone6 have demonstrated activity in this setting and are frequently used. However, efficacy data are mostly from Phase II studies in this population and comparative studies are lacking.7
Microtubule agents
Chemotherapy agents acting on microtubules have demonstrated activity against multiple cancers, including breast cancer. Microtubules form an integral part of many intracellular processes, including maintenance of cell structure, transport of intracellular components, cell signaling, and mitosis.7 These structures are tube-shaped filaments composed of α-tubulin and β-tubulin heterodimers, which form highly dynamic polymers. Exploitation of their vital importance in mitosis and cell division has led to development of some of the most effective systemic chemotherapy drugs, with the taxanes and vinca alkaloids being the most widely studied. Broadly, these drugs exert their antineoplastic effect on the dynamics of microtubules, with taxane drugs thought to act as microtubule stabilizers and vinca alkaloids as destabilizers. Vinca alkaloids (eg, vincristine, vinblastine, vinorelbine, vinflunine) were the first class of microtubule-targeting drugs, isolated over 50 years ago from periwinkle leaves. These agents are thought to act by binding to β-tubulin and inhibiting polymerization into microtubules. They are currently in clinical use in a wide range of cancers including lung cancer, lymphoma (Hodgkin and non-Hodgkin), leukemia, germ cell tumors, and breast cancer. The first and most widely studied taxane drug to be developed clinically was paclitaxel, an agent derived from the bark of the Pacific yew tree. Subsequently, other taxanes such as docetaxel, and more recently cabazitaxel, have been synthesized and are now also used in clinical practice. These drugs have shown activity in a range of solid tumors, including cancers of the breast, lung, stomach, bladder, and ovary, as well as germ cell tumors. Many of the microtubule-targeting agents have similar toxicities, ie, myelosuppression, peripheral neuropathy, nausea, and fatigue. The commonly prescribed taxanes, docetaxel and paclitaxel, have additional issues related to solubility. They require the use of solvents such as polysorbate 80 for docetaxel and Cremophor® EL for paclitaxel. The solvents themselves are also recognized as causing adverse effects such as hypersensitivity reactions. As noted, taxanes exert their anticancer effect by binding to tubulin and stabilizing the microtubule, preventing disassembly and leading to apoptosis and cell death. Recent work has suggested that the mechanism of action of these drugs may be more complex than previously thought, and may involve inhibiting the dynamics of microtubules.8 Hence, there has been intense interest in microtubule-directed cancer treatment in recent years, with the development of several new agents.9
Eribulin
Eribulin mesylate is a novel compound derived from halichondrin B, a large polyether macrolide found in marine sponges including Halichondria okadai. Halichondrin B was first noted to have potent anticancer activity in vitro almost 30 years ago. However, clinical development was hampered by the lack of availability of the naturally occurring compound in sufficient quantities. Marine harvesting was sponsored by the National Cancer Institute, which allowed this naturally occurring compound to be studied and ultimately led to the development of eribulin (E7389), a synthetic analog.10 Structurally, eribulin is a simplified halichondrin analog with biochemical effects similar to those of the parent compound since the active macrocyclic lactone moiety is preserved.11 Eribulin has a unique mechanism of action, with a tubulin binding site that appears to be different from the taxane and vinca binding sites on the positive end of the microtubule (Figure 1). In practical terms, eribulin exerts its cytotoxic effect by inhibiting microtubule growth and sequestering tubulin, ultimately causing G2-M cell cycle arrest and cell death through apoptosis (Figure 2).12 Eribulin has shown preclinical activity against a number of human cancer cell lines and xenografts,10 with a wide therapeutic index, making it attractive for clinical development. Notable activity was seen in breast cancer cell lines, in which setting eribulin was more potent than vinblastine or paclitaxel. In addition, eribulin demonstrated marked activity in paclitaxel-resistant ovarian cancer cell lines.13 Eribulin was also found to have extensive distribution, with prolonged elimination primarily through feces,14 and was predominantly metabolized by cytochrome P450 3A4.15 It is of particular interest that eribulin can be administered without premedication, largely as a result of the lack of need for drug vehicles such as polysorbate 80 or Cremophor EL which are responsible for many of the hypersensitivity reactions seen with taxanes.
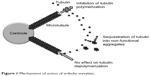  | Figure 1 Mechanisms of action of eribulin mesylate. |
  | Figure 2 Binding sites of microtubule-targeting chemotherapy agents. |
Phase I studies
Several reported Phase I studies with eribulin used a series of different dosing regimens and frequencies (Table 1).16–20 Two studies used a weekly schedule, in which treatment was given on days 1, 8, and 15 of a 28-day cycle.16,17 These studies suggested that the maximum tolerated dose of eribulin was 1.0 and 1.4 mg/m2, respectively.16,17 A similar maximum tolerated dose of 1.4 mg/m2 was seen when eribulin was administered weekly on days 1 and 8 of a 21-day cycle.18 When treatment was administered once every 21 days, the maximum tolerated dose was 2 mg/m2.19
Consistently in these studies, neutropenia was the most common dose-limiting toxicity. For example, in a study using the weekly regimen by Synold et al one patient developed dose-limiting toxicity of grade 3 febrile neutropenia and another experienced grade 4 neutropenia at the 2.0 mg/m2 dose.16 In the study by Goel et al which also used the weekly regimen, neutropenia (grade 3 or 4) was seen in three (33%) patients on day 15 at the 1.4 mg/m2 dose level, and a further two (22%) had neutropenia as a dose-limiting toxicity at this level.17 Grade 3/4 neutropenia was seen in all six patients at the 1.4 mg/m2 dose and in all three patients at the 2.0 mg/m2 dose in the study by Mukohara et al.18 Febrile neutropenia was also noted in patients at these dose levels. At the highest dose level tested by Tan et al in a 21-day cycle (4 mg/m2), all three patients developed febrile neutropenia.19 At the 2.0 mg/m2 dose established as the maximum tolerated dose in this study, febrile neutropenia was recorded in only one (14%) patient.
Pharmacokinetic analyses yielded similar results in all of these studies, ie, eribulin was characterized by rapid distribution, an extensive volume of distribution, slow to moderate clearance, and slow elimination with a terminal half-life of 36–48 hours. Renal clearance of eribulin was only 5%–10%, suggesting the major route of metabolism was hepatic. To further investigate the effect of hepatic impairment on the tolerability of eribulin, a Phase I study was performed in patients with mild to moderate hepatic impairment. The results of this study demonstrated that eribulin could be administered at doses of 1.1 mg/m2 in patients with mild hepatic dysfunction and 0.7 mg/m2 in patients with moderate dysfunction, due to increased exposure to the drug caused by delayed clearance.20 Encouraging responses, including partial responses by Response Evaluation Criteria In Solid Tumors criteria, were seen in patients in all of these studies. Given these findings and the preclinical activity seen in breast cancer cell lines, eribulin was brought to further clinical development, and the weekly schedule was used in the majority of studies due to favorable toxicity results.
Phase II studies in breast cancer
Collectively, these Phase I studies showed that eribulin was well tolerated. Subsequently, the activity of eribulin, mostly in a weekly schedule, was investigated in MBC in a series of Phase II studies (Table 2).21–25,28 Most of these studies included patients who had been extensively pretreated with chemotherapy.21–23,28 Given the potential concerns about neurotoxicity from microtubule agents, one study examined the incidence of peripheral neuropathy as the primary endpoint in a second-line treatment setting.28 More recently, two other studies have evaluated the activity of eribulin in the first-line setting for MBC.24,25
In the study by Vahdat et al21 patients with progressive MBC after anthracycline and taxane chemotherapy were initially treated with eribulin at a dose of 1.4 mg/m2 on days 1, 8, and 15 of a 28-day cycle. As a result of frequent recording of neutropenia on day 15 and subsequent dose omission, the treatment regimen was amended to 1.4 mg/m2 on days 1 and 8 every 21 days. The median number of prior chemotherapy regimens was four (1–11). In the per protocol population of 87 patients, an objective response rate (ORR) of 11.5% was achieved (95% confidence interval [CI] 5.7–20.1). All of these responses were partial; there were no recorded complete responses. The clinical benefit rate, defined as response or stable disease for >6 months, was 17.2% (95% CI 10.0–26.8). The median duration of response (DOR) was 5.6 months, progression-free survival (PFS) was 2.6 months, and median overall survival was 9.0 months. As predicted from the Phase I studies, the most common grade 3/4 toxicity was neutropenia, which occurred in 66 (64%) patients. Other toxicities included alopecia, leukopenia, and fatigue. Importantly, grade III neuropathy was seen in only five (5%) patients.
The study by Cortes et al22 enrolled 299 patients across a number of sites in the USA and Europe. Similar to the previous trial, patients in this study were also heavily pretreated, with a median of four (range 1–6) prior chemotherapy regimens. Treatment consisted of eribulin 1.4 mg/m2 on days 1 and 8 of a 21-day cycle. The ORR for the eligible patients (n=269) was 9.3% (95% CI 6.1–13.4), and the clinical benefit rate was 17.1% (95% CI 12.8–22.1). Median DOR was 4.1 months, median PFS was 2.6 months, and median overall survival was 10.4 months. Again, neutropenia was the dominant recorded grade 3/4 toxicity (54%), although febrile neutropenia was uncommon (5.5%). Grade 3 peripheral neuropathy was seen in 6.9% of patients, but the majority of patients with pre-existing neuropathy (78%) did not experience worsening of their symptoms. Overall, sensory neuropathy was reported by 78 (26.8%) patients.
A third study, in a Japanese population, was reported by Aogi et al.23 This group of 81 patients was previously treated with anthracycline and taxane chemotherapy, and had a median of three (range 1–5) previous regimens. Eribulin was administered at a dose of 1.4 mg/m2 on days 1 and 8 of a 21-day cycle. In this study, an ORR of 21.3% (95% CI 12.9–31.8) was observed, with a clinical benefit rate of 27.5% (95% CI 18.1–38.6). Median DOR was 3.9 months, median PFS was 3.7 months, and median overall survival was 11.1 months. Grade 3/4 neutropenia was seen in 77 (95.1%) patients, and febrile neutropenia in eleven (13.6%). Additional toxicities were seen at frequencies similar to those in the other studies, with sensory neuropathy recorded in 19 (23.5%) patients overall, and being grade 3 in three (3.7%) patients.
Two studies have been recently reported using eribulin in first-line treatment of MBC. In the study by McIntyre et al24 56 patients with recurrent or MBC without expression of HER2 were recruited. The treatment was delivered in a 21-day schedule similar to previous studies. The majority of patients (79%) had estrogen receptor-positive disease, and liver or lung metastases (84%). The median interval from diagnosis of breast cancer was 2.7 years. This study showed an impressive ORR of 28.6% (95% CI 17.3–42.2) and the clinical benefit rate was 51.8% (95% CI 38.0–65.3). The median DOR in this study was 5.8 months and median PFS was 6.8 months. Notably, 33 (59%) patients in this study had received an anthracycline and/or a taxane as adjuvant therapy. The activity of eribulin in this setting was similar to the overall study population. Grade 3 or greater neutropenia was recorded in 32 (50%) patients and eleven (20%) patients experienced grade 3 neuropathy. The apparently higher rate of neuropathy seen in this study may have been the result of prior antimicrotubule agents, or longer duration of therapy compared with studies in later lines of treatment.
As noted, chemotherapy is often combined with targeted therapy for patients with tumors that overexpress HER2. Therefore, the combination of eribulin with appropriate anti-HER2 agents is of significant research interest. Wilks et al25 recently reported the results of a study of eribulin in combination with the monoclonal antibody trastuzumab as first-line therapy for HER2-positive MBC. The study included 52 patients treated with eribulin on days 1 and 8 of a 21-day cycle, combined with standard doses of trastuzumab every 21 days. Almost half (48%) of the patients had received prior anthracycline or taxane chemotherapy in the adjuvant setting. The median number of cycles of eribulin administered on this study was ten. The ORR was 71.2% (95% CI 56.9–82.9), and the clinical benefit rate was 84.6% (95% CI 71.9–93.1). The median DOR was 11.1 months and median PFS was 11.6 months. Grade 3/4 toxicity was similar to previous studies, with neutropenia (26.9%) and neuropathy (38.5%) being the most common. Overall, peripheral neuropathy was observed in 31 (59.6%) patients at any grade. Although cross-study comparisons are notoriously challenging, it does appear that neuropathy was more common in these first-line studies compared with the studies in more heavily pretreated patients. One possible explanation for this is that there may have been greater drug exposure in these first-line studies, with median numbers of cycles of seven and ten24,25 compared with a median of 4–5 cycles in the older studies.21–23
As discussed, neuropathy is a common toxicity with microtubule-targeting agents. Preclinical data suggest that eribulin may cause less impairment of axonal transport due to the difference in tubulin-binding site compared with other agents,26 and in mouse models it was observed that eribulin did not worsen pre-existing neuronal damage caused by paclitaxel.27 Given these interesting results, and the observation that neuropathy rates were acceptable in clinical studies of eribulin, neuropathy was chosen as the focus of a recently reported randomized Phase II study comparing eribulin with ixabepilone.28 This study included patients who had received at least one line of chemotherapy for MBC, had previously been exposed to a taxane agent, and had grade 0 or 1 peripheral neuropathy. In total, 101 patients were randomized to treatment with eribulin or ixabepilone, and the primary endpoint of the study was comparison of neuropathy grades by treatment group. No statistically significant difference was seen in the incidence of peripheral neuropathy for those treated with eribulin (31.3%) compared with ixabepilone (44.1%). Similarly, there was no statistically significant difference in the incidence of grade 3/4 neuropathy (9.8% versus 20%), or in ORR (15.4% versus 5.8%) in patients who received eribulin or ixabepilone. However, there was a longer time to onset of neuropathy in the eribulin-treated patients (11.6 weeks versus 35.9 weeks) and fewer patients discontinued eribulin due to neuropathy (3.9% versus 18%).
Phase III studies
The activity seen in Phase II clinical trials prompted further investigation of eribulin in Phase III studies.29 The first reported study was EMBRACE (the Eisai Metastatic Breast Cancer Study Assessing Physician’s Choice Versus E7389).30 This was a global, multicenter, randomized, open-label study that included 762 patients with MBC who had progressive disease after treatment with anthracycline and taxane chemotherapy. Patients were randomized 2:1 to receive either eribulin 1.4 mg/m2 on days 1 and 8 every 21 days, or the comparator group receiving treatment of physician’s choice (TPC). In this study, TPC was defined as any single-agent chemotherapy, hormonal or biological treatment, radiotherapy, or supportive care. The primary objective of the study was to compare overall survival by treatment; secondary endpoints included PFS, ORR, and DOR. Key baseline characteristics of patients included the observation that the majority of patients (64%) had tumors that were estrogen/progesterone receptor-positive and HER2 negative (74%). In addition, more than half (51%) of the patients had three or more sites of metastatic disease. The median number of chemotherapy regimens was four (range 1–7). A large proportion of patients (73%) had been previously treated with capecitabine, and 64% came from Western Europe, North America, or Australia.
In total, 508 patients were treated with eribulin, and 254 received TPC. The TPC arm was made up of a number of therapies, with the majority of patients (96%) being treated with chemotherapy and 4% with hormonal therapy. The study met its primary objective, ie, an improvement in overall survival for patients treated with eribulin compared with TPC. The median overall survival in the eribulin-treated patients was 13.1 months compared with 10.6 months in the TPC-treated group (hazard ratio [HR] 0.81, 95% CI 0.66–0.99), a difference that was statistically significant (P=0.041). Despite the improvement in overall survival, median PFS was not significantly longer (P=0.137) with eribulin (3.7 months) than with TPC (2.2 months) in the independent review (HR 0.87, 95% CI 0.71–1.05). When the results were analyzed by investigator assessment, the median PFS was similar (3.6 versus 2.2 months) but fewer patients were censored, and the resultant benefit for eribulin appeared greater (HR 0.76, 95% CI 0.64–0.9) and reached statistical significance (P=0.002). Objective responses were significantly more frequent in patients treated with eribulin compared with TPC (12% versus 5%; P=0.002). There were three complete responses in the eribulin group and 54 partial responses, compared with ten partial responses in the TPC group. There was no significant difference in DOR. Overall toxicity rates were similar between eribulin and TPC, and the majority were grade 1/2. Grade 3/4 adverse events, which occurred more frequently with eribulin than with TPC were neutropenia (45% versus 21%), leucopenia (14% versus 6%), and peripheral neuropathy (9% versus 2%). Overall, neuropathy (any grade) was reported by 174 (35%) and 40 (16%) patients treated with eribulin and TPC, respectively. Adverse events leading to discontinuation of therapy occurred in 67 (13%) and 38 (15%) of patients treated with eribulin and TPC, respectively. Fatal adverse events occurred in 4% versus 7%, respectively.
This was a landmark study for patients with MBC and for eribulin. Critically, this was the first study to show a benefit in overall survival for any new therapy in heavily pretreated patients with MBC. It has been praised for its comparator TPC arm, which was designed to reflect clinical practice and arguably makes the result of the study more generalizable to clinical practice. This design does, however, have limitations. The relatively small number of patients treated with each chemotherapy agent in the TPC group precludes direct comparison of these individual treatments with eribulin, and additionally makes quality of life data collection and comparison impossible. The prolongation of overall survival without PFS prolongation in the EMBRACE study is also problematic, and may point to issues in the analysis or validity of the results. Generally, in randomized studies, the more active agent will delay cancer progression as well as prolong survival.
There are small numbers of agents in recent years that have prolonged overall survival without PFS prolongation. In many of these studies, alternative or confounding reasons have been found for this result (for example, more patients in one arm receiving additional lines of post-study treatments). In the case of the EMBRACE study, the authors reported that when PFS was analyzed by investigator review there was a statistically significant prolongation (3.6 versus 2.2 months, HR 0.76, P=0.002) that was not seen on independent review (3.7 versus 2.2 months, HR 0.87, P=0.137). The authors suggest that this is due to fewer patients being censored in the investigator review (127 versus 241), allowing more events to be analyzed (635 versus 521). They do not go into further detail regarding the reason for this difference. The authors report that only data regarding immediate post-study treatments were collected and that the proportion treated with further chemotherapy (54% of the eribulin group and 50% of the TPC group) and hormonal therapies (10% and 12%) were similar. Again, they do not detail what these post-study treatments were, although the similar proportions would suggest that this may not be the reason for this finding. Despite these concerns, eribulin mesylate was approved in the USA for treatment of MBC in patients who have received at least two prior chemotherapy regimens based on the results of this study.31
The possible use of eribulin in older patients was highlighted by an analysis of clinical trial data reported by Muss et al.32 This study included data collected for patients from two Phase II studies21,22 as well as the Phase III EMBRACE study.30 Toxicity and efficacy data were compared between groups of patients defined by age. The analysis showed no significant difference in outcome or in toxicity rates based on patient age, and validates eribulin as an option in patients aged over 70 years. Unfortunately, this study did not include a geriatric assessment tool, and is limited by the stringent entry criteria of the studies involved. The results of a second randomized Phase III study in MBC have also been reported.33 The 301 study compared eribulin mesylate (given in the same schedule as EMBRACE) with capecitabine (1,250 mg/m2 orally twice daily on days 1–14 every 21 days) in patients who had previously received treatment with anthracyclines and taxanes. In total, 1,102 patients were enrolled, and randomized in a 1:1 manner between groups. The patients included in this study had received two or fewer (and a maximum of three) prior chemotherapy regimens for advanced disease. The study used coprimary endpoints of overall survival and PFS, with secondary endpoints including ORR, quality of life, and overall survival at 1, 2, and 3 years. Patient characteristics were well balanced between the groups. The majority (70%) of patients received the study treatment as their first-line or second-line regimen for metastatic disease, 85% had visceral metastases, 70% had HER2-negative disease, and approximately 25% had triple-negative breast cancer.
Unfortunately, the study failed to meet either of its coprimary endpoints. There was a trend towards improvement in overall survival for patients treated with eribulin (15.9 versus 14.5 months), with a favorable HR of 0.88 (95% CI 0.77–1.01); however this failed to reach statistical significance (P=0.056). In addition, PFS was similar in both arms at 4.1 and 4.2 months (HR 0.98, 95% CI 0.86–1.11, P=0.736). This was not significantly different by investigator or independent review. Survival at 1, 2, and 3 years was numerically higher in the eribulin group, but this difference was not statistically significant. Response rates by independent review were similar between the two groups: the ORR was 11% and 12% in patients treated with eribulin and capecitabine, respectively. In prespecified subgroup analyses, there was a benefit seen in patients with HER2-negative disease (HR 0.84, 95% CI 0.72–0.098), ER-negative disease (HR 0.78, 95% CI 0.64–0.96), and triple-negative disease (HR 0.70, 95% CI 0.55–0.91). No new safety concerns were raised in this study, and toxicity profiles fit those previously recorded for these agents: neutropenia was common with eribulin (grade 3/4 in 46%) and hand/foot syndrome was common with capecitabine (45% any grade, 14% grade 3/4). Neuropathy (any grade) was seen in 13% of patients treated with eribulin and was grade 3/4 in 4% of patients. The quality of life results as measured by European Organisation for Research and Treatment of Cancer QLQ-C30 and QLQ-BR23 were presented separately.34 These data show that patients treated with eribulin had a 6.5 point greater improvement in overall quality of life (P=0.048) and a 15.3 point improvement in cognitive functioning (P<0.001) than quality of life score improvements seen in patients treated with capecitabine. Conversely, patients receiving capecitabine reported a 3.3 point greater improvement in emotional functioning (P=0.033), which may reflect the use of oral therapy.
The 301 study is considered a negative study because it did not meet its specified endpoints. However, the efficacy of eribulin was at least similar to that of capecitabine and with a different toxicity profile, which may be preferable for some patients (Table 3). Additionally, patients treated with eribulin had better patient-reported quality of life scores. The exploratory subgroup analysis suggests that further study in the HER2-negative and triple-negative populations may yield greater benefit, as there was a statistically significant prolongation in survival, but these results cannot be used to drive clinical decisions outside of a trial setting.
  | Table 3 Selected toxicities reported from Phase III studies of eribulin |
Regulatory and pharmacoeconomic assessments
The US Food and Drug Administration and the European Medicines Agency approved eribulin mesylate for the treatment of MBC patients who have received at least two prior chemotherapy regimens based largely on the results of the EMBRACE study.31,35,36 It has also received approval in many other countries, including Japan, Canada, and Australia.
In the UK, the National Institute for Health and Care Excellence subjected eribulin to a health technology assessment. Its assessment was based primarily on data from the EMBRACE study, and it was found that the most plausible estimate of the incremental cost-effectiveness ratio for eribulin versus TPC was £68,600 per quality-adjusted life year gained. The authors concluded that eribulin could not be recommended for use in the National Health Service despite acknowledgement of the overall survival benefit seen.37 Separate analyses have been carried out in Ireland and Canada, and have reported incremental cost-effectiveness ratios of €76,610 and $223,840–272,275 per quality-adjusted life year gained, respectively.38,39
Future directions
There are several ongoing studies evaluating eribulin in breast cancer (Table 4). The encouraging results seen with eribulin in pretreated patients with MBC compare favorably with published studies using other agents in this setting3–6 and have led to further research in other groups of patients with breast cancer. In the first-line setting for MBC, the current standard options for patients with HER2-negative disease include taxanes (docetaxel or paclitaxel), with reported response rates of 35%–42%40,41 and a median PFS of 6–9 months. A Phase III study comparing eribulin with paclitaxel in the first-line and second-line treatment of HER2-negative MBC is currently recruiting patients in centers in the USA.56 For HER2-positive MBC, taxanes combined with trastuzumab have yielded ORRs of 69%–76% and median PFS rates of 9–12 months.41,42 More recently, addition of the monoclonal antibody pertuzumab to docetaxel and trastuzumab improved response rates and survival in a Phase III study.43 On this background, a Phase II study of eribulin in combination with trastuzumab and pertuzumab is currently recruiting.49
To date, limited data are available regarding the safety and efficacy of eribulin combined with other agents, and there is current interest in combination therapy with novel agents44–46 as well as with existing therapies.47–50,52,54–56 Recent preclinical data have suggested synergistic activity between eribulin and S-1,63 and clinical studies of this regimen are likely to follow. The first data from Phase I studies of combination therapy have recently been reported, using trastuzumab in one Japanese study of 12 patients with MBC64 and cisplatin in a US study of 36 patients with advanced solid tumors.65 Both studies reported safety of the combination approaches without the need for reduction of eribulin dose intensity.
To date, there are no published data on eribulin in early-stage breast cancer, but studies are in progress evaluating use of this agent in the neoadjuvant setting, prior to surgery for localized disease, and in the adjuvant postoperative setting (Table 4).59–62 Although biomarker-directed therapy is increasingly common and sought after in breast cancer therapy, no predictive biomarkers for response to eribulin have been identified to date. One of the ongoing neoadjuvant studies59 is focusing on identification of potential biomarkers of therapeutic benefit from eribulin. In the future, the results of this study may help define a subpopulation in MBC who are more likely to derive benefit from this agent. This biomarker-driven approach might alleviate some of the challenges related to cost in the future.
Conclusion
MBC remains an incurable illness despite advances in treatment, and many patients with early-stage breast cancer will ultimately suffer distant recurrence of their disease. Chemotherapy continues to play a significant role in the management of the disease, despite the efficacy of targeted therapies against the estrogen receptor and HER2. Most patients with MBC are treated with anthracycline and taxane agents; however, patients will ultimately fail treatment as a result of disease progression or intolerable toxicity with these drugs, and for those patients no universal standard therapy exists. Eribulin mesylate is a novel nontaxane inhibitor of microtubule dynamics, and has demonstrated efficacy in patients with heavily pretreated MBC with a largely acceptable toxicity profile. Unlike taxanes, eribulin does not require premedication since there is no need for drug vehicles such as polysorbate 80 or Cremophor EL. In a landmark Phase III study, eribulin was the first cytotoxic agent to improve overall survival in heavily pretreated patients with MBC. It has induced responses in patients whose disease is resistant to other microtubule-targeting drugs, and appears to have a lower rate of neuropathy than many of the other agents in this class. Its development highlights both the utility of naturally occurring compounds in medical therapy as well as the importance of synthetic drug development. Future research is needed to optimize the role of eribulin in the treatment of MBC, in terms of both patient selection and its place in the therapeutic sequence. Finally, the potential role of this drug in early breast cancer is currently being explored.
Disclosure
The authors report no conflicts of interest in this work.
References
International Agency for Research on Cancer, World Health Organization. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. International Agency for Research on Cancer. 2013. Available from: http://globocan.iarc.fr. Accessed July 10, 2014. | ||
Li CI, Malone KE, Daling JR. Differences in breast cancer stage, treatment, and survival by race and ethnicity. Arch Intern Med. 2003;163:49–56. | ||
Blum JL, Jones SE, Buzdar AU, et al. Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol. 1999;17:485–493. | ||
Modi S, Currie VE, Seidman AD, et al. A phase II trial of gemcitabine in patients with metastatic breast cancer previously treated with an anthracycline and taxane. Clin Breast Cancer. 2005;6:55–60. | ||
Zelek L, Barthier S, Riofro M, et al. Weekly vinorelbine is an effective palliative regimen after failure with anthracyclines and taxanes in metastatic breast carcinoma. Cancer. 2001;92:2267–2272. | ||
Perez EA, Lerzo G, Pivot X, et al. Efficacy and safety of ixabepilone (BMS-247550) in a phase II study of patients with advanced breast cancer resistant to an anthracycline, a taxane, and capecitabine. J Clin Oncol. 2007;25:3407–3414. | ||
Murphy CG, Seidman AD. Evolving approaches to metastatic breast cancer previously treated with anthracyclines and taxanes. Clin Breast Cancer. 2009;9:S58–S65. | ||
Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–265. | ||
Morris PG, Fornier MN. Microtubule active agents: beyond the taxane frontier. Clin Cancer Res. 2008;14:7167–7172. | ||
Towle MJ, Salvato KA, Budrow J, et al. In vitro and in vivo anticancer activities of synthetic macrocyclic ketone analogues of halichondrin B. Cancer Res. 2001;61:1013–1021. | ||
Dabydeen DA, Burnett JC, Bai R, et al. Comparison of the activities of the truncated halichondrin B analog NSC 707389 (E7389) with those of the parent compound and a proposed binding site on tubulin. Mol Pharmacol. 2006;70:1866–1875. | ||
Kuznetsov G, Towle MJ, Cheng H, et al. Induction of morphological and biochemical apoptosis following prolonged mitotic blockage by halichondrin B macrocyclic ketone analog E7389. Cancer Res. 2004;64: 5760–5766. | ||
Kuznetsov G, TenDyke K, Yu MJ, Littlefield BA, Yu J, Littlefield BA. Antiproliferative effects of halichondrin B analog eribulin mesylate (E7389) against paclitaxel-resistant human cancer cells in vitro. Abstract C58 presented at the annual meeting of the American Association for Cancer Research, April 14–18, 2007, Los Angeles, CA, USA. | ||
Newman S. Eribulin, a simplified ketone analog of the tubulin inhibitor halichondrin B, for the potential treatment of cancer. Curr Opin Investig Drugs. 2007;8:1057–1066. | ||
Zhang ZY, King BM, Pelletier RD, Wong YN. Delineation of the interactions between the chemotherapeutic agent eribulin mesylate (E7389) and human CYP3A4. Cancer Chemother Pharmacol. 2008;62: 707–716. | ||
Synold TW, Morgan RJ, Newman EM, et al. A phase I pharmacokinetic, target validation study of the novel anti-tubulin agent E7389: a California Cancer Consortium trial. J Clin Oncol. 2005;23 Suppl 200: Abstr 3036. | ||
Goel S, Mita AC, Mita M, et al. A Phase I study of eribulin mesylate (E7389), a mechanistically novel inhibitor of microtubule dynamics, in patients with advanced solid tumors. Clin Cancer Res. 2009;15:4207–4212. | ||
Mukohara T, Nagai S, Mukai H, et al. Eribulin mesylate in patients with refractory cancers: a Phase I study. Invest New Drugs. 2012;30: 1926–1933. | ||
Tan AR, Rubin EH, Walton DC, et al. Phase I study of eribulin mesylate (E7389) administered once every 21 days in patients with advanced solid tumors. Clin Cancer Res. 2009;15:4213–4218. | ||
Devriese LA, Witteveen PO, Marchetti S, et al. Pharmacokinetics of eribulin mesylate in patients with solid tumors and hepatic impairment. Cancer Chemother Pharmacol. 2012;70:823–832. | ||
Vahdat LT, Pruitt B, Fabian CJ, et al. Phase II study of eribulin mesylate, a halichondrin B analog, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2009;27:2954–2961. | ||
Cortes J, Vahdat L, Blum JL, et al. Phase II study of the halichondrin B analog eribulin mesylate in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline, a taxane, and capecitabine. J Clin Oncol. 2010;28:3922–3928. | ||
Aogi K, Iwata H, Masuda N, et al. A phase II study of eribulin in Japanese patients with heavily pretreated metastatic breast cancer. Ann Oncol. 2012;23:1441–1448. | ||
McIntyre K, O’Shaughnessy J, Schwartzberg L, et al. Phase 2 study of eribulin mesylate as first-line therapy for locally recurrent or metastatic human epidermal growth factor receptor 2-negative breast cancer. Breast Cancer Res Treat. 2014;146:321–328. | ||
Wilks S, Puhalla S, O’Shaughnessy J, et al. Phase 2, multicenter, single-arm study of eribulin mesylate with trastuzumab as first-line therapy for locally recurrent or metastatic HER2-positive breast cancer. Clin Breast Cancer. June 2, 2014. [Epub ahead of print]. | ||
LaPointe NE, Morfini G, Brady ST, Feinstein SC, Wilson L, Jordan MA. Effects of eribulin, vincristine, paclitaxel and ixabepilone on fast axonal transport and kinesin-1 driven microtubule gliding: implications for chemotherapy-induced peripheral neuropathy. Neurotoxicology. 2013;37:231–239. | ||
Wozniak KM, Wu Y, Farah MH, Littlefield BA, Nomoto K, Slusher BS. Neuropathy-inducing effects of eribulin mesylate versus paclitaxel in mice with preexisting neuropathy. Neurotox Res. 2013;24:338–344. | ||
Vahdat L, Garcia A, Vogel C, et al. Eribulin mesylate versus ixabepilone in patients with metastatic breast cancer: a randomized Phase II study comparing the incidence of peripheral neuropathy. Breast Cancer Res Treat. 2013;140:341–351. | ||
Twelves C, Cortes J, Vahdat LT, et al. Phase III trials of eribulin mesylate (E7389) in extensively pretreated patients with locally recurrent or metastatic breast cancer. Clin Breast Cancer. 2010;10:160–163. | ||
Cortes J, O’Shaughnessy J, Loesch D, et al. Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet. 2011;377:914–923. | ||
US Food and Drug Administration. Halaven™ (eribulin mesylate) injection. 2010. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/201532lbl.pdf. Accessed July 10, 2014. | ||
Muss H, Cortes J, Vahdat LT, et al. Eribulin monotherapy in patients aged 70 years and older with metastatic breast cancer. Oncologist. 2014; 19:318–327. | ||
Kaufman PA, Awada A, Twelves C, et al. A phase III, open-label, randomized, multicenter study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with anthracyclines and taxanes. Cancer Res. 2012;72(24 Suppl):Abstr S66. | ||
Cortes J, Awada A, Kaufman PA, et al. Quality of life (QoL) in patients (pts) with locally advanced or metastatic breast cancer (MBC) previously treated with anthracyclines and taxanes who received eribulin mesylate or capecitabine: a phase III, open-label, randomized study. J Clin Oncol. 2013;31 Suppl:Abstr 1050. | ||
European Medicines Agency. Assessment report for Halaven (eribulin). 2011. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002084/WC500105115.pdf. Accessed July 10, 2014. | ||
Pean E, Klaar S, Berglund EG, et al. The European Medicines Agency review of Eribulin for the treatment of patients with locally advanced or metastatic breast cancer: summary of the scientific assessment of the Committee for Medicinal Products for Human Use. Clin Cancer Res. 2012;18:4491–4497. | ||
National Institute for Health and Clinical Excellence. Final appraisal determination on eribulin for the treatment of locally advanced or metastatic breast cancer. 2012. Available from: http://www.nice.org.uk/nicemedia/live/13238/57079/57079.pdf. Accessed July 10, 2014. | ||
National Centre for Pharmacoeconomics. Cost effectiveness of eribulin (Halaven®) for the treatment of patients with locally advanced breast cancer or metastatic breast cancer who have progressed after at least two chemotherapeutic regimens for advanced disease. 2012. Available from: http://www.ncpe.ie/wp-content/uploads/2011/03/Eribulin-Halaven-Summary1.pdf. Accessed July 10, 2014. | ||
Pan-Canadian Oncology Drug Review. Initial economic guidance report Eribulin (Halaven) for metastatic breast cancer. 2012. Available from: http://www.pcodr.ca/idc/groups/pcodr/documents/pcodrdocument/pcodr-halaven-in-egr.pdf. Accessed July 10, 2014. | ||
Rivera E, Mejia JA, Arun BK, et al. Phase 3 study comparing the use of docetaxel on an every-3-week versus weekly schedule in the treatment of metastatic breast cancer. Cancer. 2008;112:1455–1461. | ||
Seidman AD, Berry D, Cirrincione C, et al. Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: final results of Cancer and Leukemia Group B protocol 9840. J Clin Oncol. 2008;26:1642–1649. | ||
John M, Hinke A, Stauch M, et al. Weekly paclitaxel plus trastuzumab in metastatic breast cancer pretreated with anthracyclines – a phase II multipractice study. BMC Cancer. 2012;12:165. | ||
Baselga J, Cortés J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. | ||
Rugo H. Phase Ib/II study of PLX 3397 and eribulin in patients with metastatic breast cancer. Available from: http://clinicaltrials.gov/show/NCT01596751. Accessed November 25, 2014. | ||
Corcept Therapeutics. Mifepristone and eribulin in patients with metastatic triple negative breast cancer or ovarian cancer. Available from: http://clinicaltrials.gov/show/NCT02014337. Accessed November 25, 2014. | ||
Polyphor Ltd. Dose escalation of POL6326 in combination with eribulin in patients with metastatic breast cancer. Available from: http://clinicaltrials.gov/show/NCT01837095. Accessed November 25, 2014. | ||
Lee H. Moffitt Cancer Center and Research Institute. A Phase I dose escalation study of eribulin plus weekly carboplatin for metastatic breast patients. Available from: http://clinicaltrials.gov/show/NCT01795586. Accessed November 25, 2014. | ||
City of Hope Medical Center. Eribulin mesylate and everolimus in treating patients with triple-negative metastatic breast cancer. Available from: http://clinicaltrials.gov/show/NCT02120469. Accessed November 25, 2014. | ||
Dana-Farber Cancer Institute. Phase II study of eribulin mesylate, trastuzumab, and pertuzumab in women with metastatic, unresectable locally advanced, or locally recurrent HER2-positive breast cancer. Available from: http://clinicaltrials.gov/show/NCT01912963. Accessed November 25, 2014. | ||
German Breast Group. Efficacy and tolerability of eribulin plus lapatinib in patients with metastatic breast cancer (E-VITA). Available from: http://clinicaltrials.gov/show/NCT01534455. Accessed November 25, 2014. | ||
University of Washington. Eribulin mesylate in treating patients with previously treated metastatic breast cancer. Available from: http://clinicaltrials.gov/show/NCT01908101. Accessed November 25, 2014. | ||
Arcagy/Gineco Group. First line metastatic breast cancer treatment (ESMERALDA). Available from: http://clinicaltrials.gov/show/NCT01941407. Accessed November 25, 2014. | ||
Medica Scientia Innovation Research. Monotherapy with eribulin in HER2 negative metastatic breast cancer as a first line treatment (MERIBEL). Available from: http://clinicaltrials.gov/show/NCT02061085. Accessed November 25, 2014. | ||
Asan Medical Center. Eribulin plus gemcitabine vs paclitaxel plus gemcitabine in HER2-negative metastatic breast cancer. Available from: http://clinicaltrials.gov/show/NCT02263495. Accessed November 25, 2014. | ||
Dana-Farber Cancer Institute. Eribulin in HER2 negative metastatic BrCa. Available from: http://clinicaltrials.gov/show/NCT01827787. Accessed November 25, 2014. | ||
Consorzio Oncotech. Safety and efficacy study of eribulin in combination with bevacizumab for second-line treatment HER2- MBC patients (GIM11-BERGI). Available from: http://clinicaltrials.gov/show/NCT02175446. Accessed November 25, 2014. | ||
Tkaczuk K. Trastuzumab and pertuzumab alone or in combination with hormonal therapy or chemotherapy with eribulin in women aged 60 and over with HER2/Neu overexpressed locally advanced or MBC. Available from: http://clinicaltrials.gov/show/NCT02000596. Accessed November 25, 2014. | ||
Eisai Inc. A randomized Phase III trial of eribulin compared to standard weekly paclitaxel as first- or second-line therapy for locally recurrent or metastatic breast cancer. Available from: http://clinicaltrials.gov/show/NCT02037529. Accessed November 25, 2014. | ||
SOLTI Breast Cancer Research Group. Pharmacogenomic study of neoadjuvant eribulin for HER2 non-overexpressing breast cancer (NeoEribulin). Available from: http://clinicaltrials.gov/show/NCT01669252. Accessed November 25, 2014. | ||
Eisai Inc. Eribulin in combination with capecitabine for adjuvant treatment in estrogen receptor-positive early stage breast cancer. Available from: http://clinicaltrials.gov/show/NCT01439282. Accessed November 25, 2014. | ||
Emory University. Neoadjuvant trial of eribulin followed by dose dense doxorubicin and cyclophosphamide for HER2-negative, locally advanced breast cancer. Available from: http://clinicaltrials.gov/show/NCT01498588. Accessed November 25, 2014. | ||
M.D. Anderson Cancer Center. Neoadjuvant study of sequential eribulin followed by FAC compared to sequential paclitaxel followed by FEC in early stage breast cancer not overexpressing HER-2. Available from: http://www.clinicaltrials.gov/show/NCT0159302. Accessed November 25, 2014. | ||
Terashima M, Sakai K, Togashi Y, et al. Synergistic antitumor effects of S-1 with eribulin in vitro and in vivo for triple-negative breast cancer cell lines. Springer Plus. 2014;3:417. | ||
Mukai H, Saeki T, Shimada K, et al. Phase 1 combination study of eribulin mesylate with trastuzumab for advanced or recurrent human epidermal growth factor receptor 2 positive breast cancer. Invest New Drugs. September 23, 2014. [Epub ahead of print]. | ||
Koczywas M, Frankel PH, Synold TW, et al. Phase I study of the halichondrin B analogue eribulin mesylate in combination with cisplatin in advanced solid tumors. Br J Cancer. October 28, 2014. [Epub ahead of print]. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



