Back to Journals » Infection and Drug Resistance » Volume 14
Eradication Rates for Esomeprazole and Lansoprazole-Based 7-Day Non-Bismuth Concomitant Quadruple Therapy for First-Line Anti-Helicobacter pylori Treatment in Real World Clinical Practice
Authors Hung KT, Yang SC, Wu CK , Wang HM, Yao CC, Liang CM , Tai WC , Wu KL, Kuo YH , Lee CH , Chuah SK
Received 31 January 2021
Accepted for publication 6 March 2021
Published 25 March 2021 Volume 2021:14 Pages 1239—1246
DOI https://doi.org/10.2147/IDR.S304711
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Kuo-Tung Hung,1 Shih-Cheng Yang,1 Cheng-Kun Wu,1,2 Hsing-Ming Wang,1 Chih-Chien Yao,1 Chih-Ming Liang,1,2 Wei-Chen Tai,1,2 Keng-Liang Wu,1,2 Yuan-Hung Kuo,1,2 Chen-Hsiang Lee,2,3 Seng-Kee Chuah1,2
1Division of Hepatogastroenterology, Department of Internal Medicine, Chang Gung Memorial Hospital, Kaohsiung, Taiwan; 2Chang Gung University, College of Medicine, Taoyuan, Taiwan; 3Division of Infectious Disease, Department of Internal Medicine, Chang Gung Memorial Hospital, Kaohsiung, Taiwan
Correspondence: Seng-Kee Chuah; Chih-Ming Liang
Division of Hepatogastroenterology, Chang Gung Memorial Hospital, 123, Ta-Pei Road, Niao-Sung Hsiang, Kaohsiung, 833, Taiwan
Tel + 886-7-7317123 ext. 8301
Fax + 886-7-7322402
Email [email protected]; [email protected]
Purpose: Non-bismuth concomitant quadruple therapy is commonly administered in Taiwan, achieving an acceptable efficacy as a first-line anti-Helicobacter pylori treatment. This study compared the eradication rates between esomeprazole- and lansoprazole-based non-bismuth concomitant quadruple therapy for first-line anti-H. pylori treatment.
Patients and Methods: This study included 206 H. pylori-infected naïve patients between July 2016 and February 2019. The patients were prescribed with either a 7-day non-bismuth containing quadruple therapy (esomeprazole, 40 mg twice daily; amoxicillin, 1 g twice daily; and metronidazole, 500 mg twice daily; and clarithromycin, 500 mg twice daily for 7 days [EACM group]; lansoprazole, 30 mg twice daily; amoxicillin, 1 g twice daily; metronidazole, 500 mg twice daily; and clarithromycin, 500 mg twice daily [LACM group]). Then, the patients were asked to perform urea breath tests 8 weeks later.
Results: The eradication rates in the EACM group were 86.1% (95% confidence interval [CI], 77.8%– 92.2%) and 90.6% (95% CI, 82.9%– 95.6%) in the intention-to-treat (ITT) and the per-protocol (PP) analyses, respectively. Moreover, the eradication rates in the LACM group were 90.1% (95% CI, 82.6%– 95.2%) and 92.6% (95% CI, 85.5%– 96.9%) in the ITT and the PP analyses, respectively. Consequently, the LACM group exhibited more diarrhea patients than the EACM group (7.1% versus 1.0%, p = 0.029), but all symptoms were mild. Univariate analysis in this study showed that metronidazole-resistant strains were the clinical factor affecting the eradications (95.3% versus 78.9%, p = 0.044). Moreover, a trend was observed in dual clarithromycin- and metronidazole-resistant strains (91.5% versus 66.7%, p = 0.155).
Conclusion: The eradication rates between esomeprazole and lansoprazole-based non-bismuth concomitant quadruple therapy for first-line H. pylori treatment were similar in this study. Both could achieve a > 90% report card in the PP analysis.
Keywords: Helicobacter pylori, esomeprazole, lansoprazole, concomitant therapy, antibiotic resistance
Introduction
Helicobacter pylori infection is an important worldwide public health issue with a prevalence of 45.2%–84.2%.1 Patients can develop chronic gastritis, atrophic gastritis, intestinal metaplasia, dysplasia, gastric cancer, and peptic ulcer disease when infected.2–6 Hence, successful eradication of H. pylori reduces the recurrence of peptic ulcers and gastric cancer.7–9 The current guidelines and consensus of H. pylori management suggested a concomitant therapy (standard proton pump inhibitor (PPI); amoxicillin, 1 g twice daily; metronidazole, 500 mg twice daily; and clarithromycin, 500 mg twice daily) as first-line regimens.8,10,11 One of the crucial key points for successful H. pylori eradication is securing and lengthening the duration of high intra-gastric pH. A strong acid-suppressing effect drug, vonoprazan, maintains a much higher intra-gastric pH than the traditional PPIs. However, this drug is not available worldwide.12,13
In real-world practice, a twice daily PPI is currently prescribed to patients because it is allowed by the National Health Insurance policy. Therefore, whether different PPIs influence and affect the outcome of H. pylori eradication in this concomitant regimen is an important concern among physicians and patients. Therefore, this study was conducted to compare the eradication rates and adverse effects of esomeprazole- and lansoprazole-based 7-day non-bismuth concomitant quadruple therapy for first-line anti-Helicobacter pylori treatment.
Patients and Methods
Patients
We retrospectively analyzed a total of 206 H. pylori-infected naïve patients from our prospectively collected patients’ registrar profile who were ≥ 20 years old between July 2016 and July 2019 at outpatient clinics in Kaohsiung Chang Gung Memorial Hospital, Taiwan. All patients received an endoscopy that showed either peptic ulcers or gastritis. H. pylori infection was diagnosed by histological assessment of endoscopic biopsy specimens or of gastric mucosa or rapid urease test. All patients were treated with a 7-day non-bismuth concomitant quadruple therapy (esomeprazole, 40 mg twice daily; amoxicillin, 1 g twice daily; metronidazole, 500 mg twice daily; and clarithromycin, 500 mg twice daily for 7 days [EACM group, n = 105] or lansoprazole, 30 mg twice daily; amoxicillin, 1 g twice daily; metronidazole 500 mg twice daily; and clarithromycin 500 mg twice daily [LACM group, n = 101]). For peptic ulcer patients, PPIs or mucosa protectants was prescribed for 4–6 weeks after 7-day non-bismuth concomitant quadruple therapy. However, in a few patients, PPI maybe need for 8 weeks if symptoms were not totally improved. An endoscope follow-up examination would be performed then. No matter how, all of them stop PPI for 2 weeks before taking a UBT test. Patients with a history of previous H. pylori eradication, antibiotics administration within 3 months before endoscopy, gastric malignancy, lost to follow-up, or demonstrated incomplete records were excluded.
Culture and Antimicrobial Susceptibility Testing
H. pylori strains were tested for susceptibility to amoxicillin, clarithromycin, levofloxacin, tetracycline, and metronidazole using the Epsilometer test method (AB Biodisk, Solna, Sweden). H. pylori strains exhibited MIC values of ≥0.5, ≥1, ≥1, ≥4, and ≥8 mg/L, which were considered to be the resistance breakpoints for amoxicillin, clarithromycin, levofloxacin, tetracycline, and metronidazole, respectively, according to the European Committee on Antimicrobial Susceptibility Testing.14
Outcome Measurements and Follow-Up
Follow-up studies to assess treatment responses were carried out for 1 week for drug compliance and adverse events assessment and 8 weeks later for H. pylori testing by urea breath tests. The primary outcome of this study was the eradication rate by intention-to-treat (ITT) and per-protocol (PP) analyses of the two therapeutic groups. Eradication therapy failure was confirmed after treatment by a positive 13C-urea breath test 8 weeks after treatment. Poor compliance was the failure to finish 80% of all medication due to adverse effects.15,16 The secondary outcomes were drug compliance and adverse events. In the PP analysis, patients who were lost during follow-up or did not follow the protocol or with unknown H. pylori status posttherapy were excluded. Adverse events (abdominal pain, constipation, diarrhea, dizziness, headache, nausea/vomiting, and skin rash) were compared.
Statistical Analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences, version 22, for windows. Moreover, a P value of <0.05 was considered statistically significant. The 95% confidence interval (CI) was constructed by normal approximation. Univariate logistic regressions were performed to predict successful eradication.
Results
Baseline Characteristics and H. pylori Eradication Rates
The patient deposition is shown in Figure 1. Six and three patients were lost to follow-up, respectively, in the EACM and LACM groups among the 206 patients enrolled in the ITT. Finally, 99 and 98 patients were included in EACM and LACM groups for PP analysis, respectively. The baseline characteristics were similar between the two groups in age, gender, social habits, and endoscopic findings (Table 1). The eradication rates in the EACM group were 86.1% (95% CI, 77.8%–92.2%) and 90.6% (95% CI, 82.9%–95.6%) in the ITT and PP analyses, respectively. Moreover, the eradication rates in the LACM group were 90.1% (95% CI, 82.6%–95.2%) and 92.6% (95% CI, 85.5%–96.9%) in the ITT and PP analyses, respectively (Table 2). The adverse events were also similar between the two groups (11.1% versus 10.2%, p = 0.837; Table 2). However, more diarrhea symptoms were observed in the LACM than in the EACM group (7.1% versus 1.0%, p = 0.029; Table 3). Other adverse events included abdominal pain (4.0% and 3.1%), nausea sensation (3.1% and 2.0%), dizziness (1% in both groups), and headache (3% and 1%). Univariate analysis showed that metronidazole-resistant strains were the clinical factor affecting the eradications in this study (95.3% versus. 78.9%, p = 0.044). A trend was observed in dual clarithromycin- and metronidazole-resistant strains (91.5% versus 66.7%, p = 0.155; Table 4).
 |
Table 1 Demographic Data and Endoscopic Appearance of Two Patient Groups |
 |
Table 2 The Major Outcomes of Two Period’s Groups |
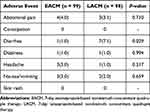 |
Table 3 Adverse Events of Two Groups |
 |
Table 4 Univariate Analysis of the Clinical Factors Influencing the Efficacy of H. pylori Eradication Therapy |
Antibiotic Resistance
The H. pylori strains were tested for susceptibility to antibiotics in 68 patients, the positive culture rate was 91.2% (62/68). Antibiotic resistances were 14.5%, 30.6%, and 35.5% clarithromycin, metronidazole, and levofloxacin, respectively. Moreover, 4.8% of them exhibited dual resistant clarithromycin and metronidazole. No antibiotic-resistant strain to amoxicillin and tetracycline was noted in this study (Figure 2).
Among patients with the amoxicillin- and clarithromycin-susceptible strains, the H. pylori eradication rate was 90.6% (48/53) and 88.9% (8/9) for those with clarithromycin-resistant strains. The eradication rate was 78.9% (15/19) for patients with metronidazole resistance assigned to both the concomitant therapy groups.
Discussion
Conferring to the Maastricht V and Taiwanese consensus, concomitant therapy for 7–14 days was recommended as one of the first-line therapies for H. pylori eradication.8,11 Achieving an acceptable eradication rate for naïve H. pylori infection was its benefit with the advantages of convenience and easy-to-adhere one-stage combination formulation instead of the two-stage sequential and hybrid therapies.17–20 The differences between PPIs in H. pylori eradication triple therapy were compared, and the results were debatable after several decades.21,22 Most head to head comparison studies were based on comparisons among standard triple therapy. The H. pylori eradication rate in esomeprazole was better than the first-generation PPIs (omeprazole, lansoprazole, and pantoprazole) in the standard triple therapy.23 This result was often explained by the prominent effect of esomeprazole to control gastric pH level and the CYP2C19 effect on metabolization of PPIs. However, rare studies existed comparing eradication rates between different kinds of PPI in non-bismuth concomitant quadruple therapy for first-line H. pylori.
The eradication rates between esomeprazole- and lansoprazole-based non-bismuth containing quadruple therapy for first-line H. pylori treatment were similar in this study and achieved a 90% report card in the PP analysis. Heterogeneous results regarding the head to head comparisons between esomeprazole and lansoprazole have been reported in the literature. Wilder-Smith et al reported that the stronger acid suppression performance in esomeprazole compared with lansoprazole in standard dose (esomeprazole [40 mg daily] versus lansoprazole [30 mg daily]).24 Graham et al reported similar potencies of esomeprazole and lansoprazole at a 20 mg:45 mg ratio after standardizing PPI potency in terms of the duration of intra-gastric pH > 4/24 h (pH4 time).25 One other report documented the impact of the CYP2C19 genotype on pharmacokinetic parameters for esomeprazole presenting with a smaller effect on the area under the curve (AUC) than other PPIs (omeprazole and lansoprazole).26 The latter could explain why the esomeprazole-based concomitant therapy was not superior in treatment success compared with lansoprazole-based concomitant treatment regimens in the current study. Other possible reasons could be that the rate of CYP2C19 rapid metabolizers was proven to be higher in Europe and North America (56%–81%) compared with the Asian population (27%–38%).27 The lower potent PPIs, such as lansoprazole, could be enough for acid suppression in the concomitant regiment in Asia. In addition, metronidazole is relatively stable in low pH gastric juice compared to clarithromycin.28,29 The additional metronidazole in the quadruple than in the triple therapy may overcome the influence of lower acid suppression in the LACM group. In China, Chen et al reported no difference between esomeprazole (20 mg BID) and lansoprazole (30 mg BID) in the eradication rate of H. pylori in the 14-day bismuth-furazolidone quadruple regiment.30 Boltin et al reported the esomeprazole did not show a significant trend over omeprazole among subjects receiving quadruple therapy.31 These reports were similar with to the current findings. Thus, concomitant quadruple therapies could achieve good eradication efficacy in case of less potency in acid suppression of PPIs.
Studies reported that dual clarithromycin and metronidazole resistance undermines the efficacy of concomitant therapy. Cure rates with sequential, hybrid, and concomitant therapy will always be <90% when the rates of dual resistant strains are >5%, >9%, or >15%, respectively.8,32 Thus, antibiotic resistance is one of the most important factors that determine eradication success. The data in this study also showed a lower eradication rate in the dual resistant group compared with the dual-sensitive group (91.5% vs 66.7%, p = 0.155). A progressively higher resistance rate was observed for clarithromycin (11.8–20.4%, p = 0.039) and metronidazole (25.6–42.3%, p < 0.001) among patients who received first-line eradication therapy in 7 years was observed in the cohort antibiotics resistance study in Taiwan. Furthermore, the primary dual resistance to clarithromycin and metronidazole also significantly increased in a linear trend from 2.4% to 10.4% (p = 0.009).33 The eradication rate of non-bismuth concomitant quadruple therapy in first-line therapy could be expected to drop further to < 90% in Taiwan as time goes by. Therefore, continuously monitoring regional resistance rates was mandatory. Recent studies reported a novel potassium-competitive acid blocker (vonoprazan) that provides a stronger and longer-lasting effect on gastric acid suppression than other PPIs.34 Vonoprazan-based regimens is more useful than the PPI-based regimen as a first-lineH. pylori eradication therapy.35 Furthermore, the effectiveness for patients infected with clarithromycin-resistant strains was demonstrated in patients living in areas where clarithromycin-resistant strain prevalence is >15%.36
This study exhibits several limitations. First, antibiotic susceptibility tests were not performed in all patients, and only 68 of 206 patients exhibited an antibiotic susceptibility test. However, no difference in antibiotic resistance exists between the EACM and LACM groups. Second, this was a retrospective study in a single medical center.
Conclusion
The eradication rates between esomeprazole- and lansoprazole-based non-bismuth concomitant quadruple therapy for first-line H. pylori treatment were similar in this study. Both could achieve a >90% report card in the PP analysis.
Data Sharing Statement
No data will be shared except besides what is included in the manuscript.
Ethics Approval and Informed Consent
This protocol was approved by the institutional review board and the Ethics Committee of Chang Gung Memorial Hospital (IRB-202002020B0D001). The Ethics Committee waived the requirement for informed consent for this retrospective study and all the data were analyzed anonymously. None of our patients were minors or children. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution’s human research committee.
Acknowledgments
The authors would like to acknowledge Miss Ching-Yi Lin for the assistance in this study.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest exist in this work.
References
1. Mentis A, Lehours P, Mégraud F. Epidemiology and diagnosis of Helicobacter pylori infection. Helicobacter. 2015;20:1–7. doi:10.1111/hel.12250
2. Correa P. Helicobacter pylori and gastric carcinogenesis. Am J Surg Pathol. 1995;19:S37.
3. Correa P, Piazuelo MB. The gastric precancerous cascade. J Dig Dis. 2012;13:2–9. doi:10.1111/j.1751-2980.2011.00550.x
4. Watari J, Chen N, Amenta PS, et al. Helicobacter pylori associated chronic gastritis, clinical syndromes, precancerous lesions, and pathogenesis of gastric cancer development. World J Gastroenterol. 2014;20:5461–5473. doi:10.3748/wjg.v20.i18.5461
5. Cancer IAfRo. IARC monographs on the evaluation of carcinogenic risks to humans. Schistosomes, liver flukes and Helicobacter pylori. Vol 61. Lyon: World Health Organization, International Agency for Researchon Cancer (IARC); 1994.
6. Hopkins RJ, Girardi LS, Turney EA. Relationship between Helicobacter pylori eradication and reduced duodenal and gastric ulcer recurrence: a review. gastroenterology. 1996;110:1244–1252. doi:10.1053/gast.1996.v110.pm8613015
7. Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection—the Maastricht V/Florence consensus report. Gut. 2017;66:6–30.
8. Lee YC, Chiang TH, Chou CK, et al. Association between Helicobacter pylori eradication and gastric cancer incidence: a systematic review and meta-analysis. Gastroenterology. 2016;150:1113–1114. e5. doi:10.1053/j.gastro.2016.01.028
9. Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112:212–239. doi:10.1038/ajg.2016.563
10. Sheu BS, Wu MS, Chiu CT, et al. Consensus on the clinical management, screening‐to‐treat, and surveillance of Helicobacter pylori infection to improve gastric cancer control on a nationwide scale. Helicobacter. 2017;22:e12368. doi:10.1111/hel.12368
11. Mori H, Suzuki H. Role of acid suppression in acid-related diseases: proton pump inhibitor and potassium-competitive acid blocker. J Neurogastroenterol Motil. 2019;25:6–14. doi:10.5056/jnm18139
12. Abadi ATB, Ierardi E. Vonoprazan and Helicobacter pylori treatment: a lesson from Japan or a limited geographic phenomenon? Front Pharmacol. 2019;10:316. doi:10.3389/fphar.2019.00316
13. The European Committee on Antimicrobial Susceptibility Testing – EUCAST. Available from: https://www.eucast.org/ast_of_bacteria/previous_versions_of_documents/.
14. Chuah SK, Liang CM, Lee CH, et al. A randomized control trial comparing 2 levofloxacin-containing second-line therapies for Helicobacter pylori eradication. Medicine. 2016;95:e3586. doi:10.1097/MD.0000000000003586
15. Yao CC, Kuo CM, Hsu CN, et al. First-line Helicobacter pylori eradication rates are significantly lower in patients with than those without type 2 diabetes mellitus. Infect Drug Resist. 2019;12:1425–1431. doi:10.2147/IDR.S194584
16. Tai WC, Liang CM, Lee CH, et al. Seven-day nonbismuth containing quadruple therapy could achieve a grade “A” success rate for first-line Helicobacter pylori eradication. Biomed Res Int. 2015;2015:623732. doi:10.1155/2015/623732
17. Tai WC, Liang CM, Kuo CM, et al. 14-day esomeprazole-and amoxicillin-containing high dose dual therapy achieves high eradication rate in the first line anti-helicobacter pylori treatment in Taiwan: a Prospective Randomized Trial. J Antimicrob Chemother. 2019;74:1718–1724. doi:10.1093/jac/dkz046
18. Wu DC, Hsu PI, Wu JY, et al. Sequential and concomitant therapy with 4 drugs are equally effective for eradication of H. pylori Infection. Clin Gastroenterol Hepatol. 2010;8:36–41. doi:10.1016/j.cgh.2009.09.030
19. Tsay FW, Wu DC, Yu HC, et al. A randomized controlled trial shows that both 14- day hybrid and bismuth quadruple therapies cure most patients with Helicobacter pylori infection in populations with moderate antibiotic resistance. Antimicrob Agents Chemother. 2017;61. doi:10.1128/AAC.00140-17
20. Anagnostopoulos GK, Tsiakos S, Margantinis G, Kostopoulos P, Arvanitidis D. Esomeprazole versus omeprazole for the eradication of Helicobacter pylori infection: results of a randomized controlled study. J Clin Gastroenterol. 2004;38:503–506. doi:10.1097/01.mcg.0000129061.54277.c6
21. Choi HS, Park DI, Hwang SJ, et al. Double‐dose, new‐generation proton pump inhibitors do not improve Helicobacter pylori eradication rate. Helicobacter. 2007;12:638–642. doi:10.1111/j.1523-5378.2007.00556.x
22. Hsu PI, Lai K-H, Lin C-K, et al. A prospective randomized trial of esomeprazole- versus pantoprazole-based triple therapy for Helicobacter pylori eradication. Am J Gastroenterol. 2005;100:2387–2392. doi:10.1111/j.1572-0241.2005.00264.x
23. McNicholl A, Linares P, Nyssen O, Calvet X, Gisbert J. Meta‐analysis: esomeprazole or rabeprazole vs. first‐generation pump inhibitors in the treatment of Helicobacter pylori infection. Aliment Pharmacol Ther. 2012;36:414–425. doi:10.1111/j.1365-2036.2012.05211.x
24. Wilder-Smith C, Lind T, Lundin C, Naucler E, Nilsson-Pieschl C, Röhss K. Acid control with esomeprazole and lansoprazole: a comparative dose–response study. Scand J Gastroenterol. 2007;42:157–164. doi:10.1080/00365520601075845
25. Graham DY, Lu H, Dore MP. Relative potency of proton‐pump inhibitors, Helicobacter pylori therapy cure rates, and meaning of double‐dose PPI. Helicobacter. 2019;24:e12554. doi:10.1111/hel.12554
26. Sahara S, Sugimoto M, Uotani T, et al. Twice‐daily dosing of esomeprazole effectively inhibits acid secretion in CYP2C19 rapid metabolisers compared with twice‐daily omeprazole, rabeprazole or lansoprazole. Aliment Pharmacol Ther. 2013;38:1129–1137. doi:10.1111/apt.12492
27. Shimatani T, Inoue M, Kuroiwa T, et al. Acid‐suppressive effects of rabeprazole, omeprazole, and lansoprazole at reduced and standard doses: a crossover comparative study in homozygous extensive metabolizers of cytochrome P450 2C19. Clin Pharmacol Ther. 2006;79:144–152. doi:10.1016/j.clpt.2005.09.012
28. Erah P, Goddard A, Barrett D, Shaw P, Spiller R. The stability of amoxycillin, clarithromycin and metronidazole in gastric juice: relevance to the treatment of Helicobacter pylori infection. J Antimicrob Chemother. 1997;39:5–12. doi:10.1093/jac/39.1.5
29. Goddard AF, Jessa MJ, Barrett DA, et al. Effect of omeprazole on the distribution of metronidazole, amoxicillin, and clarithromycin in human gastric juice. Gastroenterology. 1996;111:358–367. doi:10.1053/gast.1996.v111.pm8690200
30. Chen L, He J, Wang L, et al. Efficacies of different proton pump inhibitor-based 14- day bismuth–furazolidone quadruple regimens for the initial eradication of Helicobacter pylori in the southeast coastal region of China: an open-label, randomized clinical trial. Clin Exp Med. 2018;18:569–576. doi:10.1007/s10238-018-0510-9
31. Boltin D, Levi Z, Gingold-Belfer R, et al. Comparative effect of proton-pump inhibitors on the success of triple and quadruple therapy for Helicobacter pylori infection. Dig Dis. 2020;38:408–414.
32. Graham DY, Lee YC, Wu MS. Rational Helicobacter pylori therapy: evidence-based medicine rather than medicine-based evidence. Clin Gastroenterol Hepatol. 2014;12(177–186):e3. doi:10.1016/j.cgh.2013.05.028
33. Liang CM, Tai WC, Hsu PI, et al. Trend of changes in antibiotic resistance in Helicobacter pylori from 2013 to 2019: a multicentre report from Taiwan. Therap Adv Gastroenterol. 2020;13:1756284820976990. doi:10.1177/1756284820976990
34. Sakurai Y, Mori Y, Okamoto H, et al. Acid‐inhibitory effects of vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole 10 mg in healthy adult male subjects‐a randomised open‐label cross‐over study. Aliment Pharmacol Ther. 2015;42:719–730. doi:10.1111/apt.13325
35. Maruyama M, Tanaka N, Kubota D, et al. Vonoprazan-based regimen is more useful than PPI-based one as a first-line Helicobacter pylori eradication: a randomized controlled trial. Can J Gastroenterol Hepatol. 2017;2017. doi:10.1155/2017/4385161
36. Sugimoto M, Yamaoka Y. Role of vonoprazan in Helicobacter pylori eradication therapy in Japan. Front Pharmacol. 2018;9:1560. doi:10.3389/fphar.2018.01560
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


