Back to Journals » Journal of Pain Research » Volume 11
Epidural dexmedetomidine infusion for perioperative analgesia in patients undergoing abdominal cancer surgery: randomized trial
Authors Hetta DF , Fares KM, Abedalmohsen AM , Abdel-Wahab AH , Abo Elfadl GM , Ali WN
Received 28 January 2018
Accepted for publication 4 September 2018
Published 30 October 2018 Volume 2018:11 Pages 2675—2685
DOI https://doi.org/10.2147/JPR.S163975
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Erica Wegrzyn
Diab Fuad Hetta,1 Khaled Mohamed Fares,1 Abualauon Mohamed Abedalmohsen,2 Amani Hassan Abdel-Wahab,2 Ghada Mohammed Abo Elfadl,2 Wesam Nashat Ali2
1Anesthesia and Pain Management, South Egypt Cancer Institute, 2Anesthesia and Intensive Care, Faculty of Medicine, Assuit University, Assuit, Egypt
Objective: To assess the postoperative analgesic efficacy of epidural dexmedetomidine added to bupivacaine infusion for patients undergoing major abdominal cancer surgery.
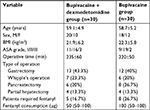
Methods: Patients scheduled for major upper abdominal cancer surgery were allocated to group bupivacaine (n =32), in which patients received epidural bupivacaine infusion (6 mL/h bupivacaine 0.1%) for 48 hours postoperatively, or group bupivacaine + dexmedetomidine (n=32), in which patients received epidural dexmedetomidine added to bupivacaine infusion (6 mL/h of bupivacaine 0.1% + dexmedetomidine, 0.5 µg/mL) for 48 hours postoperatively. The cumulative morphine consumption, the time to first analgesic request, and the VAS pain score were evaluated.
Results: The cumulative morphine consumption was significantly reduced in group bupivacaine + dexmedetomidine compared with group bupivacaine: mean ± SD of 10.40±5.16 mg vs 23.23±8.37 mg with an estimated difference (95% CI) of –12.83 (−16.43, –9.24), (P<0.001). The time to the first analgesic demand was significantly delayed in group bupivacaine + dexmedetomidine compared with group bupivacaine: median (IQR) of 6 (1.75, 8.25) h vs 1 (0, 4) h, (P<0.001). The mean collapsed over time of overall VAS pain scores at rest and movement was significantly reduced in group bupivacaine + dexmedetomidine compared with group bupivacaine : mean ± SE of 1.6±0.08 vs 2.38±0.08 with an estimated difference (95% CI) of −0.8 (−1, –0.86), (P<0.001), and mean ± SE of 2.17±0.07 vs 3.25±0.07 with an estimated difference (95% CI) of −1.1 (−1.27, – 0.89), (P<0.001), respectively.
Conclusion: Epidural infusion of dexmedetomidine added to bupivacaine for patients undergoing major abdominal cancer surgery significantly reduced morphine consumption, delayed time to first analgesic supplementation, and decreased pain intensity during the first 48 hours postoperatively without harmful derangement on hemodynamics.
Keywords: dexmedetomidine, epidural, postoperative pain
Introduction
Major abdominal cancer surgery is associated with extensive tissue destruction that induces severe postoperative pain. Epidural analgesia is the most preferred technique among the various existing analgesic methods.1 It has the potential to provide early mobilization, accelerated recovery of gastrointestinal function, and reduction of pulmonary and cardiovascular morbidity in the early postoperative period after abdominal surgery.2
Administration of local anesthetics at effective doses raises the concerns about adverse events, such as hypotension, bradycardia, and motor weakness. So, several local anesthetic adjuvants such as morphine,3 clonidine,4 ketamine,5 neostigmine,6 magnesium,7 and dexamethasone8 have been introduced for epidural usage with varying degrees of efficacy. Opioids are considered the reference standard of those adjuvants. Unfortunately, opioids carry the risks of respiratory depression, delayed intestinal recovery, pruritus, and postoperative nausea and vomiting.
The selectivity of dexmedetomidine to the α2-receptors is eight times of its prototype, clonidine. Accordingly, dexmedetomidine is a more powerful sedative and analgesic drug than clonidine with less hemodynamic derangements from the α1-receptor activation.9
Dexmedetomidine has been primarily used for intravenous sedation in intensive care settings.10 The unique analgesic properties of dexmedetomidine have encouraged the anesthesiologists to use it perineurally.11 Previous studies have declared that dexmedetomidine potentiates local anesthetic effect when administered by neuraxial route.12–16 Unfortunately, the aforementioned studies have administered dexmedetomidine as a single injection that could not provide long-lasting analgesia sufficient for the relief of severe pain associated with major abdominal cancer surgery.
The objective of the current study was to assess the analgesic efficacy of continuous infusion of epidural dexmedetomidine.
Methods
The current study was approved from the institutional review board of South Egypt Cancer Institute, Assuit University, and each participant was informed and written consent was obtained for the procedure and the study. The trial was registered in Australian New Zealand Clinical Trials Registry, trial ID: ACTRN12615000288527.
Sixty-four adult patients, scheduled for major upper abdominal cancer surgery and classified as American Society of Anesthesiologists (ASA) physical status class I–III, were enrolled. Exclusion criteria included treatment with α-adrenergic antagonists, history of arrhythmias, contraindications to placement of an epidural catheter (eg, coagulopathy, local infection, and vertebral anomalies), patients known to be allergic to bupivacaine, dexmedetomidine, or morphine, and patients treated with regular chronic pain medications.
The randomization of patients was achieved by a statistician through a computer-generated list of random numbers and sealed envelopes. Patients were allocated to two groups, group bupivacaine (n=32), patients received perioperative epidural analgesia with bupivacaine only, and group bupivacaine + dexmedetomidine (n=32), patients received perioperative epidural analgesia with bupivacaine and dexmedetomidine.
Epidural catheter insertion
After intravenous infusion of 1 L of normal saline, the patients were placed in the sitting position and sedated with combination of intravenous midazolam, 2 mg, and fentanyl, 50 µg. Under a strict aseptic precaution, thoracic spines from seven to ten were identified (the inferior angle of the scapula is corresponding to the seventh thoracic vertebra). The preferred insertion site was T8–T9. However, if it was not possible to introduce the needle at the chosen site, one space above or below was selected. Three milliliters of 1% lidocaine was infiltrated at the epidural needle insertion site where Tuohy 18 G epidural needle was introduced using the paramedian approach. The epidural space was identified by loss of resistance using saline. A test dose, 3 mL of 2% lidocaine containing 1:200,000 adrenaline was injected to detect intrathecal or intravascular misplacement. Lastly, epidural catheter was threaded through the epidural needle.
After initial blind placement of the epidural catheter, we injected 2 mL of a nonionic contrast dye through the catheter, then anteroposterior and lateral fluoroscopic images were obtained for further confirmation of the desired epidural location. An anesthesiologist (not involved in the study) prepared the coded study drug solutions and then handed them over to the attending senior staff anesthesiologist for epidural administration.
According to the randomization code for each patient, for group bupivacaine + dexmedetomidine, we injected the patients with a bolus dose of 6 mL of bupivacaine, 0.1% + dexmedetomidine, 0.5 µg/mL via the epidural catheter before skin incision, followed by a continuous epidural infusion of 6 mL/h of bupivacaine 0.1% + dexmedetomidine, 0.5 µg/mL throughout the operative period and continued for 48 hours postoperatively, and for group bupivacaine, we injected the patients with a bolus dose of 6 mL of bupivacaine, 0.1% through the epidural catheter before skin incision, followed by a continuous epidural infusion of 6 mL/h of bupivacaine, 0.1% through the operative period and sustained for 48 hours postoperatively. The epidural administered medications were prepared as follow, for bupivacaine group, 10 mL of bupivacaine 0.5% dissolved in 40 mL normal saline to obtain bupivacaine concentration of 0.1% and for group bupivacaine + dexmedetomidine, 10 mL of bupivacaine 0.5%+1 mL (25 µg) dexmedetomidine (Precedex®; Hospira, Lake Forest, IL, USA) dissolved in 39 mL normal saline to obtain bupivacaine concentration of 0.1% and dexmedetomidine concentration of 0.5 µg/mL and delivered through a 50 mL syringe pump (Perfusor® compact S; B Braun, Melsungen, Germany).
General anesthesia was standardized for all patients. Induction of anesthesia was done by intravenous fentanyl 2 µg/kg and propofol 1–2 mg/kg. Endotracheal intubation was achieved by cisatracurium 0.15 mg/kg. Maintenance of anesthesia was done by isoflurane and cisatracurium 0.03 mg/kg on demand. Additional intraoperative analgesia consisted of administration of intravenous boluses of fentanyl 50 µg according to the attending anesthesiologist’s decision. At the end of surgery, muscle relaxation was reversed using neostigmine 0.05 mg/kg and atropine 0.01 mg/kg. Hypotension was determined as a systolic blood pressure <85 mmHg and was managed with intravenous ephedrine 0.1 mg/kg. Bradycardia was determined as a heart rate slower than 50 beats/min and was managed with atropine 0.01 mg/kg.
After the end of surgery, the patients were admitted to the postanesthesia care unit. The level of sedation was monitored using the Observer’s Assessment of Alertness/Sedation Scale (OAA/S).17 Scores ≤3 were considered excessive sedation. All patients were followed up for 2 weeks postoperatively for the detection of a possible neurotoxicity of epidural dexmedetomidine injection. The neurological assessment was done after the first and the second week postoperatively for the detection of any sensory or motor affection.
Postoperative analgesia comprises of patient-controlled intravenous analgesia; set to provide 1 mg boluses of morphine on demand without a background infusion with a lockout period of 5 minutes and no fixed dose limit. All assessments of pain and sedation were made by an intensive care unit resident who was not involved in the study. If the patient had a VAS pain score >5, unusually high morphine consumption or high injection pressure, an X-ray with contrast was done for the detection of possible epidural catheter dislodgement.
Our primary end point was the cumulative morphine consumption during the first postoperative 48 hours. The secondary end points were a) time to first analgesic request, b) 11-point VAS measured during rest and movement at 2, 6, 12, 16, 24, 36, and 48 hours postoperatively. All these data were recorded by nurses blinded to the study protocol.
Statistical analysis
Statistical analysis was carried out on a personal computer using SPSS version 20 software. The data were checked for normality using the Anderson–Darling test prior to statistical analysis. The primary outcome, the cumulative morphine consumption during the first postoperative 48 hours was normally distributed, so ensuing comparisons between groups were done by the unpaired Student’s t-test. The secondary outcome variable, the time to first analgesic request was not normally distributed; therefore, it was expressed as medians (IQR) and consequent comparison between groups was done by the Mann–Whitney U-test. The VAS pain score was normally distributed using the Anderson–Darling test. We assessed the effects of group and the group-by-time interaction on mean VAS pain score over time (2, 6, 12, 16, 24, 36, and 48 hours postoperatively) using a linear mixed effects model adjusting for within-subject correlation with an autoregressive-1 (AR-1) correlation structure.
Qualitative data were reported as counts and percentages, and differences between groups were analyzed with the chi-squared test or Fisher’s exact test, as appropriate, where continuous data were described as mean ± SD and (95% confidence interval). The repeated measures over time of intraoperative blood pressure and heart rate measured at preoperative, 10, 30, 60, and 90 minutes and end of operation; the postoperative blood pressure and heart rate measured at 0, 2, 6, 12, 16, 24, 36, and 48 hours postoperatively, and OAA/S score measured at 0, 2, 6, 12, 16, 24, 36, and 48 hours postoperatively were analyzed using a linear mixed effects model. The type I error was controlled with the Bonferroni correction when conducting the multiple tests. P<0.05 was considered statistically significant.
Based on a preliminary pilot study of 10 patients in each group, we reported a mean difference of 9.2 mg of total postoperative 48 hours morphine consumption and a SD of 12.5 for group bupivacaine and 11.9 for group bupivacaine + dexmedetomidine. Therefore, it was estimated that a minimum sample size of 29 patients in each study group would achieve a power of 80%, assuming a type I error of 0.05. We enrolled 64 patients to allow for dropouts.
Results
Seventy-five patients scheduled for major upper abdominal cancer surgery were assessed for the study eligibility and 64 patients were eligible and involved in the study. Four patients were excluded from the study (one patient was excluded due to failure of localization of epidural space and the other three were excluded due to dislodged epidural catheter). Sixty patients (30 patients in each group) remained for analysis (Figure 1).
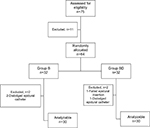  | Figure 1 Flow diagram of patients Abbreviations: Group B, bupivacaine group; Group BD, bupivacaine + dexmedetomidine group. |
Demographic data, patient’s characteristics, and intraoperative fentanyl consumption were similar between groups (Table 1).
The quantity of the cumulative morphine consumption during the first postoperative 48 hours was significantly reduced in group bupivacaine + dexmedetomidine compared with group bupivacaine (mean ± SD) (10.40±5.16 mg vs 23.23±8.37 mg), with an estimated difference (95% CI) of –12.83 (−16.43, –9.24), (P<0.001).
The time to the first analgesic demand was significantly delayed in group bupivacaine + dexmedetomidine compared with group bupivacaine, [median (IQR)] 6 (1.75, 8.25) h vs 1 (0, 4) h, (P<0.001).
The linear mixed effects model revealed a significant reduction of the mean collapsed over time (2, 6, 12, 16, 24, 36, and 48 hours postoperatively) of overall VAS pain scores at rest in group bupivacaine + dexmedetomidine compared with group bupivacaine (mean ± standard error [SE]) (1.6±0.08 vs 2.38±0.08), with an estimated difference (95% CI) of −0.8 (−1, –0.86), (P<0.001), with significant time and group-by-time interaction effect. Moreover, the mean collapsed over time (2, 6, 12, 16, 24, 36, and 48 hours postoperatively) of overall VAS pain scores at movement was significantly reduced in group bupivacaine + dexmedetomidine compared with group bupivacaine (mean ± SE) (2.17±0.07 vs 3.25±0.07), with an estimated difference (95% CI) of −1.1 (−1.27, –0.89), (P<0.001), with significant time and group-by-time interaction effect (Table 2). Further comparison between groups at each time point was made and revealed significant reduction of VAS pain score during rest and movement at all measured time points as shown in Table 3.
  | Table 3 Postoperative pain score Note: P<0.05 was considered statistically significant. Abbreviations: VAS_M, visual analog pain scale at movement; VAS_R, visual analog pain scale at rest. |
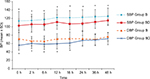
Regarding the intraoperative hemodynamics measured at preoperative, 10, 30, 60, and 90 minutes and end of operation, there was a significant reduction in the mean collapsed over time of overall intraoperative systolic blood pressure in group bupivacaine + dexmedetomidine compared with group bupivacaine, (mean ± SE) (107±2 vs 115±2) mmHg, with an estimated difference (95% CI) of −8 (−14, –2), (P=0.006), with significant group-by-time interaction effect. Similarly, there was a significant reduction of the mean collapsed over time of overall intraoperative diastolic blood pressure in group bupivacaine + dexmedetomidine compared with group bupivacaine (62±1.6 vs 69±1.6) mmHg, with an estimated difference (95% CI) of –7 (−11, –2), (P=0.041), with significant group-by-time interaction effect (Figure 2). We reported four cases in group bupivacaine + dexmedetomidine that developed hypotension (SBP <85 mmHg) and successfully treated with ephedrine, 15 mg. However, dexmedetomidine did not significantly reduce the mean collapsed over time of overall intraoperative heart rate (group bupivacaine + dexmedetomidine vs group bupivacaine), (mean ± SE) (69±1.7 vs 74±2) beats/min, with an estimated difference (95% CI) of −5 (−9, 0.2), (P=0.062), with significant group-by-time interaction effect (Figure 3; Table 2). Furthermore, comparison between groups at each time point revealed no significant changes of mean ± SD intraoperative heart rate at 10 and 30 minutes (bupivacaine + dexmedetomidine vs bupivacaine) (74.13±11.21 vs 70.83±9.40), with an estimated difference (95% CI) of 3.30 (−2.1, 8.6), (P=0.222) and (68.03±9 vs 72.43±8.98), with an estimated difference (95% CI) of −4.40 (−9, 0.3), (P=0.063), respectively. However, dexmedetomidine reduced significantly intraoperative heart rate at the following time points, 60 and 90 minutes and end of operation (bupivacaine + dexmedetomidine vs bupivacaine) (64.70±8.48 vs 74.63±8.34), with an estimated difference (95% CI) of −9.93 (−14.3,–5.6), (P<0.001), (65.53±12.08 vs 74.80±10.08), with an estimated difference (95% CI) of –9.26 (−15,–3.5), (P=0.002), and (65.60±8.74 vs 76.73±10.21), with an estimated difference (95% CI) of –11.13 (−16, –6.2), (P<0.001), respectively (Table 4). One patient in group bupivacaine + dexmedetomidine developed bradycardia (heart rate <50 beats/min) and successfully treated with atropine 0.6 mg.
  | Table 4 Intraoperative heart rate Notes: Data are presented as mean ± SD and mean difference (95% CI). P<0.05 was considered statistically significant. end, end of surgery. |
With regard to the postoperative hemodynamics measured at 0, 2, 6, 12, 16, 24, 36, and 48 hours postoperatively, we detected a significant reduction of the mean collapsed over time of overall postoperative systolic blood pressure in group bupivacaine + dexmedetomidine compared with group bupivacaine (mean ± SE) (104±2 vs 119±2) mmHg, with an estimated difference (95% CI) of −15 (−21, –9), (P<0.001), with significant group-by-time interaction effect. Likewise, there was a significant reduction of the collapsed mean over time of overall postoperative diastolic blood pressure in group bupivacaine + dexmedetomidine compared with group bupivacaine (mean ± SE) (62±1.3 vs 70±1.3) mmHg, with an estimated difference (95% CI) of −8 (−11, –4), (P<0.001), with significant group-by-time interaction effect (Figure 4). Furthermore, there was a significant reduction of the mean collapsed over time of overall postoperative heart rate in group bupivacaine + dexmedetomidine compared with group bupivacaine (mean ± SE) (67±1.3 vs 75±1.3) beats/min, with an estimated difference (95% CI) of −8 (−12, –4), (P<0.001), with significant group-by-time interaction effect (Figure 5; Table 2). Two cases in group bupivacaine + dexmedetomidine developed postoperative hypotension (SBP <85 mmHg) and successfully treated with ephedrine, 10 mg and 20 mg, respectively, and one patient developed postoperative bradycardia (heart rate <50 beats/min) and successfully treated with atropine 0.6 mg.
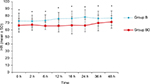  | Figure 5 Postoperative HR. Note: *P<0.05, considered statistically significant. Abbreviations: Group B, bupivacaine group; Group BD, bupivacaine + dexmedetomidine group; HR, heart rate. |
Concerning dexmedetomidine-related sedation, there was a significant reduction of the collapsed mean over time measured at 0, 2, 6, 12, 16, 24, 36, and 48 hours postoperatively of overall OAA/S scores in group bupivacaine + dexmedetomidine compared with group bupivacaine (mean ± SE) (4.4±0.05 vs 4.8±0.05), with an estimated difference (95% CI) of −0.35 (−0.49, –0.21), (P<0.001), without significant group-by-time interaction effect.
In relation to other drawbacks of dexmedetomidine, we detected three patients in group bupivacaine + dexmedetomidine who complained of dry mouth.
Three cases were excluded from the study due to dislodged epidural catheter and one case in group bupivacaine + dexmedetomidine was accidentally taken out by the patient. In group bupivacaine, we detected two cases, one case consumed unusually higher doses of opioid in the first two postoperative hours “20 mg” and X-ray with contrast was requested and proved to be paravertebrally migrated and another case the epidural catheter migrated subcutaneously.
No further complications attributable neither to the epidural block nor to the block medications were detected in both groups within 2 week follow-up period.
Discussion
The current study revealed that epidural infusion of dexmedetomidine added to bupivacaine for patients undergoing major upper abdominal cancer surgery significantly reduced morphine consumption, delayed time to first analgesic supplementation, and decreased pain intensity during the first 48 hours postoperatively.
Dexmedetomidine has been used as an adjuvant to local anesthetics in a wide diversity of regional blocks. It has been administered intravenously in conjunction with spinal anesthesia and resulted in improvement of the quality of sensory and motor block and delayed the time to first analgesic supplementation.18 Wahlander et al claimed that intravenous dexmedetomidine administration is a potentially effective analgesic adjunct to thoracic epidural infusion of 0.125% bupivacaine.19 Moreover, Memiş et al showed that dexmedetomidine as an adjunct to lidocaine improves the quality of regional IV anesthesia.20 Furthermore, it had been declared that dexmedetomidine is a useful local anesthetic adjunct for peripheral and neuraxial blocks.11–15,21–23
Indeed, all the previously mentioned studies have administered dexmedetomidine as a single injection, which in no way could provide long-lasting analgesia sufficient for relief of the severe pain associated with major upper abdominal cancer surgery that continues for several days postoperatively.
The antinociceptive effects of dexmedetomidine are in part caused by its action on spinal α2-adrenoceptors, while the sedative effects predominantly result from its supraspinal action.24,25 The poor diffusion of dexmedetomidine through the blood–brain barrier makes its administration via the neuraxial route a reasonable option that might provide locally mediated analgesia with less unwanted side effects.
To the best of our body of knowledge, there were no previous human studies that administered dexmedetomidine infusion through the epidural route for postoperative pain relief. Thus, the dosage of dexmedetomidine used in this study was inspired from the study by Asano et al,26 who stated that the potency of administered dexmedetomidine and clonidine through the epidural route was correlated with their binding affinity to the spinal α2-adrenoreceptors (the binding capacity of dexmedetomidine is approximately 10 times that of clonidine). A previous study showed that epidural clonidine infusion has intrinsic analgesic efficacy at a dose range of 0.5–2 µ/kg/h after major abdominal surgeries.27 Moreover, Kanazi et al had revealed that 3 μg of intrathecal dexmedetomidine and 30 μg of intrathecal clonidine, have a similar analgesic efficacy on the quality of spinal anesthesia.14 Based on these assumptions, the epidural dexmedetomidine dosage might be 0.05–0.2 µg/kg/h, but during preparation of this study protocol, we tried multiple concentrations of bupivacaine and dexmedetomidine and we found that a concentration of bupivacaine 0.1% + dexmedetomidine, 0.5 µg/mL, infused at a rate of 6 mL/h at the thoracic epidural level provided the best analgesic effect with minimal hemodynamic derangement. It deserves mentioning that dexmedetomidine had been administered through epidural infusion in a dose of 0.2 µg/kg/h during the intraoperative period only, and they claimed that it improves oxygenation and reduces the shunt fraction during one lung ventilation.28
The analgesia produced by the α2-adrenergic agonists is probably due to their action on multiple levels on pain pathway including supraspinal,29 ganglionic,30 spinal,31 and peripheral nerves.32 Therefore, neuraxially administered dexmedetomidine produces analgesia by suppressing the release of C-fiber transmitters and by hyperpolarization of postsynaptic dorsal horn neurons.33
In this context, Ishii et al suggested that dexmedetomidine hyperpolarizes the membrane potentials of the substantia gelatinosa neurons of spinal dorsal horn via G-protein-mediated activation of K+ channels.34 Similarly, Brummett et al illustrated that the mechanism of analgesia produced by dexmedetomidine is likely through inhibiting the propagation of firing of pain impulses via blockade of hyperpolarization-activated cation current (Ih).35
The concentration and the infusion rates of epidural bupivacaine in the current study were consistent with similar studies for abdominal surgeries.36,37 Lower concentrations of bupivacaine <0.1% have been used in previous studies; however, these studies used opioid adjuvants to obtain effective analgesia especially during movement.38 For patients subjected to major abdominal cancer surgery, effective physiotherapy is an essential perioperative care that could not be obtained by the sole administration of these lower concentrations.
In agreement with previous studies,18,27,39,40 a significant reduction in the perioperative heart rate was observed in group bupivacaine + dexmedetomidine, and we detected three cases where patients developed bradycardia and they were successfully managed with atropine. Likewise, a significant reduction in systolic and diastolic blood pressure occurred in group bupivacaine + dexmedetomidine perioperatively. The reduction in hemodynamics is related to the binding of dexmedetomidine to the α2-receptors within the central nervous system that resulted in a reduction of tonic levels of sympathetic outflow and facilitation of vagal activity.41 Moreover, the reduction in hemodynamics is accentuated with the thoracic epidural-induced sympathectomy and the debilitated elderly cancer patients.
In this context, epidural dexmedetomidine infusion at doses mentioned in our study was not associated with any neurological deficits within 2-week follow-up period. In agreement with our report, Eisenach et al did not find any neurological deficits in a 1-week follow-up period in sheep receiving intrathecal dexmedetomidine at a dose of 100 µg.23
Study limitations and future studies
The epidural infusions were limited only to 48 hours postoperatively, and the pain of major cancer surgery may be extended to the fifth or sixth postoperative day; moreover, some patients in dexmedetomidine group still have pain and consumed opioid, so future studies with extended period and higher doses are needed. In addition, the efficacy of dexmedetomidine should be compared against opioid adjuvants such as morphine or fentanyl.
Conclusion
Epidural infusion of dexmedetomidine may be useful or have a more expansive role for abdominal cancer-related surgery that could plausibly change clinical practice. It may potentiate the action of epidural opioid; moreover, it may be of value for patients on chronic preoperative opioid therapy, which is common in patients with cancer. Furthermore, the mild sedative effect of dexmedetomidine may decrease the postoperative agitation and delirium, which is not uncommon in geriatric patients subjected to major cancer surgery. Dexmedetomidine significantly reduced morphine consumption, delayed time to first analgesic supplementation, and decreased pain intensity during the first 48 hours postoperatively without harmful derangement on hemodynamics.
Disclosure
The authors report no conflicts of interest in this work.
References
Werawatganon T, Charuluxanun S. Patient controlled intravenous opioid analgesia versus continuous epidural analgesia for pain after intra-abdominal surgery. Cochrane Database Syst Rev. 2005;1(1):CD004088. | ||
Mohamad MF, Mohammad MA, Hetta DF, Ahmed EH, Obiedallah AA, Elzohry AAM. Thoracic epidural analgesia reduces myocardial injury in ischemic patients undergoing major abdominal cancer surgery. J Pain Res. 2017;10:887–895. | ||
Hjortsø NC, Lund C, Mogensen T, Bigler D, Kehlet H. Epidural morphine improves pain relief and maintains sensory analgesia during continuous epidural bupivacaine after abdominal surgery. Anesth Analg. 1986;65(10):1033–1036. | ||
Gordh T. Epidural clonidine for treatment of postoperative pain after thoracotomy. A double-blind placebo-controlled study. Acta Anaesthesiol Scand. 1988;32(8):702–709. | ||
Subramaniam B, Subramaniam K, Pawar DK, Sennaraj B. Preoperative epidural ketamine in combination with morphine does not have a clinically relevant intra- and postoperative opioid-sparing effect. Anesth Analg. 2001;93(5):1321–1326. | ||
Lauretti GR, de Oliveira R, Reis MP, Juliâo MC, Pereira NL. Study of three different doses of epidural neostigmine coadministered with lidocaine for postoperative analgesia. Anesthesiology. 1999;90(6):1534–1538. | ||
Farouk S. Pre-incisional epidural magnesium provides pre-emptive and preventive analgesia in patients undergoing abdominal hysterectomy. Br J Anaesth. 2008;101(5):694–699. | ||
Thomas S, Beevi S. Epidural dexamethasone reduces postoperative pain and analgesic requirements. Can J Anaesth. 2006;53(9):899–905. | ||
Dyck JB, Maze M, Haack C, Vuorilehto L, Shafer SL. The pharmacokinetics and hemodynamic effects of intravenous and intramuscular dexmedetomidine hydrochloride in adult human volunteers. Anesthesiology. 1993;78(5):813–820. | ||
Bhana N, Goa KL, Mcclellan KJ. Dexmedetomidine. Drugs. 2000;59(2):263–268. | ||
Fritsch G, Danninger T, Allerberger K, et al. Dexmedetomidine added to ropivacaine extends the duration of interscalene brachial plexus blocks for elective shoulder surgery when compared with ropivacaine alone: a single-center, prospective, triple-blind, randomized controlled trial. Reg Anesth Pain Med. 2014;39(1):37–47. | ||
Salgado PF, Sabbag AT, Silva PC, et al. Synergistic effect between dexmedetomidine and 0.75% ropivacaine in epidural anesthesia. Rev Assoc Med Bras. 2008;54(2):110–115. | ||
El-Hennawy AM, Abd-Elwahab AM, Abd-Elmaksoud AM, El-Ozairy HS, Boulis SR. Addition of clonidine or dexmedetomidine to bupivacaine prolongs caudal analgesia in children. Br J Anaesth. 2009;103(2):268–274. | ||
Kanazi GE, Aouad MT, Jabbour-Khoury SI, et al. Effect of low-dose dexmedetomidine or clonidine on the characteristics of bupivacaine spinal block. Acta Anaesthesiol Scand. 2006;50(2):222–227. | ||
Mohamed AA, Fares KM, Mohamed SA. Efficacy of intrathecally administered dexmedetomidine versus dexmedetomidine with fentanyl in patients undergoing major abdominal cancer surgery. Pain Physician. 2012;15(4):339–348. | ||
Zeng XZ, Xu YM, Cui XG, Guo YP, Li WZ. Low-dose epidural dexmedetomidine improves thoracic epidural anaesthesia for nephrectomy. Anaesth Intensive Care. 2014;42(2):185–190. | ||
Chernik DA, Gillings D, Laine H, et al. Validity and reliability of the Observer’s Assessment of Alertness/Sedation Scale: study with intravenous midazolam. J Clin Psychopharmacol. 1990;10(4):244–251. | ||
Abdallah FW, Abrishami A, Brull R. The facilitatory effects of intravenous dexmedetomidine on the duration of spinal anesthesia: a systematic review and meta-analysis. Anesth Analg. 2013;117(1):271–278. | ||
Wahlander S, Frumento RJ, Wagener G, et al. A prospective, double-blind, randomized, placebo-controlled study of dexmedetomidine as an adjunct to epidural analgesia after thoracic surgery. J Cardiothorac Vasc Anesth. 2005;19(5):630–635. | ||
Memiş D, Turan A, Karamanlioğlu B, Pamukçu Z, Kurt I. Adding dexmedetomidine to lidocaine for intravenous regional anesthesia. Anesth Analg. 2004;98(3):835–840. | ||
Hetta DF, Kamal EE, Mahran AM, Ahmed DG, Elawamy A, Abdelraouf AM. Efficacy of local dexmedetomidine add-on for spermatic cord block anesthesia in patients undergoing intrascrotal surgeries: randomized controlled multicenter clinical trial. J Pain Res. 2017;10:2621–2628. | ||
Elhakim M, Abdelhamid D, Abdelfattach H, Magdy H, Elsayed A, Elshafei M. Effect of epidural dexmedetomidine on intraoperative awareness and post-operative pain after one-lung ventilation. Acta Anaesthesiol Scand. 2010;54(6):703–709. | ||
Eisenach J, Shafer S, Bucklin B, Jackson C, Kallio A. Pharmacokinetics and pharmacodynamics of intraspinal dexmedetomidine in sheep. Anesthesiology. 1994;80:1349–1359. | ||
Pertovaara A. Antinociception induced by alpha-2-adrenoceptor agonists, with special emphasis on medetomidine studies. Prog Neurobiol. 1993;40(6):691–709. | ||
Maze M, Tranquilli W. Alpha-2 adrenoceptor agonists: defining the role in clinical anesthesia. Anesthesiology. 1991;74(3):581–605. | ||
Asano T, Dohi S, Ohta S, Shimonaka H, Iida H. Antinociception by epidural and systemic alpha(2)-adrenoceptor agonists and their binding affinity in rat spinal cord and brain. Anesth Analg. 2000;90(2):400–407. | ||
de Kock M, Wiederkher P, Laghmiche A, Scholtes JL. Epidural clonidine used as the sole analgesic agent during and after abdominal surgery. A dose-response study. Anesthesiology. 1997;86(2):285–292. | ||
Kar P, Durga P, Gopinath R. The effect of epidural dexmedetomidine on oxygenation and shunt fraction in patients undergoing thoracotomy and one lung ventilation: a randomized controlled study. J Anaesthesiol Clin Pharmacol. 2016;32(4):458–464. | ||
Doze VA, Chen BX, Maze M. Dexmedetomidine produces a hypnotic-anesthetic action in rats via activation of central alpha-2 adrenoceptors. Anesthesiology. 1989;71(1):75–79. | ||
Mccallum JB, Boban N, Hogan Q, Schmeling WT, Kampine JP, Bosnjak ZJ. The mechanism of alpha2-adrenergic inhibition of sympathetic ganglionic transmission. Anesth Analg. 1998;87(3):503–510. | ||
Eisenach JC, Hood DD, Curry R. Intrathecal, but not intravenous, clonidine reduces experimental thermal or capsaicin-induced pain and hyperalgesia in normal volunteers. Anesth Analg. 1998;87(3):591–596. | ||
Poree LR, Guo TZ, Kingery WS, Maze M. The analgesic potency of dexmedetomidine is enhanced after nerve injury: a possible role for peripheral alpha2-adrenoceptors. Anesth Analg. 1998;87(4):941–948. | ||
Lawhead RG, Blaxall HS, Bylund DB. Alpha-2A is the predominant alpha-2 adrenergic receptor subtype in human spinal cord. Anesthesiology. 1992;77(5):983–991. | ||
Ishii H, Kohno T, Yamakura T, Ikoma M, Baba H. Action of dexmedetomidine on the substantia gelatinosa neurons of the rat spinal cord. Eur J Neurosci. 2008;27(12):3182–3190. | ||
Brummett CM, Hong EK, Janda AM, Amodeo FS, Lydic R. Perineural dexmedetomidine added to ropivacaine for sciatic nerve block in rats prolongs the duration of analgesia by blocking the hyperpolarization-activated cation current. Anesthesiology. 2011;115(4):836–843. | ||
Perotti L, Cusato M, Ingelmo P, et al. A Comparison of differences between the systemic pharmacokinetics of levobupivacaine and ropivacaine during continuous epidural infusion: A Prospective, randomized, multicenter, double-blind controlled Trial. Anesth Analg. 2015;121(2):348–356. | ||
Wu Y, Liu F, Tang H, et al. The analgesic efficacy of subcostal transversus abdominis plane block compared with thoracic epidural analgesia and intravenous opioid analgesia after radical gastrectomy. Anesth Analg. 2013;117(2):507–513. | ||
Ganapathi S, Roberts G, Mogford S, Bahlmann B, Ateleanu B, Kumar N. Epidural analgesia provides effective pain relief in patients undergoing open liver surgery. Br J Pain. 2015;9(2):78–85. | ||
Oriol-López SA, Maldonado-Sanchez KA, Hernandez-Bernal CE, Castelazo-Arredondo JA, Moctezuma RL. Dexmedetomidina epidural en anestesia regional para reducir la ansiedad [Epidural dexmedetomidine in regional anesthesia to reduce anxiety]. Rev Mex Anest. 2008;31:271–277. Spanish. | ||
Bazin M, Bonnin M, Storme B, et al. Addition of clonidine to a continuous patient-controlled epidural infusion of low-concentration levobupivacaine plus sufentanil in primiparous women during labour. Anaesthesia. 2011;66(9):769–779. | ||
Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93(2):382–394. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.