Back to Journals » OncoTargets and Therapy » Volume 9
ERCC1 and XRCC1 but not XPA single nucleotide polymorphisms correlate with response to chemotherapy in endometrial carcinoma
Authors Chen L, Liu MM, Liu H, Lu D, Zhao XD, Yang XJ
Received 20 April 2016
Accepted for publication 7 August 2016
Published 14 November 2016 Volume 2016:9 Pages 7019—7028
DOI https://doi.org/10.2147/OTT.S110976
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Min Li
Liang Chen,1 Mei-Mei Liu,1 Hui Liu,1 Dan Lu,2 Xiao-Dan Zhao,3 Xue-Jing Yang4
1Department of Gynecology and Obstetrics, 2Department of Oncology, 3Department of Clinical Laboratory, The 2nd Affiliated Hospital, Harbin Medical University, 4Nursing Department, Harbin Chest Hospital, Harbin, People’s Republic of China
Abstract: Our study aimed to investigate the correlation between single nucleotide polymorphisms of ERCC1/XRCC1/XPA genes and postoperative chemotherapy efficacy and prognosis of endometrial carcinoma. Our study included 108 patients with endometrial carcinoma and 100 healthy participants. ERCC1 rs11615/XRCC1 rs25487/XPA rs1800975 gene polymorphisms were detected by polymerase chain reaction–restriction fragment length polymorphism. Then the chemotherapy efficacy and toxic effects of the patients were assessed. The genotype and allele frequency of ERCC1 rs11615/XRCC1 rs25487 in the case group were significantly different from that in the control group (all P<0.05). The patients with AA + GA in ERCC1 rs11615 had an increased risk of endometrial carcinoma than those with GG, and the risk of endometrial carcinoma for patients with AA + GA was also higher in comparison with patients with GG genotype in XRCC1 rs25487 (all P<0.05). GG on both ERCC1 rs11615/XRCC1 rs25487 had a higher effective rate of chemotherapy than GA + AA (all P<0.05). ERCC1 rs11615/XRCC1 rs25487 gene polymorphisms were linked with toxic effects in liver, kidney, and nervous system. ERCC1 rs11615/XRCC1 rs25487, muscular invasion, and tumor stage were independent risk factors for the prognosis of endometrial carcinoma (all P<0.05). However, no significant associations were observed between XPA rs1800975 polymorphism and chemotherapy efficacy and prognosis of endometrial carcinoma (all P>0.05). These results indicated that ERCC1 and XRCC1 but not XPA polymorphisms correlate with response to chemotherapy in endometrial carcinoma.
Keywords: ERCC1, XRCC1, XPA, single nucleotide polymorphism, endometrial carcinoma, chemotherapy, efficacy, toxic effects
Introduction
Endometrial carcinoma, the fourth most prevalent gynecologic malignancy, is a disease driven predominantly by the neoplastic proliferation of endometrial epithelial cells.1 The high-risk group suffering from endometrial carcinoma is postmenopausal women, with up to 86% of patients aged >50 years.2 At present, although the etiology has not been completely clear, this disease may be attributed to the changes in endogenous and exogenous hormones that lead to the unopposed estrogen hypothesis. Namely, exposure of the endometrium to high levels of estrogen and low progesterone contributes to the development of endometrial cell and further enhances the risk of cancer.1 Interestingly, operation is the principal way for patients with endometrial carcinoma, such as hysterectomy and bilateral salpingo-oophorectomy, followed by other auxiliary treatments.3 In recent years, along with the improvement in molecular biological fields, it has been found that DNA repair polymorphism participates in the development of tumors, such as base excision repair (BER) and nucleotide excision repair (NER).4 The polymorphism of certain repair pathway can affect DNA repair capacity, and thereby influence risk rate of cancer as well as therapeutic efficacy.5
XRCC1 is an important DNA repair gene and plays a critical role in the process of BER.6 Located on chromosome 19q13.2-13.3, 33 kb-long XRCC1 embraces 17 exons and encodes a 70 kDa protein.7 The mutation of the 399 Gln allele located on the XRCC1 rs25487 greatly affects the BER function, which is closely associated with a higher incidence of lung cancer.8 ERCC1 is believed to be an essential polypeptide of the NER system, which promotes the repairing of damaged DNA through removal of the DNA–platinum adduct that inhibits DNA synthesis in cancer cells.9 A previous study showed that ERCC1 C118T synonymous single nucleotide polymorphism (SNP; No rs11615) affected the ERCC1 mRNA and protein expression levels that in return influence sensitivity to platinum-based chemotherapy in lung cancer.10 XPA functions to assist DNA excision repair to maintain genomic integrity by identifying damage position as well as interacting with many core repair factors.11 XPA A23G polymorphism, also known as XPA (-4) G-to-A polymorphism (rs1800975), is associated with the response to DNA damage and high risk of non-small-cell lung cancer and gastric cancer.12,13 Up to now, less attention has been paid to the relationship between DNA damage gene polymorphism and endometrial carcinoma. Given this, the purpose of this study is to assess the prognostic value of ERCC1/XRCC1/XPA gene polymorphisms in patients with endometrial adenocarcinoma undergoing two cycles of chemotherapy. This study will not only help to understand the mechanism of this disease but also help to facilitate the prevention of this tumor.
Patients and methods
Patients
From January 2010 to December 2012, a total of 108 patients with endometrial carcinoma undergoing operative treatments in The 2nd Affiliated Hospital, Harbin Medical University, were enrolled into a case group with a mean age of 52.91±7.35 years (range: 35–65). The surgical cases were divided into stages according to the standards of Federation of Gynecology and Obstetrics (FIGO),14 including 44 cases in I stage, 18 cases in II stage, 46 cases in III stage, and zero case in IV stage. And there were 88 (81.5%) cases with endometrioid carcinoma and 20 (18.5%) cases with nonendometrioid carcinoma. All patients underwent extrafascial hysterectomy with bilateral adnexectomy and pelvic cavity abdominal aorta lymph node dissection. Also, all patients with complete clinical data neither accept any treatment before hospital admission nor suffer from other malignant tumors. They had no mental disorder and unconscious disorders, blood coagulation dysfunction, severe cardiac, cerebral vessels disease, and liver and kidney dysfunction. Meanwhile, the patients did not accept other antineoplastic treatments before treatment with cisplatin plus fluorouracil. The specific dosages of chemotherapeutics were cisplatin (75 mg/m2) VD d1 and fluorouracil (500 mg/m2). Therapeutic evaluations of the patients who were administered with 5 mg granisetron as antiemesis and 15 mg furosemidum intravenously (3–4 weeks as a cycle) were performed after 2 weeks in accordance with the principles of response evaluation criteria in solid tumors (RECIST). Another 110 cases confirmed by physical examination in the corresponding period were randomly collected as a control group with a mean age of 50.00±8.52 years (ra1). All patients and their families knew the study plan and have signed the written informed consents. Also, this study (NCT: ChiCTR-RRC-16009412) was approved by the ethics committee of The 2nd Affiliated Hospital, Harbin Medical University.
DNA extraction
Before chemotherapy, from two group of patients, 3 mL of venous blood was collected in a tube containing sodium citrate, which is an anticoagulant. Each tube was labeled with the number and name of the subject. After 10 minutes of centrifugation (3,000 rpm), the DNA kit (Takara, Japan) was applied to draw the whole blood DNA in peripheral blood, and the DNA concentration detector (NANODROP2000; Thermo Fischer Scientific, Waltham, MA, USA) was employed to detect the purity of DNA and the concentration of DNA at an optical density (OD) of 260 nm and 280 nm. The mean concentration was detected as 100±20 ng/L and the DNA purity was determined in 1.6–1.8 according to the ratio of A260 nm/A280 nm. The extracted DNA was stored at −40°C for further use.
Detections of single nucleotide polymorphisms
The primer was purchased from Nanjing Lejin Biotech Co., Ltd. (Nanjing, People’s Republic of China) (Table 1). A polymerase chain reaction (PCR) kit was bought from Vazyme Biotech Co., Ltd. (Nanjing, People’s Republic of China), with 20 mL general reaction system containing 1 mL of extracted DNA, 1 mL of upstream and 1 mL of downstream primers (10 pmol/mL), 10 mL of PCR mixture and 7 mL of deionized water. The reaction conditions include: predenaturation at 95°C for 5 minutes, then with a total of 40 circulations of denaturation at 95°C for 30 seconds, annealing at 65°C for 40 seconds, extension at 72°C for 7 minutes and final extension at 72°C for 10 minutes. After 3.5% agarose gel electrophoresis (containing 0.5 mg/mL ethidium bromide), 4 mL amplification products and 1 mL bromophenol blue sample buffer solution were mixed with each other, and then the sample was added. The amplification bands were observed and photographed when the mixture was subject to 0.5× Tris–boric acid–EDTA buffer solution and 70 V electrophoresis for 15 minutes under an ultraviolet lamp to test whether PCR was successful. The products of ERCC1 rs11615/XRCC1 rs25487/XPA rs1800975 underwent enzyme digestion of BsrDI (37°C), MspI (37°C), and cleavage SfcI (37°C), which were purchased from New England Biolabs, Ipswich, MA, USA, and the operation procedures were carried out according to the instructions. The enzyme-digested products were separated in 3.5% agarose gel electrophoresis at 120 V for 40 minutes, and the band was observed in an ultraviolet lamp. The sample (10%) was sequenced bidirectionally (Sangon Biotech Co., Ltd., Shanghai, China) to test the results of PCR–restriction fragment length polymorphism.
  | Table 1 Primer sequences of ERCC1 rs11615, XRCC1 rs25487, and XPA rs1800975 polymorphisms |
Efficacy evaluation
The patients were reexamined according to the evaluation standards of RECIST15 after two-cycle chemotherapy. Complete remission (CR) meant that the whole measureable diseases have completely disappeared for 4 weeks; partial response (PR) meant that the maximum diameter of the lesions reduced <30% and maintained for <4 weeks; no-change (NC) was between PR and progressive disease (PD) and PD meant that the maximum diameter overtopped 20% or new lesions appear. Patients with CR and PR belong to efficient chemotherapy cases, while patients with stable disease and PD belong to inefficient ones.
Evaluation of toxic effects
Relapses in patients were recorded during follow-up after chemotherapy, and chemotherapy toxic reaction of the patients was defined according to the evaluation standards of toxic reaction recommended by the World Health Organization.16 The grading standards of nausea and vomiting were as follows: 0, no nausea and vomiting symptoms; I, vomiting less than two times every day, which did not affect daily life; II, vomiting less than or equivalent to five times, which slightly affected daily life, and III–IV, vomiting more than five times, which was addressed by remaining in bed and taking medicine according to indications.
Follow-up
A total of 108 patients with endometrial carcinoma were followed up, with the follow-up rate reaching 93.5%. It ended because of death or censoring time with a follow-up of 1–3 years (mean follow-up of 2.5 years). The patients were followed up by telephone, petition, and outpatient review to understand the postoperative survivals and recovery status of patients, for example, whether the patients relapsed or survived. If relapsed, what were the sites and treatments? If died, what were the specific causes and time of death?
Statistical analysis
The SPSS 20.0 integrated software (SPSS Inc., Chicago, IL, USA) was employed for data analysis. Measurement data were exhibited as mean ± SD; independent-sample t-test was used for comparison between two groups, and analysis of variance was used for comparison among more than two groups. Welch was used when measurement data failed to satisfy homogeneity of variance. Least significant difference was applied to compare homogeneity of variance among groups. Tamhane’s T2 test was used for multiple comparisons between groups. Pearson chi-square test was employed to compare the frequency distribution of genotype and allele among groups. Logistic regression analysis was used to confirm the relationships between ERCC1/XRCC1/XPA gene polymorphisms and the postoperative chemotherapy efficacy of endometrial carcinoma. Hardy–Weinberg balance was tested by chi-square test, and Kaplan–Meier was performed in survival analysis. The Cox risk ratio model was adopted in multiplicity. Moreover, P was bilaterally inspected and P<0.05 indicated statistically significant.
Results
Clinical characteristics of subjects
No significant difference was found between the case group and control group concerning the age, body mass index (BMI), and menopause (all P>0.05). Among the 108 patients with endometrial carcinoma, 88 presented with endometrioid carcinoma and 20 presented with nonendometrioid carcinoma. Furthermore, 53 patients presented no muscular invasion or <1/2 invasion, while 55 exhibited over 1/2 muscular invasion. The maximum lesion diameter was <1 cm in 29 patients, between 1 cm and 2 cm in 45 patients and >2 cm in 34 patients.
Frequency distribution of ERCC1/XRCC1/XPA gene polymorphisms
Allele frequency examined by Hardy–Weinberg equilibrium was tested by chi-square test. The Hardy–Weinberg equilibrium test showed that the allele frequency of ERCC1 rs11615/XRCC1 rs25487/XPA rs1800975 in the control group was in balance (P>0.05), signaling a strong group representation.
The electropherogram of ERCC1 rs11615 is displayed in Figure 1A. There was a statistical difference regarding genotype and allele frequency between the case group and the control group (all P<0.05, Table 2), which implied that ERCC1 rs11615 polymorphism might be associated with the risk of endometrial carcinoma. Compared with the patients with GG in ERCC1 rs11615, the patients with AA + GA had an increased risk of endometrial carcinoma (GA vs GG, OR =3.964, 95% CI =2.013–7.805, P<0.05; AA vs GG, OR =6.098, 95% CI =1.252–29.700, P<0.05; AA + GA vs GG, OR =4.215, 95% CI =2.218–8.010, P<0.05).
The electropherogram of XRCC1 rs25487 is outlined in Figure 1B. There were significant differences regarding genotype and allele frequency between the case group and control group (all P<0.05), which implied that XRCC1 rs25487 polymorphism might be lined with the risk of endometrial carcinoma. Compared with the patients with GG in XRCC1 rs25487, the patients with AA + GA had an increased risk of endometrial carcinoma (GA vs GG, OR =1.806, 95% CI =1.014–3.215, P<0.05; AA vs GG, OR =2.512, 95% CI =1.021–6.178, P<0.05; AA + GA vs GG, OR =1.947, 95% CI =1.136–3.336, P<0.05; A vs G, OR =1.738, 95% CI =1.148–2.629, P<0.05). Therefore, AA + GA genotype of XRCC1 was a risk factor for endometrial carcinoma.
The electropherogram of XPA rs1800975 is illustrated in Figure 1C. No significant difference was found regarding genotype and allele frequency between the case group and control group (all P>0.05).
Correlations of ERCC1/XRCC1/XPA gene polymorphisms with clinical characteristics
After the stratified analysis of the age, BMI, and pathologic type of the 108 patients, no significant difference was revealed between the AA + GA frequency and GG frequency of ERCC1 rs11615/XRCC1 rs25487, and there was also no difference between the GG + GA frequency and AA frequency of XPA rs1800975 (all P>0.05, Table 3). When the stratified analysis was made based on menopause, muscular invasion, maximum lesion diameter, tumor stage and lymphatic metastasis, the AA + GA frequency and GG frequency of ERCC1 rs11615/XRCC1 rs25487 were significantly different (all P<0.05), while the the GG + GA frequency and AA frequency of XPA rs1800975 were not significant different (P>0.05).
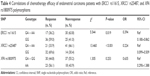
Correlations of ERCC1/XRCC1/XPA gene polymorphisms with chemotherapy efficacy
Among the total 108 cases, 53 cases had clinical response to chemotherapy, achieving 49.1% of effective rate. The statistical data are listed in Table 4.
The effective rate of GA + AA in ERCC1 rs11615 attained 36.2%, significantly lower than 59% of GG (P<0.05), signifying that GG was more sensitive to chemotherapeutic drugs.
Moreover, in XRCC1 rs25487, GA + AA reached the effective rate of 33.9%, lower than 69.6% of GG (P<0.05), the latter of which demonstrated a more sensitivity to chemotherapy.
The AA and GA + GG in XPA gene exhibited 44.8% and 56.1% effective rate, respectively. There was no significant difference among the three groups (all P>0.05).
Correlations of ERCC1/XRCC1/XPA gene polymorphisms with toxic effects
In both ERCC1 rs11615 and XRCC1 rs25487, compared with the patients with GG genotype, the digestive system and hematological system of patients with GA + AA genotype showed no significant difference in toxic effects (P>0.05), while there were significant differences between the two genotypes concerning toxic effects in liver, kidney, and nervous system (both P<0.05). The toxic effects of patients with GA + GG and AA in XPA rs1800975 demonstrated no statistical difference (all P>0.05, Table 5).
  | Table 5 Correlations of toxic effects in endometrial carcinoma patients with ERCC1 rs11615, XRCC1 rs25487, and XPA rs1800975 polymorphisms |
Survival analysis
The follow-up for endometrial carcinoma patients spanned for 1–3 years with a mean of 2.5 years. The overall survival rates of 1, 2, and 3 years after the surgery were 93.5%, 78.7%, and 70.4%, respectively. In ERCC1 rs11615, among the patients with GG genotype, ten cases (16.4%) died and six cases lost contact, while among the patients with GA + AA, 22 cases (46.8%) died and five cases lost contact (all P<0.05, Figure 2A). In XRCC1 rs25487, among the patients with GG genotype, three cases (6.5%) died and seven cases lost contact, while among the patients with GA + AA, 29 cases (46.8%) died and four cases lost contact (all P<0.05, Figure 2B). In XPA rs1800975, among the patients with AA genotype, 14 cases (34.1%) died and four cases lost contact, while among the patients with GA + AA, 18 cases (26.9%) died and seven cases lost contact (all P>0.05, Figure 2C).
The Cox regression analysis in Table 6 revealed that the tumor stage and genes of ERCC1 rs11615 and XRCC1 rs25487 were independent risk factors of the prognosis of endometrial carcinoma (all P<0.05). Specifically, the GG genotype in ERCC1 rs11615 and XRCC1 rs25487, and I + II stages were protective factors of the prognosis of endometrial carcinoma, while muscular invasion failed to be correlated with the prognosis of endometrial carcinoma.
  | Table 6 Cox regression analysis |
Discussion
In recent years, endometrial carcinoma is a growing global concern with important public health implications on women. Besides, the prevalence of endometrial carcinoma is tending to increase due to a long-term, excessive estrogen exposure without the protection of progesterone and poor postoperative treatment. Existing evidence shows that genetic characteristics may contribute to the development of endometrial carcinoma. More importantly, the DNA repair gene genetic polymorphisms are significantly involved with the risk of cancer occurrence.17 Therefore, the study is designed to investigate the significance of DNA repair gene genetic polymorphisms (XRCC1, ERCC1, and XPA) on the risk occurrence of endometrial carcinoma and evaluation of chemotherapy efficacy in order to provide a theoretical basis for the prevention and treatment of the disease.
Initially, our findings revealed that gene polymorphism of XRCC1 rs25487 was a risk factor for endometrial carcinoma. Namely, the patients with AA + GA had an increased risk of endometrial carcinoma when compared with the patients carrying GG genotype at XRCC1 rs25487. To the best of our knowledge, XRCC1 plays an essential role in the BER pathway and functions as a scaffold protein, which bonds with DNA repair complex. XRCC1 gene was identified by its function to restore the DNA repair capacity in the Chinese hamster ovary mutant cell line EM9 and to interact with poly(ADP-ribose) polymerase and DNA ligase III for recognizing and rejoining DNA strand breaks, as well as with DNA polymerase β and apurinic/apyrimidinic endonuclease I.18–22 The most extensively studied SNPs of XRCC1 gene are Arg399Gln (G>A, rs25487) and Arg194Trp (C>T, rs1799782), which have been reported to be associated with an altered DNA repair activity. At the same time, BER pathway is the main DNA repair pathway, which removes oxidized and alkylated bases. Various enzymes, including OGG1, XRCC1, and PARP1, participate in BER, which contribute to polymorphisms related to the risk of cancer.8 A functional SNP in XRCC1 rs25487 with a G to A base alteration results in an arginine to glutamine substitution. A study reported by Duell et al23 discovered that the minor allele (A) for XRCC1 rs25487, the 399Gln allele, was connected to an increased frequency of glycophorin mutation, enhanced DNA adduct levels, higher frequency of baseline sister chromatid exchange, as well as increased sensitivity to ionizing radiation, all of which might emerge due to weakened BER function. Therefore, our study speculated that the mutation GA of XRCC1 rs25487 can cause the alteration of amino acid Arg-Gln, which is located in 399th codon, resulting in an increased risk of endometrial carcinoma by reducing the binding ability of XRCC1, PARP, DNA polymerase β, and ligase III.
Meanwhile, continuous analysis was performed for the value of XRCC1 genetic polymorphism on the efficacy of chemotherapy and overall survival of prognosis. The results demonstrated that the patients with GG genotype were more sensitive to chemotherapy and had higher survival capacity. Interestingly, cancer patients are commonly resistant to chemotherapy, and this resistance has been lined with enhanced NER in cancer tissues, and most chemotherapy drugs will lead to the recognition and activation of the apoptotic program in DNA damage identification of molecular as well as DNA repair process by forming platinum–DNA adducts, such as anticancer drugs of platinum agents.24 Also, cancer cells may resist against the platinum-based chemotherapy if their DNA repair ability is increased to remove those DNA adducts due to the function of platinum agents. Therefore, we assumed that genetic polymorphism of XRCC1 rs25487 reduced repair ability of DNA damage and promoted tumor growth and metastasis, thus affecting the sensitivity of tumor cells to chemotherapeutic drugs. Consistent with our study, Gurubhagavatula et al25 reported that XRCC1 (Arg399Gln) DNA repair gene may act as an important prognostic factor in patients with advanced lung cancer.
More importantly, we found that the risk of endometrial carcinoma in the patients with the AA + GA genotype was increased when compared to GG genotype in ERCC1 rs11615. ERCC1 protein XPF and ERCC4 can recognize damaged DNA 5′-end and function as 5′–3′ endonuclease of nucleic acids, thereby reducing the possibility of structural chromosome aberrations and guarantying genomic instability.26 Recently, an epidemiological study suggested that inhibition of expression of ERCC1 played an essential role in reducing the ability of DDP–DNA adduct repair, after which the tumor incidence increased with low resistance in drugs.27 Furthermore, Ma et al28 reported that the ERCC1 rs11615 polymorphism contributed to the clinical outcomes of patients with gastrointestinal cancer, such as gastric cancer and colorectal cancer who were treated by oxaliplatin-based chemotherapy. Therefore, we may assume that influence of the alteration of AA + GA genotype decreased the nuclear nucleotide repair ability. Therefore, the increased risk of occurrence of endometrial carcinoma was observed in the patients with AA + GA genotype of ERCC1 rs11615. Also, continuous data were analyzed to investigate the relationship between the ERCC1 rs11615 genetic polymorphisms and chemotherapy efficiency. The results demonstrated that the patients with GG genotype were more sensitive to chemotherapeutic drugs. Similarly, Viguier et al29 showed that the expressions of ERCC1 protein were changed when ERCC1 codon 118 C (rs11615) was transferred to T, reducing the ability of DNA repair and weakening the impact of ERCC1 on DNA damage caused by platinum drugs. Besides, the data also indicated that the patients with ERCC1 codon 118 T/T genotype were more sensitive to platinum-based chemotherapy than the patients with C/T and C/C genotype.29,30 All the results revealed that the patients with wild-type C/C were more sensitive to chemotherapeutic drugs than heterozygous C/T and mutant T/T in ERCC1 118. Thus, we may propose that wild-type GG of ERCC1 rs11615 promoted the chemotherapeutic drugs to target the tumor cells and inhibited the replication and transcription of DNA, resulting in apoptosis of tumor cells.
Conclusion
Our study elucidated that ERCC1 rs11615 and XRCC1 rs25487 but not XPA rs1800975 polymorphisms correlate with response to chemotherapy in endometrial carcinoma. Therefore, the related genetic polymorphisms of DNA damage repair have implicated the pathogenesis of endometrial carcinoma, thus providing a theoretical basis of individualized treatment for this disease. However, our results demonstrated that there was no significant difference between XPA genetic polymorphisms and the occurrence and platinum-based chemotherapy efficiency of endometrial carcinoma. Therefore, further studies, with larger sample size, including clinical staging and postoperative follow-up data of the selected cases, are required to obtain the results reflecting relevance of XPA genetic polymorphisms and endometrial carcinoma.
Acknowledgment
We would like to sincerely extend our gratitude to the reviewers for their constructive comments.
Disclosure
The authors report no conflicts of interest in this work.
References
Karageorgi S, Hankinson SE, Kraft P, De Vivo I. Reproductive factors and postmenopausal hormone use in relation to endometrial cancer risk in the Nurses’ Health Study cohort 1976–2004. Int J Cancer. 2010;126(1):208–216. | ||
Wallace AE, Gibson DA, Saunders PT, Jabbour HN. Inflammatory events in endometrial adenocarcinoma. J Endocrinol. 2010;206(2):141–157. | ||
Myatt SS, Wang J, Monteiro LJ, et al. Definition of microRNAs that repress expression of the tumor suppressor gene FOXO1 in endometrial cancer. Cancer Res. 2010;70(1):367–377. | ||
Rajaraman P, Hutchinson A, Wichner S, et al. DNA repair gene polymorphisms and risk of adult meningioma, glioma, and acoustic neuroma. Neuro Oncol. 2010;12(1):37–48. | ||
Kalikaki A, Kanaki M, Vassalou H, et al. DNA repair gene polymorphisms predict favorable clinical outcome in advanced non-small-cell lung cancer. Clin Lung Cancer. 2009;10(2):118–123. | ||
Mutamba JT, Svilar D, Prasongtanakij S, et al. XRCC1 and base excision repair balance in response to nitric oxide. DNA Repair (Amst). 2011;10(12):1282–1293. | ||
Zhang L, Wang Y, Qiu Z, Luo J, Zhou Z, Shu W. The XRCC1 Arg194Trp polymorphism is not a risk factor for glioma: a meta-analysis involving 1,440 cases and 2,562 controls. Exp Ther Med. 2012;4(6):1057–1062. | ||
Wang X, Ma KW, Zhao YG, Wang GJ, Li W. XRCC1 rs25487 polymorphism is associated with lung cancer risk in epidemiologically susceptible Chinese people. Genet Mol Res. 2015;14(4):15530–15538. | ||
Lee SH, Noh KB, Lee JS, et al. Thymidylate synthase and ERCC1 as predictive markers in patients with pulmonary adenocarcinoma treated with pemetrexed and cisplatin. Lung Cancer. 2013;81(1):102–108. | ||
Wei SZ, Zhan P, Shi MQ, et al. Predictive value of ERCC1 and XPD polymorphism in patients with advanced non-small cell lung cancer receiving platinum-based chemotherapy: a systematic review and meta-analysis. Med Oncol. 2011;28(1):315–321. | ||
Chisholm-Burns M, Pinsky B, Parker G, et al. Factors related to immunosuppressant medication adherence in renal transplant recipients. Clin Transplant. 2012;26(5):706–713. | ||
Qian B, Zhang H, Zhang L, Zhou X, Yu H, Chen K. Association of genetic polymorphisms in DNA repair pathway genes with non-small cell lung cancer risk. Lung Cancer. 2011;73(2):138–146. | ||
Palli D, Polidoro S, D’Errico M, et al. Polymorphic DNA repair and metabolic genes: a multigenic study on gastric cancer. Mutagenesis. 2010;25(6):569–575. | ||
Saida T, Tanaka YO, Matsumoto K, Satoh T, Yoshikawa H, Minami M. Revised FIGO staging system for cancer of the ovary, fallopian tube, and peritoneum: important implications for radiologists. Jpn J Radiol. 2016;34(2):117–124. | ||
Duffaud F, Therasse P. Nouvelles recommandations pour l’évaluation de la réponse tumorale dans les tumeurs solides [New guidelines to evaluate the response to treatment in solid tumors]. Bull Cancer. 2000;87(12):881–886. French. | ||
Tsuchida Y, Therasse P. Response evaluation criteria in solid tumors (recist): new guidelines. Med Pediatr Oncol. 2001;37(1):1–3. | ||
Figueroa JD, Malats N, Real FX, et al. Genetic variation in the base excision repair pathway and bladder cancer risk. Hum Genet. 2007;121(2):233–242. | ||
Thompson LH, Brookman KW, Jones NJ, Allen SA, Carrano AV. Molecular cloning of the human XRCC1 gene, which corrects defective DNA strand break repair and sister chromatid exchange. Mol Cell Biol. 1990;10(12):6160–6171. | ||
Thompson LH, West MG. XRCC1 keeps DNA from getting stranded. Mutat Res. 2000;459(1):1–18. | ||
Vidal AE, Boiteux S, Hickson ID, Radicella JP. XRCC1 coordinates the initial and late stages of DNA abasic site repair through protein-protein interactions. EMBO J. 2001;20(22):6530–6539. | ||
Al Mutairi FM, Alanazi M, Shalaby M, Alabdulkarim HA, Pathan AA, Parine NR. Association of XRCC1 gene polymorphisms with breast cancer susceptibility in Saudi patients. Asian Pac J Cancer Prev. 2013;14(6):3809–3813. | ||
Cui Z, Yin Z, Li X, Wu W, Guan P, Zhou B. Association between polymorphisms in XRCC1 gene and clinical outcomes of patients with lung cancer: a meta-analysis. BMC Cancer. 2012;12:71. | ||
Duell EJ, Holly EA, Bracci PM, Wiencke JK, Kelsey KT. A population-based study of the Arg399Gln polymorphism in X-ray repair cross-complementing group 1 (XRCC1) and risk of pancreatic adenocarcinoma. Cancer Res. 2002;62(16):4630–4636. | ||
Bosken CH, Wei Q, Amos CI, Spitz MR. An analysis of DNA repair as a determinant of survival in patients with non-small-cell lung cancer. J Natl Cancer Inst. 2002;94(14):1091–1099. | ||
Gurubhagavatula S, Liu G, Park S, et al. XPD and XRCC1 genetic polymorphisms are prognostic factors in advanced non-small-cell lung cancer patients treated with platinum chemotherapy. J Clin Oncol. 2004;22(13):2594–2601. | ||
Park CH, Sancar A. Formation of a ternary complex by human XPA, ERCC1, and ERCC4 (XPF) excision repair proteins. Proc Natl Acad Sci U S A. 1994;91(11):5017–5021. | ||
Takahata C, Masuda Y, Takedachi A, Tanaka K, Iwai S, Kuraoka I. Repair synthesis step involving ERCC1-XPF participates in DNA repair of the Top1-DNA damage complex. Carcinogenesis. 2015;36(8):841–851. | ||
Ma SC, Zhao Y, Zhang T, Ling XL, Zhao D. Association between the ERCC1 rs11615 polymorphism and clinical outcomes of oxaliplatin-based chemotherapies in gastrointestinal cancer: a meta-analysis. Onco Targets Ther. 2015;8:641–648. | ||
Viguier J, Boige V, Miquel C, et al. ERCC1 codon 118 polymorphism is a predictive factor for the tumor response to oxaliplatin/5-fluorouracil combination chemotherapy in patients with advanced colorectal cancer. Clin Cancer Res. 2005;11(17):6212–6217. | ||
Ryu JS, Viguier J, Praz F. Genetic effect of ERCC1 codon 118 polymorphism and confounding factors. Clin Cancer Res. 2006;12(15):4784. [author reply 4784–4785]. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.