Back to Journals » Veterinary Medicine: Research and Reports » Volume 10
Equine glandular gastric disease: prevalence, impact and management strategies
Authors Banse HE, Andrews FM
Received 15 January 2019
Accepted for publication 26 March 2019
Published 16 July 2019 Volume 2019:10 Pages 69—76
DOI https://doi.org/10.2147/VMRR.S174427
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Young Lyoo
Heidi E Banse, Frank M Andrews
Equine Health Studies Program, Department of Veterinary Clinical Sciences, School of Veterinary Medicine, Louisiana State University, Baton Rouge LA, USA
Abstract: Equine glandular gastric disease (EGGD) is an increasingly recognized disease of the glandular mucosa of the equine stomach. Diagnosis is confirmed by gastric endoscopy and scored based upon one of several different endoscopic scoring systems. Prevalence appears to be variable, depending upon breed and discipline. Primary identified risk factors include exercise frequency, and stress; therefore, management strategies are focused on reducing exercise and stress. Limiting grain intake and increasing pasture turnout may also be helpful preventative measures. Pharmacologic treatment consists primarily of an approved omeprazole product with or without misoprostol or sucralfate. Further research into the pathophysiology of EGGD may allow for identification of other targeted treatments.
Keywords: gastric ulcer, inflammation, exercise, stress, omeprazole, misoprostol
Introduction
The stomach of the horse is comprised of two distinct regions, the squamous and glandular mucosa, separated by the margo plicatus. The glandular mucosa lines the ventral portion of the stomach and consists of gastric glands that secrete hydrochloric acid, pepsinogen, histamine, mucous, and sodium bicarbonate. The dorsal portion of the stomach is covered by squamous epithelium. Due to differences in risk factors associated with these regions, disease of the stomach has been divided based upon lesion location: equine glandular gastric disease (EGGD) refers to disease of the glandular portion of the stomach, and equine squamous gastric disease (ESGD) refers to disease of the squamous portion of the stomach.1 Equine gastric ulcer syndrome (EGUS) refers to disease of any portion of the stomach1 and is the umbrella term used. In this review, we focus on disease prevalence, impact and management strategies of EGGD, which has been considered by some as an emerging disease in horses.
Diagnosis
Presently, there are no studies specifically describing clinical signs of EGGD. Therefore, clinical signs of EGGD are considered under the umbrella of EGUS and are variable and vague. Clinical signs include poor body condition or weight loss, poor performance, behavioral abnormalities (nervousness or aggression), inappetence or overt signs of mild to moderate intermittent colic.1 Unfortunately, due to the nonspecific clinical signs associated with EGUS, diagnosis based on clinical signs is unreliable. Blood sucrose and salivary cortisol response to adrenocorticotropin (ACTH) have been evaluated for their diagnostic usefulness in EGGD, but both tests were unreliable.2,3 Endoscopic examination enabling visualization of the lesions in the stomach is the only method to achieve a definitive diagnosis.
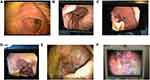
When performing endoscopy for diagnosis of EGGD, it is important to evaluate the visible glandular mucosa in the body of the stomach prior to proceeding to the pylorus, as occasionally the scope might abrade the mucosa during passage, which might appear to be a hemorrhagic glandular lesion (authors’ personal experience). During endoscopy, glandular lesions are scored based either quantitatively or qualitatively appearance using one or more of the following criteria: appearance, number, presumed depth, and distribution. Examples of EGGD lesions using available scoring systems are presented in Figure 1.
There are three available scoring systems. The most recent scoring system1 proposed using a qualitative or descriptive evaluation including lesion severity, appearance, and distribution (Table 1). This scoring system appears to be most useful for separating out the difference appearance of lesions using descriptive terms (nodular, fibrinosuppurative, hemorrhagic) that are not included in the other scoring systems. However, whether the differences in appearance can predict different pathologies remains to be determined. Another scoring system evaluates lesions based upon two separate scoring systems (0-4 number and 0-4 severity), which creates a more quantitative method but unfortunately does not include a method for scoring hyperemia and does not describe appearance.4 Finally, a simple 1–4 practitioner’s scale has been proposed,5 based upon the original Equine Gastric Ulcer Syndrome Council consensus6 and modified for EGGD which includes presence or absence of hyperemia and EGGD lesion number and severity scores, but does not include appearance description (Table 2).5 Other papers have used a simple 0–2 scoring system.7 Unfortunately, none of the reported scoring systems have been systematically evaluated, so their relationship to the histopathologic diagnosis of EGGD remains unknown. Furthermore, the use of variable scoring systems makes comparisons among studies challenging. Regardless of the scale used, present data suggest that hyperemia is a common finding8 and should be included separately during assessment. Until lesion histopathology is better characterized, a grading scheme whereby glandular hyperemia is considered either present or absent (regardless of appearance or severity of other lesions) might help provide more comprehensive assessment of EGGD.
 |
Table 1 ECEIM consensus qualitative scoring system |
 |
Table 2 EGUS council score |
Gastric biopsy is an ancillary tool that may, in some instances, be helpful in identification of type of cellular infiltrate in EGGD lesions. However, findings to date suggest that in general, gastric biopsy has limited diagnostic usefulness for histopathologic evaluation. In a study evaluating gastric biopsy sample depth obtained with two different commercially available biopsy forceps (1.8 and 2.4 mm) in horses (postmortem), submucosa was present in <70% of samples.9 Only 2/23 biopsies collected from healthy-appearing mucosa of live horses (using a 2.2 mm cupped biopsy forcep) contained submucosa (Banse, unpublished data).
Prevalence and risk factors
Prevalence of EGGD is variable and may in part depend upon breed and performance discipline, among other factors. In general, EGGD prevalence appears to be higher among sport horses and Warmblood show jumpers,8,10,11 compared to other breeds and disciplines.12–18 However, variability exists between studies and to date, only one study directly compares multiple breeds.10 One study in sport horses in the UK did not find any association between signalment and EGGD;11 however, another study in horses presenting to a hospital demonstrated that Warmbloods were at increased risk relative to other breeds.19 In Warmblood show jumpers in Canada, increased number of days spent exercising each week and lower performance level (national versus international) were at increased risk to develop EGGD.8 Another study in a small number of polo horses in Canada suggested that number of years playing polo was inversely associated with EGGD.20 In Thoroughbred racehorses in the UK and Australia, exercising 5–7 days per week increased risk of EGGD, when compared to exercising 1–4 days per week.18 Racing below expectations was also associated with an increased risk for EGGD,18 indicating that EGGD may be associated with decreased performance.
Pathophysiology
There remains limited understanding of the pathophysiology of equine glandular gastric disease. Factors that have been proposed to contribute to spontaneous EGGD include breakdown of mucosal defense, bacterial colonization, stress, and inflammation.
The glandular mucosa is constantly exposed to hydrochloric acid, and unlike the squamous epithelium, has a number of protective factors to prevent mucosal damage. Therefore, it has been proposed that breakdown of protective factors, rather than exposure to hydrochloric acid alone, may be a key factor in development of EGGD.1 Furthermore, recent data suggests that EGGD is poorly responsive to standard acid suppressive treatment (proton pump inhibitors; PPIs) compared to ESGD, suggesting acid injury alone is unlikely to be a primary cause of EGGD.21,22 Prostaglandins are considered a critical part of the mucosal barrier, contributing to mucous secretion, blood flow, bicarbonate secretion, and inhibition of acid secretion.23 However, in two studies using an experimental (nonsteroidal anti-inflammatory drug-induced) model of EGGD, glandular mucosal prostaglandins (PGE and/or PGI) did not decrease following treatment with 1–7 days of phenylbutazone.24,25 Furthermore, there was no association between NSAID use and glandular ulcers in racehorses in training.14 These findings suggest that a decrease in PGE may not be primarily responsible for development of EGGD. Another mechanism that appears to be key to mucosal defense is the hydrophobic surface of the stomach, which prevents back diffusion of hydrochloric acid.26,27 Disruption of this layer leads to increased back diffusion of hydrochloric acid and mucosal necrosis.26
The role of bacteria in formation or persistence in EGGD remains unclear. Helicobacter pylori is a common cause of gastritis in dogs and people. Helicobacter equorum, an equine-specific Helicobacter, has been isolated from feces of horses.28 However, this Helicobacter is urease negative.28 Formation of ammonia from breakdown of urea is critical to locally neutralizing stomach acid and allowing for Helicobacter colonization of the gastric mucosa (reviewed in29). Furthermore, in horses, Helicobacter spp have been inconsistently isolated from both normal and abnormal mucosa, with no consistent relationship to EGGD.30–32 Taken together, these findings suggest that Helicobacter spp. are unlikely to be a key contributor to EGGD. However, other alterations in the gastric bacterial population (ie, other than Helicobacter) have been demonstrated to contribute to gastritis in people.33 A study in a small number (N=10) of Thoroughbred racehorses with differing diets, drug histories, found associations between management factors and microbial communities,30 but not between EGGD severity and microbial communities. Additional studies in horses with EGGD that are under a more similar management scheme might help clarify these findings.
Recent data has suggested that increased stress or sensitivity to stress may contribute to development or persistence of EGGD. The presence of increased cortisol after exercise in both Warmbloods and Thoroughbreds suggests that exercise can be stressful.34,35 Furthermore, Warmblood horses had increased salivary cortisol concentration at competitions, when compared to the same concentrations in the home environment,36 suggesting competition in unfamiliar environments might be stressful for horses. Finally, horses with EGGD had an increased response to ACTH stimulation compared to horses without EGGD.37 The relationship between cortisol, stress, and formation of gastric lesions is complex. In rats, circulating cortisol was not associated with induction of stress ulcer, but stress ulcers were associated with a decrease in prostaglandins.38 In another experimental study in rats, hydrocortisone had a biphasic effect on prostaglandin levels, with low doses inhibiting and high doses stimulating prostaglandin production.39 Furthermore, exogenous steroids have been shown to reduce gastric ulcer healing in experimentally induced gastric ulcer model in rats due to decreased cellular proliferation at the ulcer edge.40 This was ameliorated by administration of misoprostol, suggesting that inhibition of prostaglandin synthesis may contribute to impaired healing.40 These findings suggest that stress may have a local effect on prostaglandin production that contributes to ulcer formation and/or healing.
Inflammation appears to be a common finding in the glandular mucosa.41 Preliminary data suggests that inflammation of the glandular mucosa is frequently lymphoplasmacytic9 (Banse, unpublished data). In humans and dogs with Helicobacter-negative gastritis, inflammation is similarly lymphocytic in nature, and is associated with inflammatory bowel disease, suggesting an immune-mediated component.42–44 The relationship between gastric and intestinal inflammation in horses remains to be established.
Management and treatment strategies
To date, management and treatment strategies have largely focused on what is known about risk factors for ESGD. However, this may not be appropriate, since pathophysiology and risk factors for the two diseases likely differ, as discussed above. The relationship between the presence or severity of ESGD and presence or severity of EGGD is inconsistent, suggesting that the two types of gastric disease may require different treatment or management strategies.1,11,18,20
Based upon identified risk factors, it seems that decreasing exercise duration or frequency may help decrease development of disease. Furthermore, minimizing stress may help decrease EGGD formation. However, evaluating what an individual horse finds stressful can be challenging. In the future, monitoring response to ACTH may allow for evaluation of the efficacy of different management changes in decreasing stress.
Across the three studies specifically evaluating management factors associated with EGGD, dietary factors were not retained in the final multivariable models, suggesting that dietary factors may be less important for control of EGGD when compared to ESGD. However, findings from the univariate models in these studies suggests that decreased pasture turnout or increased grain concentrate frequency may be associated with EGGD.8,20 Therefore, increasing pasture turnout and decreasing grain concentrates might be useful management strategies for preventing EGGD.
Pharmacological interventions for EGGD include acid suppressant therapies PPI, histamine type-2 antagonists (H2 antagonists), coating agents (sucralfate), and synthetic prostaglandins (misoprostol) (Table 3). PPIs irreversibility-bind to the H+/K+ ATPase (the proton pump) in the parietal cell, which is the terminal step in acid secretion. Because of their irreversible binding to the proton pump and their effects on the terminal step in acid secretion, they have a long duration of action. In contrast, H2 antagonists bind to histamine receptors on the parietal cell and decrease only histamine-mediated stimulation of the proton pump, without affecting other pathways (gastrin and acetylcholine acid) that stimulate acid secretion. Therefore, H2 antagonists are less effective at decreasing gastric acid secretion, when compared to PPIs (Figure 2).
 |
Table 3 Doses of medications commonly used to treat EGGD |
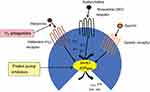 |
Figure 2 Primary pathways contributing to gastric acid secretion of the parietal cell, and site of action of H2 antagonists and PPIs. Abbreviation: PPIs, proton pump inhibitors. |
The most common PPI used in horses is omeprazole. Its efficacy for treatment of ESGD is well established and it is currently Federal Drug Administration (FDA)-approved for treatment and prevention of EGUS.45,46 However, it appears less effective for treatment of EGGD.21,22 This may in part be due to the low bioavailability of the oral formulation of omeprazole in horses, feeding practices and timing of dosing and feeding. Free choice hay decreases omeprazole absorption and duration of acid suppression.47,48 A compounded intramuscular formulation has recently been reported to result in improved EGGD healing rates;7 however, direct comparisons between this product and oral omeprazole have not been performed. Unless safety and treatment superiority of the compounded omeprazole can be demonstrated, the FDA-approved oral omeprazole formulations are preferred in the USA.
Esomeprazole is another PPI that has been shown to suppress acid production in horses, although studies evaluating efficacy in EGGD healing have not been reported.49,50 Ranitidine, an H2 antagonist, has been evaluated primarily in squamous disease and found to be less effective than omeprazole for healing of ESGD.51 Although not labeled for use in horses, intravenous formulations of esomeprazole, omeprazole, and ranitidine are available for cases in which oral administration is not feasible (ie, refluxing patients).49,52 However, caution should be used when implementing treatment in critically ill patients. In humans, proton pump inhibitors have been associated with increased risk for enteric infection.53 Similarly, proton pump inhibitors and H2-antagonists may increase risk of diarrhea in foals.54
Sucralfate is a sucrose sulfate-aluminum complex that binds to the ulcer bed, creating a physical barrier that protects the mucosa from acid and prevents the degradation of mucus. It increases viscosity of the mucus layer and increases hydrophobicity of the mucus layer along the gastric surface, limiting hydrogen ion back diffusion.55 Sucralfate binds to exposed subepithelium, allowing for healing.55–57 Furthermore, it stimulates mucus and bicarbonate secretion, and may stimulate PGE synthesis.55,56,58 Sucralfate is frequently used in conjunction with omeprazole for treatment of ESGD. Omeprazole plus sucralfate has been demonstrated to lead to healing in 63% of horses with EGGD Grade ≥2, and improvement of at least one grade in 83% of horses with EGGD Grade ≥2.59 When using multiple oral medications, medications should be administered at different times (generally at least 1 hour, for sucralfate, 2 hours) to decrease risk of impaired bioavailability.
Misoprostol is a synthetic prostaglandin E analogue (E-prostanoid receptor 2,3,4 agonist), which might enhance protective mechanisms of the glandular mucosa, including enhancing blood flow, increasing mucous and bicarbonate secretion, and decreasing acid production.23 The role of impaired prostaglandin synthesis in development of NSAID-induced or spontaneous EGGD remains to be established. As discussed above, to date there is no evidence that PGE or PGI levels are decreased following administration of phenylbutazone. However, a small study suggested that a prostaglandin E analogue was an effective preventative for NSAID-induced glandular disease.60 Furthermore, misoprostol has been shown to decrease lipopolysaccharide (LPS)-induced inflammatory cytokine production by equine leukocytes61 and inhibited equine neutrophil function.62 These findings suggest that misoprostol may help decrease glandular gastric inflammation. A small clinical trial demonstrated the misoprostol was superior to omeprazole plus sucralfate in healing or improving EGGD.63 The ideal treatment or treatment combination for healing of EGGD remains to be determined.
There is presently no evidence that changes in the gastric microbiome30 or secondary bacterial colonization play a key role in development or persistence of EGGD. One study evaluating 63 stomachs (36 with glandular lesions and 21 with hyperemic, erosive, or ulcerative lesions) found a solitary lesion with Enterococcus and Escherichia colonizations.32 In a study evaluating the use of trimethoprim-sulfadimidine for the treatment of EGGD, trimethoprim sulfadimidine combined with omeprazole was not superior to omeprazole in the healing of EGGD.64 Therefore, at present, antimicrobials are not recommended for treatment of EGGD. Further studies evaluating the role of gastric bacteria in development or persistence of EGGD may help to clarify the role of antimicrobials in treatment.
Sea buckthorn berry, a supplement, was found to prevent glandular lesions from forming in horses while stalled for 4 weeks and subjected to a 1-week intermittent feed deprivation model, suggesting it may be a useful adjunctive prevention strategy for EGGD.65 Although the exact mechanism by which sea buckthorn berry may be effective for EGGD is unknown, it has been speculated that high concentrations of antioxidants within the sea buckthorn berry may attenuate oxidative stress within the glandular mucosa, thus preventing EGGD.65
Conclusions
Equine glandular gastric disease remains a poorly understood disorder of the equine stomach. Present data suggests that management for prevention of EGGD should be directed at decreasing exercise and stress, and potentially limiting grain intake and increasing pasture turnout. Treatment recommendations include omeprazole with or without sucralfate or misoprostol. Further research into pathophysiology may allow for development of additional targeted, effective treatments.
Disclosure
Dr Banse has previously provided consultancy services for Boehringer Ingelheim Animal Health. Dr Andrews has previously performed research funded by Merial and Boehringer Ingelheim Animal Health. Dr Banse reports grants from Boehringer Ingelheim Animal Health, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Sykes B, Hewetson M, Hepburn R, Luthersson N, Tamzali Y. European College of Equine Internal Medicine Consensus Statement—equine gastric ulcer syndrome in adult horses. J Vet Intern Med. 2015;29(5):1288–1299. doi:10.1111/jvim.13578
2. Sauer FJ, Bruckmaier RM, Ramseyer A, Vidondo B, Scheidegger MD, Gerber V. Diagnostic accuracy of post-ACTH challenge salivary cortisol concentrations for identifying horses with equine glandular gastric disease. J Anim Sci. 2018;96(6):2154–2161. doi:10.1093/jas/sky074
3. Hewetson M, Sykes BW, Hallowell GD, Tulamo R-M. Diagnostic accuracy of blood sucrose as a screening test for equine gastric ulcer syndrome (EGUS) in adult horses. Acta Vet Scand. 2017;59(1):15. doi:10.1186/s13028-017-0347-3
4. MacAllister C, Andrews F, Deegan E, Ruoff W, Olovson SG. A scoring system for gastric ulcers in the horse. Equine Vet J. 1997;29(6):430–433.
5. Sykes B, Jokisalo J. Rethinking equine gastric ulcer syndrome: part 1–terminology, clinical signs and diagnosis. Equine Vet Educ. 2014;26(10):543–547. doi:10.1111/eve.2014.26.issue-10
6. Anon. Recommendations for the diagnosis and treatment of equine gastric ulcer syndrome (EGUS)‐The Equine Gastric Ulcer Council. Equine Vet Educ. 1999;11:262–272. doi:10.1111/j.2042-3292.1999.tb00961.x.
7. Sykes B, Kathawala K, Song Y, et al. Preliminary investigations into a novel, long‐acting, injectable, intra‐muscular formulation of omeprazole in the horse. Equine Vet J. 2017;14:795–801. doi:10.1111/evj.12688
8. Pedersen S, Cribb A, Windeyer M, Read E, French D, Banse H. Risk factors for equine glandular and squamous gastric disease in show jumping Warmbloods. Equine Vet J. 2018;50:747–751. doi:10.1111/evj.12949
9. Crumpton S, Baiker K, Hallowell G, Habershon‐Butcher J, Bowen I. Diagnostic value of gastric mucosal biopsies in horses with glandular disease. Equine Vet J. 2015;47(S48):9. doi:10.1111/evj.12486_18
10. Luthersson N, Nielsen K, Harris P, Parkin T. The prevalence and anatomical distribution of equine gastric ulceration syndrome (EGUS) in 201 horses in Denmark. Equine Vet J. 2009;41(7):619–624.
11. Hepburn R Endoscopic examination of the squamous and glandular gastric mucosa in sport and leisure horses: 684 horses (2005–2011).
12. Dionne RM, Vrins A, Doucet MY, Pare J. Gastric ulcers in standardbred racehorses: prevalence, lesion description, and risk factors. J Vet Intern Med. 2003;17(2):218–222.
13. Begg L, O‘Sullivan C. The prevalence and distribution of gastric ulceration in 345 racehorses. Aust Vet J. 2003;81(4):199–201.
14. Murray M, Schusser G, Pipers F, Gross SJ. Factors associated with gastric lesions in Thoroughbred racehorses. Equine Vet J. 1996;28(5):368–374.
15. Nieto JE, Snyder JR, Beldomenico P, Aleman M, Kerr JW, Spier SJ. Prevalence of gastric ulcers in endurance horses–a preliminary report. Vet J. 2004;167(1):33–37.
16. Tamzali Y, Marguet C, Priymenko N, Lyazrhi F. Prevalence of gastric ulcer syndrome in high‐level endurance horses. Equine Vet J. 2011;43(2):141–144. doi:10.1111/j.2042-3306.2010.00129.x
17. De Bruijn C, Schutrups A, Seesing E. Prevalence of equine gastric ulceration syndrome in standardbreds. Vet Record. 2009;164(26):814–815.
18. Sykes BW BM, Habershon-Butcher JL, Green M, Hallowell GD. Management factors and clinical implications of glandular and squamous gastric disease in horses. J Vet Intern Med. 2019;33(1):233–240.
19. Mönki J, Hewetson M, Virtala AM. Risk factors for equine gastric glandular disease: a case‐control study in a Finnish Referral Hospital Population. J Vet Intern Med. 2016;30(4):1270–1275. doi:10.1111/jvim.14370
20. Banse HE MH, Crosby C, Windeyer MC. Prevalence of and risk factors for equine glandular and squamous disease in polo horses. Can J Vet. 2018;59(8):880–884.
21. Sykes B, Sykes K, Hallowell G. A comparison of three doses of omeprazole in the treatment of equine gastric ulcer syndrome: A blinded, randomised, dose–response clinical trial. Equine Vet J. 2015;47(3):285–290. doi:10.1111/evj.12287
22. Sykes B, Sykes K, Hallowell G. A comparison of two doses of omeprazole in the treatment of equine gastric ulcer syndrome: a blinded, randomised, clinical trial. Equine Vet J. 2014;46(4):416–421. doi:10.1111/evj.12191
23. Wallace JL, Prostaglandins N. gastric mucosal protection: why doesn‘t the stomach digest itself? Physiol Rev. 2008;88(4):1547–1565. doi:10.1152/physrev.00004.2008
24. Pedersen S, Cribb A, Read E, French D, Banse H. Phenylbutazone induces equine glandular gastric disease without decreasing prostaglandin E2 concentrations. J Vet Pharmacol Ther. 2018;41(2):239–245. doi:10.1111/jvp.12464
25. Meschter C, Gilbert M, Krook L, Maylin G, Corradino R. The effects of phenylbutazone on the morphology and prostaglandin concentrations of the pyloric mucosa of the equine stomach. Vet Pathol. 1990;27(4):244–253. doi:10.1177/030098589002700405
26. Goddard PJ, Kao Y-CJ, Lichtenberger LM. Luminal surface hydrophobicity of canine gastric mucosa is dependent on a surface mucous gel. Gastroenterology. 1990;98(2):361–370.
27. Hills B, Butler B, Lichtenberger L. Gastric mucosal barrier: hydrophobic lining to the lumen of the stomach. Am J Physiol Gastrointest Liver Physiol. 1983;244(5):G561–G568. doi:10.1152/ajpgi.1983.244.5.G561
28. Moyaert H, Decostere A, Vandamme P, et al. Helicobacter equorum sp. nov., a urease-negative Helicobacter species isolated from horse faeces. Int J Syst Evol Microbiol. 2007;57(2):213–218. doi:10.1099/ijs.0.64279-0
29. Follmer C. Ureases as a target for the treatment of gastric and urinary infections. J Clin Pathol. 2010;63(5):424–430. doi:10.1136/jcp.2009.072595
30. Dong H-J, Ho H, Hwang H, et al. Diversity of the gastric microbiota in Thoroughbred racehorses having gastric ulcer. J Microbiol Biotechnol. 2016;26(4):763–774. doi:10.4014/jmb.1507.07054
31. Contreras M, Morales A, García‐Amado M, De Vera M, Bermúdez V, Gueneau P. Detection of Helicobacter‐like DNA in the gastric mucosa of Thoroughbred horses. Lett Appl Microbiol. 2007;45(5):553–557. doi:10.1111/j.1472-765X.2007.02227.x
32. Husted L, Jensen TK, Olsen SN, Mølbak L. Examination of equine glandular stomach lesions for bacteria, including Helicobacter spp by fluorescence in situ hybridisation. BMC Microbiol. 2010;10(1):84. doi:10.1186/1471-2180-10-84
33. Li -X-X, Wong GL-H, To K-F, et al. Bacterial microbiota profiling in gastritis without Helicobacter pylori infection or non-steroidal anti-inflammatory drug use. PLoS One. 2009;4(11):e7985. doi:10.1371/journal.pone.0007985
34. Freestone J, Wolfsheimer K, Kamerling S, Church G, Hamra J, Bagwell C. Exercise induced hormonal and metabolic changes in Thoroughbred horses: effects of conditioning and acepromazine. Equine Vet J. 1991;23(3):219–223.
35. Cayado P, Muñoz‐Escassi B, Dominguez C, et al. Hormone response to training and competition in athletic horses. Equine Vet J. 2006;38(S36):274–278. doi:10.1111/j.2042-3306.2006.tb05552.x
36. Munk R, Jensen R, Palme R, Munksgaard L, Christensen JW. An exploratory study of competition scores and salivary cortisol concentrations in Warmblood horses. Domest Anim Endocrinol. 2017;61:108–116. doi:10.1016/j.domaniend.2017.06.007
37. Scheidegger MD, Gerber V, Bruckmaier R, van der Kolk JH, Burger D, Ramseyer A. Increased adrenocortical response to adrenocorticotropic hormone (ACTH) in sport horses with equine glandular gastric disease (EGGD). Vet J. 2017;228:7–12. doi:10.1016/j.tvjl.2017.09.002
38. Gitlin N, Ginn P, Kobayashi K, Arakawa T. The relationship between plasma cortisol and gastric mucosa prostaglandin levels in rats with stress ulcers. Aliment Pharmacol Ther. 1988;2(3):213–220. doi:10.1111/j.1365-2036.1988.tb00690.x
39. Avunduk C, Eastwood G, Polakowski N, Burstein S. Hydrocortisone has a biphasic effect on rat gastric mucosal prostaglandin generation in vivo: inhibition at low doses, stimulation at high doses. Prostaglandins Leukot Essent Fatty Acids. 1992;45(4):329–332. doi:10.1016/0952-3278(92)90091-V
40. de Kaski MC, Rentsch R, Levi S, Hodgson H. Corticosteroids reduce regenerative repair of epithelium in experimental gastric ulcers. Gut. 1995;37(5):613–616.
41. Martineau H, Thompson H, Taylor D. Pathology of gastritis and gastric ulceration in the horse. Part 2: a scoring system. Equine Vet J. 2009;41(7):646–651.
42. Sonnenberg A, Melton SD, Genta RM. Frequent occurrence of gastritis and duodenitis in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2011;17(1):39–44. doi:10.1002/ibd.21356
43. Sonnenberg A, Genta R. Low prevalence of Helicobacter pylori infection among patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2012;35(4):469–476. doi:10.1111/j.1365-2036.2011.04969.x
44. Wigs G, Washabau R, Day M, et al. Endoscopic, biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. J Vet Intern Med. 2010;24(1):10–26.
45. Andrews F, Sifferman R, Bernard W, et al. Efficacy of omeprazole paste in the treatment and prevention of gastric ulcers in horses. Equine Vet J. 1999;31(S29):81–86. doi:10.1111/j.2042-3306.1999.tb05176.x
46. MacAllister C, Sifferman R, McClure S, et al. Effects of omeprazole paste on healing of spontaneous gastric ulcers in horses and foals: a field trial. Equine Vet J. 1999;31(S29):77–80. doi:10.1111/j.2042-3306.1999.tb05175.x
47. Daurio C, Holste J, Andrews F, et al. Effect of omeprazole paste on gastric acid secretion in horses. Equine Vet J. 1999;31(S29):59–62. doi:10.1111/j.2042-3306.1999.tb05171.x
48. Sykes B, Underwood C, Greer R, McGowan C, Mills P. The effects of dose and diet on the pharmacodynamics of omeprazole in the horse. Equine Vet J. 2017;49(4):525–531. doi:10.1111/evj.12630
49. Videla R, Sommardahl C, Elliott S, Vasili A, Andrews F. Effects of intravenously administered esomeprazole sodium on gastric juice pH in adult female horses. J Vet Intern Med. 2011;25(3):558–562. doi:10.1111/j.1939-1676.2011.0716.x
50. Huxford K, Dart A, Perkins N, Bell R, Jeffcott L. A pilot study comparing the effect of orally administered esomeprazole and omeprazole on gastric fluid pH in horses. N Z Vet J. 2017;65(6):318–321. doi:10.1080/00480169.2017.1359125
51. Lester GD, Smith RL, Robertson ID. Effects of treatment with omeprazole or ranitidine on gastric squamous ulceration in racing Thoroughbreds. J Am Vet Med Assoc. 2005;227(10):1636–1639.
52. Andrews FM, Sommardahl CS, Frank N, Buchanan BR, Elliott SB. Effect of intravenously administered omeprazole on gastric juice pH in adult horses. J Vet Intern Med; 2006; 20(5):1202–1206.
53. Bavishi C, Dupont H. Systematic review: the use of proton pump inhibitors and increased susceptibility to enteric infection. Aliment Pharmacol Ther. 2011;34(11‐12):1269–1281. doi:10.1111/j.1365-2036.2011.04874.x
54. Furr M, Cohen N, Axon J, et al. Treatment with histamine‐type 2 receptor antagonists and omeprazole increase the risk of diarrhoea in neonatal foals treated in intensive care units. Equine Vet J. 2012;44(s41):80–86. doi:10.1111/j.2042-3306.2011.00499.x
55. Rees W. Mechanisms of gastroduodenal protection by sucralfate. Am J Med. 1991;91(2):S58–S63. doi:10.1016/0002-9343(91)90452-4
56. Szabo S, Hollander D. Pathways of gastrointestinal protection and repair: mechanisms of action of sucralfate. Am J Med. 1989;86(6):23–31.
57. Kaya N, Boyunapa H, Baris S, Kahraman H, Altintop L. Protective effects of sucralfate and omeprazole on gastric mucosal damage induced by ethanol in rats. Wien Klin Wochenschr. 1998;110(3):96–100.
58. Shorrock CJ, Rees WD. Effect of sucralfate on human gastric bicarbonate secretion and local prostaglandin E2 metabolism. Am J Med. 1989;86(6):2–4.
59. Hepburn R, Proudman C Treatment of ulceration of the gastric glandular mucosa: retrospective evaluation of omeprazole and sucralfate combination therapy in 204 sport and leisure horses.
60. Collins L, Tyler D. Experimentally induced phenylbutazone toxicosis in ponies: description of the syndrome and its prevention with synthetic prostaglandin E2. Am J Vet Res. 1985;46(8):1605–1615.
61. Martin EM, Messenger KM, Sheats MK, Jones SL. Misoprostol inhibits lipopolysaccharide-induced pro-inflammatory cytokine production by equine leukocytes. Front Vet Sci. 2017;4:160. doi:10.3389/fvets.2017.00127
62. Martin EM, Till RL, Sheats MK, Jones SL. Misoprostol inhibits equine neutrophil adhesion, Migration, and respiratory burst in an in vitro model of inflammation. Front Vet Sci. 2017;4:159. doi:10.3389/fvets.2017.00127
63. Varley G, Bowen I, Habershon‐Butcher J, Nicholls V, Hallowell G. Misoprostol is superior to combined omeprazole‐sucralfate for the treatment of equine gastric glandular disease. Equine Vet J. 2019. doi:10.1111/evj.13087
64. Sykes BW, Sykes KM, Hallowell GD. Administration of trimethoprim-sulphadimidine does not improve healing of glandular gastric ulceration in horses receiving omeprazole: a randomised, blinded, clinical study. BMC Vet Res. 2014;10(1):180. doi:10.1186/1746-6148-10-1
65. Huff N, Auer A, Garza Jr F, et al. Effect of sea buckthorn berries and pulp in a liquid emulsion on gastric ulcer scores and gastric juice pH in horses. J Vet Intern Med. 2012;26(5):1186–1191. doi:10.1111/j.1939-1676.2012.00975.x
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.