Back to Journals » Research and Reports in Tropical Medicine » Volume 10
Epilepsy diagnosis and management of children in Kenya: review of current literature
Authors Samia P, Hassell J, Hudson JA , Murithi MK, Kariuki SM , Newton CR, Wilmshurst JM
Received 11 January 2019
Accepted for publication 12 April 2019
Published 28 June 2019 Volume 2019:10 Pages 91—102
DOI https://doi.org/10.2147/RRTM.S201159
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Mario Rodriguez-Perez
Pauline Samia,1 Jane Hassell,2 Jessica-Anne Hudson,3 Maureen Kanana Murithi,1 Symon M Kariuki,4 Charles R Newton,4 Jo M Wilmshurst5
1Department of Paediatrics and Child Health, Aga Khan University, Nairobi, Kenya; 2Gertrude’s Children’s Hospital, Child development Centre, Nairobi, Kenya; 3Oxford School of Paediatrics, Department of Child Health, UK; 4Kemri–Wellcome Trust Collaborative Programme, Centre for Geographic Medicine Research Programme, Kilifi, Kenya; 5Division of Paediatric Neurology, Department of Paediatrics and Child Health, Red Cross War Memorial Children’s Hospital, Neuroscience Institute, University of Cape Town, Rondebosch, South Africa
Introduction: The growing impact of non-communicable diseases in low- to middle-income countries makes epilepsy a key research priority. We evaluated peer-reviewed published literature on childhood epilepsy specific to Kenya to identify knowledge gaps and inform future priorities.
Methodology: A literature search utilizing the terms “epilepsy” OR “seizure” as exploded subject headings AND “Kenya” was conducted. Relevant databases were searched, generating 908 articles. After initial screening to remove duplications, irrelevant articles, and publications older than 15 years, 154 papers remained for full-article review, which identified 35 publications containing relevant information. Data were extracted from these reports on epidemiology, etiology, clinical features, management, and outcomes.
Results: The estimated prevalence of lifetime epilepsy in children was 21–41 per 1,000, while the incidence of active convulsive epilepsy was 39–187 cases per 100,000 children per year. The incidence of acute seizures was 312–879 per 100,000 children per year and neonatal seizures 3,950 per 100,000 live births per year. Common risk factors for both epilepsy and acute seizures included adverse perinatal events, meningitis, malaria, febrile seizures, and family history of epilepsy. Electroencephalography abnormalities were documented in 20%–41% and neurocognitive comorbidities in more than half. Mortality in children admitted with acute seizures was 3%–6%, and neurological sequelae were identified in 31% following convulsive status epilepticus. Only 7%–29% children with epilepsy were on antiseizure medication.
Conclusion: Active convulsive epilepsy is a common condition among Kenyan children, remains largely untreated, and leads to extremely poor outcomes. The high proportion of epilepsy attributable to preventable causes, in particular neonatal morbidity, contributes significantly to the lifetime burden of the condition. This review reaffirms the ongoing need for better public awareness of epilepsy as a treatable disease and for national-level action that targets both prevention and management.
Keywords: epilepsy, children, Kenya, epidemiology, management, outcomes
Introduction
Epilepsy is the most common neurological disorder worldwide, contributing to 1% of the global disease burden.1,2 Low- and middle-income countries (LMICs), especially sub-Saharan Africa,1,2 bear the highest burden of epilepsy, with children3 being the most affected age-group. The median prevalence of active epilepsy in high-income countries (HICs) was found to be at 4.9 per 1,000 compared to 12.7 per 1,000 in rural areas of resource-limited countries.3–7 The high burden of epilepsy is attributed both to high case load associated with increased prevalence of risk factors. such as preventable brain injuries, and to poor access to health-care services due to multiple barriers, including financial cost. The chronic nature of epilepsy has wide-ranging socioeconomic impacts, including high health-resource utilization, reduced participation in education and work due to seizures, comorbidities, and stigma,behavioral problems, and psychological morbidity,all of which may contribute to overall poorer quality of life,1,8–10 in addition to increased mortality rates.1
Insufficient epilepsy diagnosis and a large treatment gap coupled with lack of appropriate infrastructure in hospitals in Kenya makes it challenging to understand the prevalence and factors associated with this disease in the country.1–8 This report presents findings from a review describing the current literature on epidemiology, diagnosis, management, and outcomes of acute seizures and epilepsy in children in Kenya, aiming to inform future priorities for research, training, and clinical service provision. We focus specifically on Kenya as the country of practice (PS, JH, MK, SMK, CRN) and collaboration (JMW, JAH) of all the authors and the site of the majority of epilepsy research for the East African community. Kenya is a lower MIC, whereas the other fivecountries in the community (Burundi, Rwanda, South Sudan, Tanzania, and Uganda) are classified as low-income. In addition to possible differences in health-care access and provision amongcountries related to income level, there will be other country-specific contextual factors that may affect the epilepsy burden in each country. We discuss factors pertinent to Kenya in this review.
Methods
In order to understand the pathways to epilepsy diagnosis and management of children in Kenya we sought to answer these questions: What is the burden of childhood epilepsy in Kenya?; What is known about the causes, presenting clinical features, management, and outcomes of these children?
A literature search was undertaken using the terms “eilepsy” OR “seizure” as exploded subject headings AND “Kenya”. No date or language restriction was used. Medline, Embase, Global Health, and CAB abstract databases and the World Health Organization Library database were searched, generating a total of 908 articles.
Titles and abstracts of all articles were screened to remove any duplicates or obviously irrelevant papers. Those published more than 15 years prior to the search were excluded. A total of 154 papers remained, for which the full article was accessed and reviewed by two authors independently (JH and JAH). Inclusion criteria were papers published 2004–2018, papers presenting data specific to Kenya, and papers presenting pediatric-specific data. Excluded were case reports, animal and in vitro studies, studies specifically addressing seizures in malaria rather than all-cause acute seizures, studies from countries other than Kenya, and studies that did not present specific pediatric data.
This review specifically focuses on pediatric epilepsy. Of note, 57 papers regarding epilepsy in adults were excluded during screening and a further nine excluded on full-text review for lack of subanalysis of the pediatric population.
Included papers were categorized according to the major theme or themes that they addressed: epidemiology, etiology, clinical features including diagnosis, management, and outcomes. Information extracted from included studies was location (region and health care–facility level), study design, number of participants, and the main findings.
Papers that followed the International League Against Epilepsy definition of epilepsy as two unprovoked seizures occurring at least 24 hours apart were considered. Papers reporting both interictal and ictal electroencephalography (EEG) findings among those with epilepsy were considered for this analysis, and also if they reported the frequency of abnormalities, such as abnormal background, focal changes, epileptiform discharges, and abnormal response to activation procedures on EEG recordings. Medical comorbidities were considered if the studies explicitly described the methods of diagnosis, such as detailed clinical examination, review of medical records, or standardized neuropsychological assessments.
Scope of published literature on childhood epilepsy
We identified a total of 35 papers that fulfilled inclusion criteria (Figure 1). Of these, 33 were from one high-output group from the Kenya Medical Research Institute–Wellcome Trust research programme based in the rural coastal county of Kilifi, a malaria-endemic area with an ethnic makeup of predominantly Bantu peoples. Most studies were based either on screening data from large population surveys or retrospective observational cohorts of children admitted to Kilifi district hospitals (Table 1). Epilepsy was the focus of 26 papers, and acute seizures or convulsive status epilepticus the focus of the remaining nine(Table 2). The most common themes were epidemiology and etiology, followed by clinical presentation, including comorbidities, management, and outcomes (Table 3).
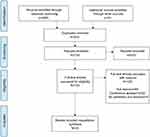 |
Figure 1 PRISMA flow diagram. |
 |
Table 1 Methodology |
 |
Table 2 Scope of published papers |
 |
Table 3 Summary of results by topic |
Results
Epidemiology
A total of 17 papers addressed prevalence or incidence of acute seizures or epilepsy (Table 2). Observed data ranges are summarized in Table 3. Twelve of the studies were cross-sectional surveys with up to 232,176 participants that identified children with epilepsy using the Ten Questions Questionnaire11 administered by field workers.
A common theme in epidemiological studies of childhood epilepsy was the exclusion of children aged <6 years, because febrile seizures are common in this age-group and would confound estimates. In studies presenting subanalyses by age-group, as expected, prevalence of epilepsy increased with age, while incidence decreased with age and the highest incidence was in the 0- to 5-year age-group.1,2
Published estimates of lifetime epilepsy prevalence in Kilifi ranged from 20.9 of 1,000 (2017, n=11,233, children aged 6–9 years)12 to 41 of 1,000 (2008, n=10,218, children aged 6–12 years).13 Prevalence of active convulsive epilepsy was estimated in Kilifi to be 2 of 1,000 children aged 0–5 years3 to 11.5 of 1,000 children aged 6–9 years.12 Estimates for incidence of active convulsive epilepsy in Kilifi ranged from 39.6 of 100,000/year (age 13–17 years)2 to 187 of 100,000/year (aged 6–12 years),11 while incidence of acute seizures was estimated at 312 of 100,000/year (<13 years)13 to 879 of 100,000/year (<5 years)14 and incidence of neonatal seizure at 443–3,950 of 100,000 live births.5,15
Etiology of epilepsy in Kenyan children
Sixteen papers addressed etiology, including seven cross-sectional surveys exploring risk factors for epilepsy or acute seizures and seven case–control studies focusing on specific associations.
The commonest association with active convulsive epilepsy was history of perinatal and/or neonatal complications, with ORs 2.88–5.70.5,7,12 Personal and family history of acute symptomatic febrile or afebrile seizures were also common predisposing factors for epilepsy.7,13 These findings were also consistent with those of a multicountry study of five sub-Saharan African sites, including Kilifi.1
Parasitic infections were also associated with epilepsy. Falciparum malaria infection is not only a risk for acute seizures but also for later development of epilepsy,15,16 with increased prevalence of epilepsy in children previously admitted with cerebral malaria (9.2%, OR, 4.4, 95% CI 1.4–13.7) or malaria complicated with seizures (11.5%, OR, 6.1, 95% CI 2.0–18.3) in malaria-endemic Kilifi.17 Other parasitic infections are implicated in the pathogenesis of epilepsy, and studies from African sites outside of Kenya that included adult patients have identified associations including Taenia solium, which causes neurocysticercosis,10 toxoplasmosis,5 onchorcerciasis,18 tuberculosis19 and T. canis.1 Other identified uninfectious predisposing conditions for childhood epilepsy include traumatic head injuries, sickle-cell disease, stroke, and acute encephalopathy.8–10,16
Risk factors for acute symptomatic seizures among children admitted to hospital were similar to those for active epilepsy identified in community surveys. Infections were common. Studies from Kilifi identified falciparum malaria as the primary diagnosis in up to 65.4% children with acute symptomatic seizures,5,6,15–21 while respiratory tract infections were the primary diagnosis in up to 27.8%5,6,22 and any infection identified in 80% of children with acute symptomatic seizures.5,6,15 Five percent of children admitted to hospital with acute symptomatic seizures hadepilepsy,14 a significant association (HR 1.53, 95% CI 1.10–2.14). The high burden of neonatal seizures was attributed to neonatal sepsis in 51%–61% cases, specifically to meningitis in 15% and to neonatal encephalopathy in 21%.14,15,20
Two Kilifi-based case–control studies examined specific genetic or biochemical risk factors for acute seizures in children admitted to hospital. There was no difference between the number of cases and controls with the HP2-2 haptoglobin genotype or with α-globin gene deletion,15 whilea meta-analysis of case–control studies identified a small increased risk of afebrile seizures in children with iron deficiency. We did not identify any other published genetic studies of people with epilepsy in Kenya.
Epilepsy characterization and comorbidities
A total of 13 papers containing data on clinical features were identified in this review, including seven cohort studies describing neurophysiology and five describing comorbidities.
Seizure semiology, electroencephalography, and imaging
In children with epilepsy, generalized tonic–clonic seizures were reported in 33.6%–70.4% and focal-onset seizures in 13.1%–78.6%.5,12,15,22 On EEG, any abnormality was reported in 20%–39% children with epilepsy5,12,14,23 and focal abnormalities in up to 61.6% of abnormal EEGs in 5- to 9-year-olds.23 In a retrospective review of 6,639 pediatric EEGs performed for suspected epilepsy and reviewed at Kenyatta National Tertiary Referral Hospital, 3 Hz spike-wave activity, a feature of childhood absence epilepsy, was seen in 7.4% (163 of 2,216) of abnormal EEGs and hypsarrhythmia, seen with infantile spasms, in 2.8% (62 of 2,216) of abnormal EEGs.23 One Kilifi study6 assessed children presenting with acute seizures and found complex (focal, prolonged, or recurrent) seizures in 84%, generalized seizures in 85%, and EEG abnormalities in 27%. Another reported convulsive status epilepticus in 35.4% children with acute seizures.13
We identified one small study of neuroimaging (brain computerized tomography) that included eleven children with focal seizures with loss of awareness; however, no abnormalities were found in this subgroup of children, possibly due to the limited sensitivity of this modality for detection of small lesions.24 There were no published studies of more advanced neuroimaging, such as magnetic resonance imaging, in this data set.
Comorbidities
A study done in rural Kenya by Kariuki et al7 showed that children with epilepsy had a greater proportion of behavioral and emotional problems (49%) thancontrols (26%).25–27 Children with active epilepsy had higher scores compared to those with inactive epilepsy. Specific behavioral and emotional problems were inability to maintain social relationships and concentration. Similar findings were observed by Kind et al,12 who found that 54% of those with lifetime epilepsy had neurobehavioral comorbidities. These included attention deficit hyperactivity disorder, autism-spectrum disorder, and cognitive impairments (OR 14.55, 36.83, and 14.55, respectively). Other behavioral problems documented in children with epilepsy included aggression, temper tantrums, and excessive crying.25–27
Epilepsy management
We identified ten papers in total. Six studies8,12,21,22,28,29 assessed whether children were on medical treatment for epilepsy, although we only able to extract pediatric-specific data from three of these.12,21,22 The other four were qualitative observational studies of caregiver attitudes towards epilepsy and the impact of these attitudes on treatment-seeking behaviors.
Drug management of epilepsy
In Kilifi-based studies, the maximum identified proportion of children with active convulsive epilepsy on antiseizure medication (ASM) detected in blood was 28.6%, of which two-thirds oflevels were within the optimal therapeutic range,30 while self-reported antiepileptic-drug use was as low as 6.9% in children (all age-groups) in a study by Kariuki et al21. No studies addressed specific ASM regimes.
Nonbiomedical beliefs and practices influencing access and care
Caregivers and parents of children with epilepsy in Kilifi categorized those with less frequent seizures as “healthy”, requiring no urgent medical attention, while those with frequent seizures were considered “sickly”, with treatment believed to be futile.31 In some communities, epilepsy is perceived to be contagious and/or associated with witchcraft.32 Traditional healers in Kilifi associated epilepsy with natural spirits known as nyagu and ancestral or evil spirits (majini).32 They described seizures occurring when spirits descend upon a child, and interpreted ictal movements as the child trying to break free from the hold of the spirits, whilevocalization prior to a seizure was interpreted as surprise at the sudden appearance of spirits.32
In Kilifi, people with epilepsy frequently seek treatment from traditional healers who may offer explanations and treatment that are concordant with perceptions held by the community.31 Traditional healers were described as having a more accessible communication style, allowed more time for consultation than biomedical practitioners, and were found to be popular, reliable, and trustworthy. Flexible payment systems, with options to pay in installments, barter trade, or pay at a later date, also contributed to the popularity of traditional healers.32,33
A single study examined the effect of biomedical health education on beliefs about epilepsy and on treatment adherence. The authors found minimal change in medication adherence following a single educational encounter; however, the intervention group had significantly fewer beliefs regarding traditional causes of epilepsy, cultural treatment, and negative stereotypes than the nonintervention group.5
Outcomes of epilepsy
Ten papers presented data on outcomes, of which five were district hospital–based cohorts of children with acute seizures, one was a hospital cohort of epilepsy patients, and four were cross-sectional surveys of children with epilepsy. All studies reported increased risk of death and neurological sequelae in both children admitted with acute seizures and children with epilepsy.
Ibinda et al2 modeled outcomes based on cross-sectional survey epidemiological data from Kilifi, and calculated that the standardized mortality ratio for children with active convulsive epilepsy ranged from 1.7 (95% uncertainty interval 1.6–1.7) in females aged 0–5 years to 16.95 (95% uncertainty interval 16.4–17.9) in males aged 6–12 years. In their prospective cross-sectional study Ngugi et al34 reported 33.3 deaths per 1,000 persons with active convulsive epilepsy per year over the study period, six times higher than the general population, while on pediatric sub-analysis, adjusted mortality ratio was up to 1.53 (95% CI 0.67–3.54) in 13- to 18-year-olds and 96.1% of deaths in this age-group were directly attributed to their epilepsy. Children with epilepsy also had a higher burden of disability-adjusted life years, with estimates ranging from 617–900 of 100,000 years2,30 and an increased disability-adjusted life-year burden in those with secondary epilepsy and in children not on ASM.2 Nonadherence to ASM and prolonged seizures were closely associated with epilepsy mortality.35,36
Childhood epilepsy was associated with increased hospital-admission rates. In a Kilifi study, the incidence of hospital admissions due to epilepsy was 45.6 per 100,000 person‐years of observation.13 The commonest causes of admission13,15 among children with epilepsy were convulsive status epilepticus (38%–41%), postictal coma, and epilepsy-related injuries, and convulsive status epilepticus was the commonest cause of in-hospital death (5.4%).15
Mortality in children admitted to hospital with acute seizures in the malaria-endemic area of Kilifi was reported in 3.1%–6%,5,15 with higher mortality found in those with medically refractory (8%)5 or neonatal seizures (10%).5 Adverse neurological outcomes were reported in 1.3% of children admitted with seizures,22 while 13% of neonates with seizures had neurological abnormalities at discharge.22 In a 3- to 4-year postadmission (to Kilifi District Hospital) follow-up study of patients with convulsive status epilepticus, 30.9% of cases vs 3.9% of matched controls (OR 11, 95% CI 5.3–22.8) had neurological impairment.35 Identified risk factors for death in children with acute seizures included coma, acidosis, hypoglycemia, bacteremia, bacterial meningitis, and focal-onset seizures. Adjusted risks for neurological sequelae were coma, status epilepticus, hypoxic ischemic encephalopathy, severe wasting, hypoglycemia, and age <12 months.15,37
Discussion
Epilepsy is a public health concern and a growing focus of research in Kenya. The emerging impact of incommunicable diseases in LMICs makes research in neurological disorders, especially epilepsy, an urgent priority. The existing population studies on childhood epilepsy in Kenya are restricted to the malaria-endemic district of Kilifi, which limits effective understanding of epidemiology of the condition on a national scale.
Burden, risk factors, and causes of epilepsy in children
Since acute-seizure data are inferred from hospital admissions, they may underestimate true incidence in the community. The burden of neonatal seizures was noted to be particularly high, and this is likely a significant underestimate, given that more than half of neonatal seizures are subclinical and can be detected only with ictal neurophysiology recording, and a significant number of births in that region still occur at home. These population data may also underestimate the true burden of childhood epilepsy in this country for various reasons, including stigma and differences in documentation of the condition arisingfrom differences in case definition.18,36
We did not identify any particular temporal trends in the prevalence or incidence of epilepsy; however, Kariuki et al30 observed a decrease in incidence of acute symptomatic seizures from 2002 to 2008, with 93.1% of this change attributed to treatment of malaria, reflecting similar findings from other studies.10,15
Kenya has both malaria-endemic and nonendemic regions. However, we did not identify any epidemiological data on epilepsy or seizure from nonendemic areas, where the frequency of these conditions may be significantly different. This limited understanding of the epidemiology of childhood seizure disorders across Kenya limits the ability to inform a national agenda for epilepsy prevention and management. Further multisite research is needed to better understand the causes of childhood seizure disorders nationwide.
Features and comorbidities of childhood epilepsy
The ethnic makeup of the Kilifi population is predominantly Bantu subgroups, with the highest proportion being Mijikenda, while representation of other prominent Kenyan ethnicities, such as the Nilotic peoples, is low. Therefore, with regard to genetic etiologies of epilepsy, Kilifi may not be representative of the wider population of Kenya. There have been no published studies from Kenya investigating the known genetic associations with seizures or epilepsy that are reported in high-income settings.
As with incidence and prevalence estimates, we found no published studies of seizure-disorder etiology from malaria-nonendemic regions of Kenya. We anticipate that the burden of perinatal complications, the commonest risk factor for childhood epilepsy, to be high nationwide, although this may vary, depending on the quality of available maternal and newborn health–care services.
The high burden of focal-onset seizures and focal EEG abnormalities implies that a significant number of Kenyan children with seizures/epilepsy may have lesional causes due to potentially preventable or curable conditions, including infections and acquired brain injuries. At present, videotelemetry is not available in Kenya, and thus ictal EEG recordings are not collected, which limits delineation of focus and semiology. International epilepsy guidance recommends neuroimaging for children with focal-onset seizures. In our experience, adherence to this guidance is variable, due to a combination of cost, access to imaging facilities, and physician knowledge.
Treatment gap and management of epilepsy in children
Data on childhood-epilepsy management in Kenya is limited. Culturally driven health-seeking behaviors contribute to a low rate of epilepsy diagnosis, as well as poor access to treatment, and are thought to be key contributors to the treatment gap.28–34 The treatment gap, which describes the percentage of people with active epilepsy with no access to adequate medical treatment, is very large in Kenya.28–33 Children have the highest incidence of epilepsy, and thus are disproportionately affected, adversely impacting their education, psychosocial development, and quality of life in a critical period of development.29 Barriers to children with epilepsy receiving regular seizure prophylaxis include underdiagnosis, lack of access to appropriate health-care facilities and medications, and local nonbiomedical belief systems and practices.33 These beliefs contribute to social exclusion of people with epilepsy by their communities. Fear of stigma is likely to discourage those affected from seeking medical attention.
Access to health care for children with epilepsy
Kenyan children with epilepsy face barriers to access in every aspect of clinical care. Lack of recognition of epilepsy by people with epilepsy, their caregivers, and health-care professionals, due in part to low awareness of the condition, have contributed to lack of treatment-seeking and consequently significant underdiagnosis and underreporting of epilepsy. Whereas no peer-reviewed studies have addressed access to health care, it has been observed that facilities for the diagnosis and management of childhood epilepsy in Kenya are severely limited. There is a marked paucity of relevant specialist skilled human resources in Kenya: only five Kenyan pediatricians have received any neurology training, and there are no pediatric-subspecialty neurosurgeons, epilepsy neurosurgeons, pediatric epileptologists or pediatric neuroradiologists.
The majority of people with epilepsy in Kenya present to dispensaries and other peripheral facilities, and are initially reviewed by clinical offices, nurses. or medical officers at presentation. Depending on the individual health-care worker’s clinical acumen and training, the diagnosis of epilepsy may be missed, especially with such presentations as childhood-absence epilepsy. Training for first-line health workers caring for children with epilepsy is limited in part due to the lack of specialists with the experience and qualifications to provide such training. Lack of training contributes to poor communication between health care workers and patients with epilepsy on the causes, diagnosis, management, and prognosis of the disorder, further widening the treatment gap.38
Health facilities in many areas lack specialized hospital equipment crucial for investigation of epilepsy, such as magnetic resonance–imaging scanners and EEG equipment. Public health-care facilities for children with epilepsy in Kenya are particularly limited, and national investment in such infrastructure and efforts to integrate epilepsy care into the existing primary health-care system is inadequate.39,40
Better-staffed and equipped hospitals are found in the larger cities, forcing the majority of those affected to travel long distances, which is costly in terms of both transport and time away from work, in addition to the direct costs of health care.34,35 Kenyans employed within the formal sector or who opt to make independent contributions are able to access the National Health Insurance Fund, which subsidizes care; however, <20% of Kenyans have such cover. The vast majority of low-income families in Kenya must make direct out-of-pocket payments for specialist care. Many families are simply unable to afford such payments, so children go untreated and are at increased risk of disability and death from epilepsy-related complications.41
An additional barrier to adequate care for children with epilepsy is access to ASMs, especially within in primary health-care facilities.39,42,43 Phenobarbital, a medication with known efficacy and low cost, is listed as a controlled drug in LMICs,40 preventing easy access. Poor quality control of ASMs has also resulted in infiltration of fake drugs into the informal lower-cost markets that are accessed by many patients: phenobarbital and phenytoin are among the drugs popular in the illegal market in Kenya.44
The multifaceted barriers to health-care access are compounded by low prioritization of epilepsy within national and global health agendas. Incommunicable diseases, including epilepsy, have not been given importance as a major public health concern. Moreover, since children with epilepsy are frequently treated within the mental-health sector, it is important to note that national mental-health policies are outdated and there exists no legislation advocating for the rights of people with mental-health issues in Kenya.38 Currently, there are no local peer-reviewed studies that address access to health care.
Recent innovations in delivery of epilepsy care in Kenya
The Ministry of Health, in collaboration with the National Epilepsy Coordination Committee, has in recent years produced guidelines for diagnosis and treatment algorithms, including types of medication, dosage, and frequency of administration and referral guidelines, indicated for each level of care from primary health-care facilities to referral hospitals.45 However, awareness and implementation of these guidelines is yet to be reviewed.
Such organizations as the Kenya Association for the Welfare of People with Epilepsy and Foundation for People with Epilepsy have assisted in creating awareness, initiation of community epilepsy-treatment programs, and facilitating support groups for people with epilepsy. Community-focused collaborative epilepsy projects involving the government, researchers, the National Epilepsy Coordination Committee, and international medical teams have also been initiated in the country. These provide a viable approach in case finding, using key informants to facilitate diagnosis.44–48 On December 1, 2018, the Government of Kenya launched a World Health Organization-supported pilot program of universal health coverage in four counties.47 The stated aims of the program include comprehensive, free-at-point-of-access coverage of all medical conditions, including chronic care.
Task-sharing is increasingly recognized as a mechanism to reduce the burden on medical specialists and overcome workforce shortages while improving the care of people with chronic conditions. A study in the Kibera informal settlement in Nairobi found that nurses were able to adhere effectively to management protocols for incommunicable diseases, including epilepsy.49
Finally, five pediatric epilepsy training (PET1) courses have been hosted since 2017 in Kenya, in conjunction with the British Paediatric Neurology Association. This course aims to improve health care–worker knowledge, diagnosis, and management of childhood epilepsy. In 2018, a full East African faculty was trained to lead PET training in the region. Evaluation of the impact of this initiative aandplans for further PET trainings are under way.
Future prospects
Early diagnosis and treatment of childhood epilepsy improves prognosis and mitigates against the impact of various of comorbidities.50,51 The most urgent priorities in the management of childhood epilepsy in Kenya are effective strategies to prevent acquired brain injuries that may result in epilepsy, early recognition and effective diagnosis of the condition, and effective and accessible management, including treatment with ASM.
In order to improve primary preventive strategies to reduce the national burden of epilepsy, we need better to understand the epidemiology and etiology of the condition nationwide, including in noncoastal and non-malaria–endemic areas and across different ethnic groups. One potential platform for such work would be the current clinical information network of 14 district hospital sites across Kenya, excluding Kilifi and representing both malaria-endemic and nonendemic areas.52 Primary preventive interventions, eg, improved perinatal care or reduced burden of infectious diseases, are out of the scope of this review.
An important step in improving seizure control will be ensuring an adequate and quality-controlled supply of all of the ASMs on the World Health Organization essential medicines list.53 There have been no randomized clinical trials of epilepsy treatments in Kenya, and these are needed to establish the most effective treatments for this population.
Epilepsy surgery has been performed successfully in a number of Kenyan patients, but there areno pediatric data, nor is a dedicated epilepsy surgery service providing comprehensive presurgical evaluation.54 Epilepsy surgery may be of particular benefit to the large cohort of Kenyan children with focal-onset seizures, as has been demonstrated in other regions.54
Finally, novel drug treatments, including cannabidiol and new technologies such as γ-knife surgery, are emerging in clinical practice in HICs, but are inaccessible to the majority of Kenyan patients.55 Partnerships between LMICs and HICs may help in capacity-building through skill training and potentially introduction of new evidence-based treatments at affordable cost.56,57
The majority of the data included in this review were obtained from one highly productive group in the country. There is a need to promote and expand on this quality of research. The sustainability of such research is essential and must be supported to grow. More researchers are needed to provide data to direct clinical needs and advise practice. Therefore, actions to improve prevention, diagnosis, and management of childhood epilepsy in Kenya will require national leadership and strategic long-term partnerships among government, health-care providers, and research institutions
The findings presented in this paper may be helpful in other LMIC settings, but it is important to consider the unique characteristics of each country, which can be contextually different from Kenya’s. For example, the increased burden of epilepsy stigma, nonadherence to treatment, and medical and psychiatric comorbidities is likely to apply in many other LMIC settings as well, but the underlying risk factors may vary. Risk factors are dependent on ecological and geographical distribution of countries, as well as their economic levels and sociocultural integration. In Kenya, malaria would be an important risk factor for epilepsy, but in other countries onchocerciasis and neurocysticercosis would be the priority target infections for programs aimed at reducing the burden of epilepsy. Sociocultural determinants of epilepsy treatment may be different across countries, and influence the development of educational interventions to improve diagnosis and management of epilepsy. In Kilifi, working with traditional healers through an education intervention helps reduce the treatment gap, but it is unknown if a similar approach would work in other settings. There may be differences in organization and resourcing of the health-care systems, meaning the suggested public health interventions for epilepsy will need different strategies of implementation and evaluation. Kenya has a devolved system of governance, which has strengthened the primary health-care systems, making it ideal for introducing and evaluating public health interventions.
Conclusion
Kenya faces a high burden of epilepsy, particularly in children, but nationwide the epidemiology of the condition is poorly understood, particularly in non-malaria–endemic areas. Cultural beliefs, stigma, and lack of access to treatment and skilled human resources are causes of delayed diagnosis and management of epilepsy. Potentially preventable etiologies, including adverse perinatal events, brain injuries, and infections, including malaria, as well as familial genetics are common risk factors and causes of epilepsy in the country. Clinical findings suggest a high prevalence of focal lesional epilepsy that may be due to preventable causes. Premature mortality, neurological sequelae, behavioral problems, frequent hospitalization, and injuries are common sequelae for children with epilepsy in Kenya, all of which point to extremely poor outcomes. Adherence to ASM reduces the number of seizures and improves overall quality of life, yet access to diagnostics and medications is highly variable and the treatment gap in Kenya remains unacceptably high.
There is urgent need for better public awareness of epilepsy, improved epidemiological understanding of the condition within Kenya, access to affordable quality health care for all those affected, and improved facilities for the diagnosis and treatment of epilepsy. A national strategy is needed to inform further research and multisector approaches to improve outcomes for children living with epilepsy.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ngugi AK, Bottomley C, Kleinschmidt I, et al. Prevalence of active convulsive epilepsy in sub-Saharan Africa and associated risk factors: cross-sectional and case-control studies. Lancet Neurol. 2013;12(3):253–263. doi:10.1016/S1474-4422(13)70003-6
2. Ibinda F, Wagner RG, Bertram MY, et al. Burden of epilepsy in rural Kenya measured in disability-adjusted life years. Epilepsia. 2014;55(10):1626–1633. doi:10.1111/epi.12741
3. Ngugi AK, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Estimation of the burden of active and life-time epilepsy : a meta-analytic approach. Epilepsia. 2010;51(5):883–890. doi:10.1111/j.1528-1167.2009.02481.x
4. Feksi AT, Kaamugisha J, Gatiti S, Sander L, Shorvon S. A comprehensive community epilepsy programme: the Nakuru Project. Epilepsy Res. 1991;8(3):252–259. doi:10.1016/0920-1211(91)90072-N
5. Carter JA, Neville BG, White S, et al. Increased prevalence of epilepsy associated with severe falciparum malaria in children. Epilepsia. 2004;45(8):978–981. doi:10.1111/j.0013-9580.2004.65103.x
6. Kariuki SM, Abubakar A, Kombe M, et al. Prevalence, risk factors and behavioural and emotional comorbidity of acute seizures in young Kenyan children : a population-based study. BMC Med. 2018;16(1):35. doi:10.1186/s12916-018-1021-y
7. Edwards T, Scott JAG, Munyoki G, et al. Active convulsive epilepsy in a rural district of Kenya: a study of prevalence and possible risk factors. Lancet Neurol. 2008;7(1):50–56. doi:10.1016/S1474-4422(07)70292-2
8. Kariuki SM, Matuja W, Akpalu A, et al. Clinical features, proximate causes, and consequences of active convulsive epilepsy in Africa. Epilepsia. 2014;55(1):76–85. doi:10.1111/epi.12392
9. Kakooza-Mwesige A, Ndyomugyenyi D, Pariyo G, et al. Adverse perinatal events, treatment gap, and positive family history linked to the high burden of active convulsive epilepsy in Uganda : a population-based study. Epilepsia Open. 2017;2(2):188–198. doi:10.1002/epi4.12048
10. Mazigo HD, Sciences A, Morona D, Sciences A, Kweka E, Waihenya R. Epilepsy and tropical parasitic infections in Sub-Saharan Africa : a review. Tanzan J Health Res. 2014;15(3):1–21. doi:10.4314/thrb.v15i2.5
11. Durkin MS, Davidson LL, Desai P, et al. Validity of the ten questions screened for childhood disability: results from population-based studies in Bangladesh, Jamaica, and Pakistan. Epidemiology. 1994;5(3):283–289.
12. Kind CJ, Newton CR, Kariuki SM. Prevalence, risk factors, and neurobehavioral comorbidities of epilepsy in Kenyan children. Epilepsia Open. 2017;2(4):388–399. doi:10.1002/epi4.12069
13. Mung’ala-Odera V, White S, Meehan R, et al. Prevalence, incidence and risk factors of epilepsy in older children in rural Kenya. Seizure. 2008;17(5):396–404. doi:10.1016/j.seizure.2007.11.028
14. Serem GK, Newton CR, Kariuki SM. Incidence, causes and phenotypes of acute seizures in Kenyan children post the malaria-decline period. BMC Neurol. 2015;15:180. doi:10.1186/s12883-015-0444-8
15. Mwaniki M, Mathenge A, Gwer S, et al. Neonatal seizures in a rural Kenyan District Hospital: aetiology, incidence and outcome of hospitalization. BMC Med. 2010;8:16. doi:10.1186/1741-7015-8-16
16. Carrizosa Moog J, Kakooza-Mwesige A, Tan CT. Epilepsy in the tropics: emerging etiologies. Seizure. 2017;44:108–112. doi:10.1016/j.seizure.2016.11.032
17. Kariuki SM, Ikumi M, Ojal J, et al. Acute seizures attributable to falciparum malaria in an endemic area on the Kenyan coast. Brain. 2011;134(Pt5):1519–1528. doi:10.1093/brain/awr051.2011
18. Bistervels IM, Kariuki SM, Newton CR. Risk of convulsive epilepsy following acute seizures in Kenyan children. Epilepsia Open. 2016;1(3–4):112–120. doi:10.1002/epi4.12013
19. Munyoki G, Edwards T, White S, et al. Clinical and neurophysiologic features of active convulsive epilepsy in rural Kenya: a population-based study. Epilepsia. 2010;51(12):2370–2376. doi:10.1111/j.1528-1167.2010.02653.x
20. Idro R, Gwer S, Kahindi M, et al. The incidence, aetiology and outcome of acute seizures in children admitted to a rural Kenyan district hospital. BMC Pediatr. 2008;8(1):5. doi:10.1186/1471-2431-8-5
21. Kariuki SM, Kakooza A, Wagner RG, et al. Prevalence and factors associated with convulsive status epilepticus in Africans with epilepsy. Neurology. 2015;84(18):1838–1845. doi:10.1212/WNL.0000000000001542
22. Jowi JO, Kidiga ZP, Gitau MG. A review of electroencephalograms done at the Kenyatta National Hospital, Nairobi. East Afr Med J. 2008;85(2):92–97.
23. Njuguna PW, Mungala-Odera V, Chong WK, Meehan RA, Newton CR. Computerized tomography scan of the brain in a community study of neurological impairment in Kenya. J Child Neurol. 2007;22(1):26–32. doi:10.1177/0883073807299972
24. Kariuki SM, Abubakar A, Kombe M, et al. Burden, risk factors, and comorbidities of behavioural and emotional problems in Kenyan children : a population-based study. Lancet Psychiatry. 2017;4(2):136–145. doi:10.1016/S2215-0366(16)30403-5
25. Kariuki SM, Abubakar A, Holding PA, et al. Epilepsy & Behavior Behavioral problems in children with epilepsy in rural Kenya. Epilepsy Behav. 2012;23(1):41–46. doi:10.1016/j.yebeh.2011.10.017
26. Abubakar A, Kariuki SM, Tumaini JD, et al. Community perceptions of developmental and behavioral problems experienced by children living with epilepsy on the Kenyan coast: a qualitative study. Epilepsy Behav. 2015;45:74–78. doi:10.1016/j.yebeh.2015.02.023
27. Ibinda F, Odermatt P, Kariuki SM, et al. Magnitude and factors associated with nonadherence to antiepileptic drug treatment in Africa: a cross-sectional multisite study. Epilepsia Open. 2017;2(2):226–235. doi:10.1002/epi4.12052
28. El Sharkawy G, Newton C, Hartley S. Attitudes and practices of families and health care personnel toward children with epilepsy in Kilifi, Kenya. Epilepsy Behav. 2006;8(1):201–212. doi:10.1016/j.yebeh.2005.09.011
29. Kendall-Taylor N, Kathomi C, Rimba K, Newton CR. Traditional healers and epilepsy treatment on the Kenyan coast. Epilepsia. 2008;49(9):1638–1639. doi:10.1111/j.1528-1167.2008.01580_1.x
30. Kariuki SM, Chengo E, Ibinda F, et al. Burden, causes, and outcomes of people with epilepsy admitted to a rural hospital in Kenya. Epilepsia. 2015;56(4):577–584. doi:10.1111/epi.12935
31. Kendall-Taylor NH, Kathomi C, Rimba K, Newton CR. Comparing characteristics of epilepsy treatment providers on the Kenyan coast: implications for treatment-seeking and intervention. Rural Remote Health. 2009;9(4):1253.
32. Mbuba KC, Ngugi A, Fegan G, et al. Risk factors associated with the epilepsy treatment gap in Kilifi, Kenya: a cross-sectional study. Lancet Neurol. 2012;11(8):688–696. doi:10.1016/S1474-4422(12)70155-2
33. Carter JA, Molyneux CS, Mbuba CK, Jenkins J, Newton CR, Hartley SD. The reasons for the epilepsy treatment gap in Kilifi, Kenya: using formative research to identify interventions to improve adherence to antiepileptic drugs. Epilepsy Behav. 2012;25(4):614–621. doi:10.1016/j.yebeh.2012.07.009
34. Ngugi AK, Bottomley C, Fegan G, et al. Premature mortality in active convulsive epilepsy in rural Kenya: causes and associated factors. Neurology. 2014;82(7):582–589. doi:10.1212/WNL.0000000000000123
35. Sadarangani M, Seaton C, Scott JA, et al. Incidence and outcome of convulsive status epilepticus in Kenyan children: a cohort study. Lancet Neurol. 2008;7(2):145–150. doi:10.1016/S1474-4422(07)70331-9
36. Palmer BS. Meta-analysis of three case controlled studies and an ecological study into the link between cryptogenic epilepsy and chronic toxoplasmosis infection. Seizure Eur J Epilepsy. 2007;16(8):657–663. doi:10.1016/j.seizure.2007.05.010
37. Ministry of Medical Services, Ministry of Public Health and Sanitation. Clinical guidelines for management and referral of common conditions at Levels 2–3: primary care. 2009:1–436. Available from: http://www.medbox.org/kenya/clinical-guidelines-for-management-and-referral-of-common-conditions-at-levels-2-3-primary-care/preview?
38. Prins A, Chengo E, Mungala Odera V, et al. Long-term survival and outcome in children admitted to Kilifi district hospital with convulsive status epilepticus. Epilepsy Res Treat. 2014;2014:643747. doi:10.1155/2014/643747
39. Bhalla D, Aziz H, Bergen D, et al. Undue regulatory control on phenobarbital—an important yet overlooked reason for the epilepsy treatment gap. Epilepsia. 2015;56(4):659–662. doi:10.1111/epi.12929
40. Jost J, Sivadier G, Ba A, et al. Quality of antiepileptic drugs in Africa: results from a pilot study (Quaeda) in Kenya and Gabon. J Neurol Sci. 2015;357:e28–e29. doi:10.1016/j.jns.2015.08.155
41. Wilmshurst JM, Kakooza-Mwesige A, Newton CR. The challenges of managing children with epilepsy in Africa. Semin Pediatr Neurol. 2014;21(1):36–41. doi:10.1016/j.spen.2014.01.005
42. Dillip A, Alba S, Mshana C, et al. Acceptability - A neglected dimension of access to health care: findings from a study on childhood convulsions in rural Tanzania. BMC Health Serv Res. 2012;12(1):1. doi:10.1186/1472-6963-12-113
43. Ibinda F, Mbuba CK, Kariuki SM, et al. Evaluation of Kilifi Epilepsy Education Programme: a randomized controlled trial. Epilepsia. 2014;55(2):344–352. doi:10.1111/epi.12498
44. Siddharthan BT, Ramaiya K, Yonga G, et al. Noncommunicable diseases in East Africa: assessing the gaps in care and identifying opportunities for improvement. Health Aff (Millwood). 2015;34(9):1506–1513. doi:10.1377/hlthaff.2015.0382
45. Kaamugishaa J, Feksi AT. Determining the prevalence of epilepsy in the semi-urban population of Nakuru, Kenya, comparing two independent methods not apparently used before in epilepsy studies. Neuroepidemiology. 1988;7(3):115–121. doi:10.1159/000110144
46. Bitta MA, Kariuki SM, Chengo E, Newton CR. An overview of mental health care system in Kilifi, Kenya: results from an initial assessment using the World Health Organization’s Assessment Instrument for Mental Health Systems. Int J Ment Health Syst. 2017;11(1):28. doi:10.1186/s13033-017-0135-5
47. Ministry of health; news: "President Uhuru launches universal health coverage pilot program December 13th 2018 Available from: http://www.health.go.ke/?p=5116.
48. Some D, Edwards JK, Reid T, et al. Task shifting the management of non-communicable diseases to nurses in Kibera, Kenya: does it work? PLoS One. 2016;11(1):1–9. doi:10.1371/journal.pone.0145634
49. Mani KS, Rangan G, Srinivas HV, Kalyanasundaram S, Narendran S, Reddy A. K. The Yelandur study: a community-based approach to epilepsy in rural South India: epidemiological aspects. Seizure. 1998;7(4):281–288. doi:10.1016/S1059-1311(98)80019-8
50. Jost J, Ratsimbazafy V, Preux PM, et al. Epilepsy management and clinical research could be improved in low and middle income countries (LMICs) by epinet database. J Neurol Sci. 2015;357:e24. doi:10.1016/j.jns.2015.08.142
51. Tuti T, Bitok M, Paton C, et al. Innovating to enhance clinical data management using non-commercial and open source solutions across a multi-center network supporting inpatient pediatric care and research in Kenya. J Am Med Inform Assoc. 2016;23(1):184–192. doi:10.1093/jamia/ocv028
52. Tuti T, Bitok M, Malla L, et al. Improving documentation of clinical care within a clinical information network: an essential initial step in efforts to understand and improve care in Kenyan hospitals. BMJ Glob Health. 2016;1(1):e000028. doi:10.1136/bmjgh-2016-000028
53. World health organization; "Ensuring medicines save lives"Available from: https://www.who.int/medicines/news/2018/ensuring_medicines_save_lives_/en/
54. Ruberti RF. Surgery of intractable epilepsy in Africa. Afr J Neurol Sci. 1997;16:1.
55. Régis J, Rey M, Bartolomei F, et al. Gamma knife surgery in mesial temporal lobe epilepsy: a prospective multicenter study. Epilepsia. 2004;45(5):504–515. doi:10.11141/j.0013-9580.2004.07903.x
56. Hopkins J, Burns E, Eden T. International twinning partnerships: an effective method of improving diagnosis, treatment and care for children with cancer in low-middle income countries. J Cancer Policy. 2013;1(1):e8–e19. doi:10.1016/j.jcpo.2013.06.001
57. Sander JW. The epidemiology of epilepsy revisited. Curr Opin Neurol. 2003;16(2). Available from: https://journals.lww.com/co-neurology/Fulltext/2003/04000/
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
