Back to Journals » Drug, Healthcare and Patient Safety » Volume 14
Epidemiology of Snake Bites Linked with the Antivenoms Production in Colombia 2008–2020: Produced Vials Do Not Meet the Needs
Authors Estrada-Gómez S , Vargas-Muñoz LJ, Higuita-Gutiérrez LF
Received 31 March 2022
Accepted for publication 13 August 2022
Published 29 September 2022 Volume 2022:14 Pages 171—184
DOI https://doi.org/10.2147/DHPS.S367757
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Siew Siang Chua
Sebastián Estrada-Gómez,1– 3,* Leidy Johana Vargas-Muñoz,2,4,* Luis Felipe Higuita-Gutiérrez4,5,*
1Grupo de Toxinologia y Alternativas Terapeuticas Alimentarias, Universidad de Antioquia UdeA, Medellin, Antioquia, Colombia; 2Tech Life Saving (TLS), Medellin, Antioquia, Colombia; 3Centro de Investigación en Recursos Naturales y Sustentabilidad, Universidad Bernardo O’Higgins, Santiago de Chile, Chile; 4Facultad de Medicina, Universidad Cooperativa de Colombia, Medellin, Antioquia, Colombia; 5Escuela de Microbiología, Universidad de Antioquia UdeA, Medellin, Antioquia, Colombia
*These authors contributed equally to this work
Correspondence: Sebastián Estrada-Gómez, Email [email protected]; [email protected]
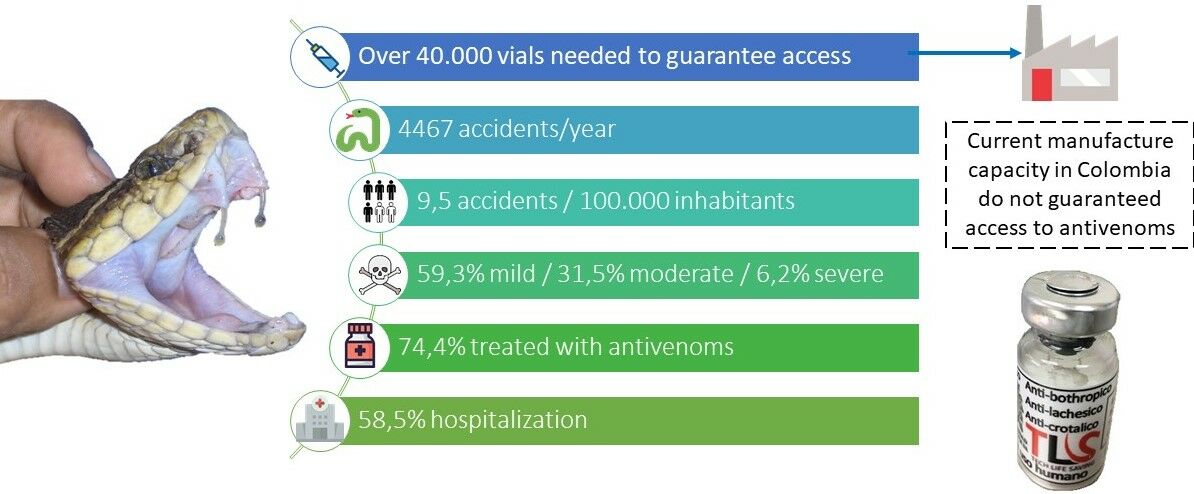
Introduction: Snakebite envenomation is a public health event of mandatory reporting in Colombia. It is considered a medical emergency in which the government must guarantee antivenom availability. We describe snakebite epidemiological figures in Colombia between 2008 and 2020 and correlate them with antivenom manufacturing figures to determine rate coverage and the need for antivenom.
Methods: We performed an ecological study based on secondary official figures from the National Health Institute, the National Institute for Surveillance of Medicines and Foods, the National Administrative Department of Statistics and the Ministry of Health and Social Protection. Absolute and relative frequencies were calculated with 95% confidence intervals, position measurements, dispersion and central tendency.
Results: Through our research, we revealed that in the last 13 years (2008– 2020), there were an average of 4467 annual snakebite envenomation cases affecting all the departments in Colombia. Antioquia reported the highest number of snakebites with 647 (95% CI 588– 706) cases per year. The population incidence per 100,000 inhabitants was 9.5; the highest rates were found in Vaupés at 116.1 and Guaviare at 79.24. During the last seven years (2014– 2020) Colombia produced an average of 21,104 antivenom vials per year, while the annual demand for antivenom is estimated at 54,440 units needed to guarantee access.
Discussion: Colombia does not produce sufficient vials to cover their needs, and this is why only 74.4% of accidents (out of the 92% not classified as dry bites) were treated, and even 9.7% of the severe accidents did not receive the specific treatment (8% of the victims were classified as dry bites). Figures support the regular antivenom shortages declared by the Ministry of Health and Social Protection in the last 13 years (11 health emergency declarations). New efforts are needed to: 1) boost the production of GMP-based high-quality antivenom, that covers the national needs and is made availability, 2) a better estimation method to calculate the need for antivenom in Colombia, and 3) implementation of production-distribution chains guaranteeing access in remote communities.
Keywords: snakebite, Colombia, antivenoms, manufacture, access, availability
Graphical Abstract:
Introduction
The 2017 sessions of the WHO (World Health Organization), Technical and Strategic Group for Neglected Tropical Diseases classified the snakebite envenomation into the Category A of the Neglected Tropical Diseases (NTD).1,2 This categorization increases the visibility of this NTD and the issues regarding antivenom production and shortages in some parts of the world.1 Additionally, antivenoms are categorized as essential drugs by the WHO, but the small number of producers worldwide and their high selling prices characterize them as semi-orphan drugs.3,4
In Colombia, snakebite envenomation is considered a priority public health issue due to the high number of annual cases, the socio-demographic and cultural characteristics of the affected population, as well as the climatic, health infrastructure and geographic conditions of the more affected regions.5 Previous reports indicate that in Colombia, the incidence of snakebites ranges between 7.0 and 9.3 per 100,000 individuals annually, with a mortality rate between 0.6% and 1.0%.6 Additionally, Colombia does not have the necessary infrastructure to guarantee the cold chain required to supply and distribute current manufactured antivenoms and fulfill the national demand for antivenoms in rural dispersed areas throughout the country. In fact, Colombia was classified in a recent study as the country in Latin America with the largest number of dangerous snake species without specific treatment available.1 This classification was made considering variables such as the access to antivenoms, the access to health centers and ecological information on poisonous species.1
According to the WHO guidelines, there are four category 1 snake species in Colombia.7 This category comprises highly venomous and widespread species that represent the highest clinical-epidemiological risk and numerous snakebites.7 Snakebites by category 1 snake species should be promptly treated with a specific antivenom, whose efficacy has been validated to neutralize the main toxicological effects of the Colombian snakes (such as hemorrhage, edema, coagulopathies, neurotoxicity, among others) both in preclinical studies8–12 and in clinical studies.13–16 Although Colombia produces specific antivenoms against category 1 snakes, the limited units manufactured and access has led health authorities to declare emergency shortages regularly. This situation contradicts the WHO recommendations, which state that each country must have a constant and sufficient antivenom production to supply its demand.7
In its antivenom production guidelines, WHO strongly recommends antivenoms to be produced in and according to the needs of each country. This is because cross-reactions are not always observed among the venoms of snakes from different regions (countries), which leads to loss of therapeutic efficacy and the importation of the antivenoms are ineffective or useless.7 A case in point is found in polyvalent antivenoms from Costa Rica (PoliVal-ICP), which are not effective against the envenomation by rattlesnakes in Colombia (Crotalus durissus cumanensis). The rattlesnake venom from Costa Rica lacks crotoxin fractions, an essential toxin for the development of clinical manifestations in Colombia (neurotoxicity).17–20 Even the Snakebite Public Health Surveillance Protocol does not recommend the use of antivenom produced in Costa Rica to treat crotalic accidents in Colombia.21 Similarly, occurs with antivenoms produced in Brazil and Mexico, which do not cover the full clinical effects of snakebites in Colombia.10
The eco-epidemiological knowledge on snakebite envenomation in a country is essential to determine the course of action regarding antivenom production and distribution, and to enable the formulation of policies guaranteeing access and satisfaction of the demand. Countries should prioritize their distribution programs in terms of: a) snake category according to WHO, and b) most affected areas by snake accidents and c) lack of access to health infrastructure. Gutierrez et al22 mentioned additional “strategies to confront snakebite envenomation” considering increase and improvement in antivenom production and quality (in-vitro and in-vivo), accessibility to antivenoms at affordable prices, effective distribution programs, continuous training programs for health staff and preventive or educational actions with the community. In terms of opportunity, Johnston et al23 showed how early administration of antivenoms was associated with short hospital stays and with the decrease of certain toxic manifestations of the Australian taipan (Oxyuranus spp.) snakebites (neurotoxicity), showing how guaranteed access to antivenoms is essential to address the problem.
The objective of this study is to analyze snakebite envenomation in Colombia according to the official figures from 2008 to 2020, correlating them with the official antivenom production stats based on the released batches of antivenoms, and the current manufacturing capacity. This information is relevant to prioritize actions in the most affected areas, identify the evolution over time of this event in Colombia and lead interventions that promote the production of antivenoms.
Materials and Methods
Type of study: ecological type according to Wakefield et al, and Morgenstern,24,25 corresponding to an epidemiological study based on the sociodemographic and clinic epidemiological aspects of the population affected by snakebites in Colombia.
Information and Data Collection
Websites: All websites and web pages below were consulted in November 2021.
Demographic Data: the demographic information comes from the figures issued by the National Administrative Department of Statistics (DANE, according to its initials in Spanish) reported in the National Agricultural Census of 2014 available at: https://www.dane.gov.co/index.php/estadisticas-por-tema/agropecuario/censo-nacional-agropecuario-2014; reported by the 2018 National Census available at: https://www.dane.gov.co/index.php/estadisticas-por-tema/demografia-y-poblacion/censo-nacional-de-poblacion-y-vivenda-2018. The information about ethnical communities was obtained from the DANE figures available at: https://www.dane.gov.co/index.php/estadisticas-por-tema/demografia-y-poblacion/grupos-etnicos/informacion-tecnica.
Snakebite statistics: the public health surveillance protocol for snakebite envenomation in Colombia works as a passive or routine surveillance system in which the snakebites are reported to the National Surveillance System (SIVIGILA, according to its initials in Spanish) on a weekly basis, by department and/or municipality.5,26 The flow of information about snakebites presents responsibility by levels starting from Primary Data Generating Units (UPGD, according to its initials in Spanish) in the local area → municipal health secretaries → district or departmental health secretaries → National Institute of Health → Ministry of Health → Pan American Health Organization → World Health Organization.5,26 The snakebite envenomation protocol and the notification chart are available following the route National Health Institute home page → A-Z Event → Snakebite. These data come directly from the UPGD or health centers, which are public or private entities that capture events of public health interest.5 Secondary sources of information included active sources of death records and registries of health services administrators.5
The official data on the snakebite envenomation were thus obtained from the official epidemiological Snakebite envenomation event report, Colombia, issued by the National Institute of Health (INS, according to its initials in Spanish) for the period 2008–2020. The information search was conducted directly on the INS official website www.ins.gov.co. The reports of the SIVIGILA and the epidemiological reports of snakebite envenomation, available at http://portalsivigila.ins.gov.co, were reviewed in the same timeline.
Available antivenom manufacturers: the information related to the national or international antivenom manufacturers meeting Good Manufacture Practices (GMP) parameters in Colombia was obtained from the National Institute for Surveillance of Medicines and Foods (INVIMA, according to its initials in Spanish) directly from the official website www.invima.gov.co.
Numbers of Batches released from antivenoms: the information about antivenoms released batches in the national territory was obtained from the INVIMA directly from its official website www.invima.gov.co. These batches are released after each of the declared products meets the standardized quality parameters along with the required documentation according with the decree number 1782 of 2014. The data were available for the period 2014–2020.
Unavailable vital medicines list: the information about unavailable vital medicines was obtained from the INVIMA, directly from the official website www.invima.gov.co.
Resolutions and declarations of emergencies: the information on resolutions and declarations of emergency made by the Ministry of Health was obtained from the issues 47,397, 47,397, 47,764, 47,772, 49,130, 49,130, 49,130, 49,490, 49,852, 50,211 and 50,573 of the Colombian National Printing Press official newspaper, available at http://jacevedo.imprenta.gov.co/buscador-diario-oficial.
Data Analysis
Absolute and relative frequencies were calculated with 95% confidence intervals for the qualitative variables, and summary measures (position, dispersion and central tendency) for the quantitative variables.27 The information was tabulated and analyzed using the Excel Microsoft Office Professional Plus 2013 software and the Statistical Package for the Social Sciences (SPSS) version 25.0.
Results and Discussion
Statistics on Snakebite Envenomation
From 2008 to 2020, a total of 58,071 cases of snakebite envenomation affecting all the territorial departments were reported in Colombia. With 334 deaths, the fatality rate of 0.57% was lower than the one reported by Leon-Nuñez et al6 in Colombia for the period 2008–2016 (from 0.6% to 1.0%). The department with the highest number of reports and deaths was Antioquia (n = 8455 and 65 respectively) and the one with the lowest number of reports was San Andrés (n = 4 cases and 0 deaths) which is coherent with the reports by Leon-Nuñez et al.6 Regarding annual behavior, it was found that the average of the annual cases was 4467, the year with the lowest number of cases was 2008 (n = 3129) and the year with the highest number was 2019 (n = 5640) reflecting a considerable increase in comparison to the figures for the period 2008–2016 reported by Leon-Nuñez et al6 (Figure 1). The fatality decrease may have been associated with an increase in the antivenom provided to treat snakebites, as explained below.
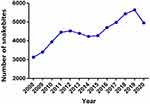 |
Figure 1 Snakebite in Colombia. Total number of snakebite envenomation cases per year between 2008 and 2020 in the Colombian territory. |
The annual rise of snakebite envenomation in Colombia (4.2%) responds to a series of multifactorial aspects that include the sum of socio-demographic, ecological, environmental and labor components.6 A constant increase in snakebite envenomation was registered since 2008, with an accumulated growth of 50.2%, a differential of 58.2% and a 4.2% annual increase of snakebites between 2008 and 2020. According to the INS epidemiological reports, this rise can be explained by two aspects: 1) an increase in the registration and reporting of the snakebites derived from the awareness and training processes of health staff; 2) climatic factors corresponding mainly to rainy seasons and/or droughts.28 The first aspect is most likely to be considered as the best explanation for the increased figures, as supported by Chippaux and Leon-Nuñez et al6,29 However, the second should be reconsidered as a direct explanation for such a rapid and marked rise of snakebite envenomation in Colombia due to the lack of experimental data to support the statement. In fact, Chippaux29 did not find any correlation between the annual incidence of snakebites and the average seasonal incidence between 2009 and 2014 in Colombia.
Two additional factors must be considered: 1) the expansion of urban areas to rural lands (periurban areas) which in Colombia have increased at a rate of 0.4% per year;30 2) and the increase of agricultural activity in rural areas (expansion of the agricultural frontier). These aspects are described in different countries in South America as an explanation for the higher incidence of bites by certain snake species.31,32 The agricultural sector in Colombia reported an average annual growth of 2.6% in the last 18 years, characterized by the increase of the planted areas in thousands of square meters.33 The expansion of these agricultural areas towards lands where snakes naturally inhabit increases the probability of snakebites. This is coherent with Gonzalez-Andrade & Chippaux,32 who state that “… agricultural activities predominate, increasing the risk of snake–human encounters”, and Chippaux,29 who states that “... the activities of human - notably agricultural - explain encounters with the snakes”.29,31 Additionally, in Colombia, the epidemiological figures show that agriculture is the main activity when snakebite envenomation occurs.6,34
Snakebite envenomation is particularly important in dispersed rural areas because the time it takes to transfer a patient bitten by a snake to a health center is greater than those close to population centers, which can reduce the favorable prognosis of treatment by 25%.1,4 It has been reported that in some regions of Colombia, especially those located in the Orinoquía and Amazonia, the mobilization time of snakebites patients to health centers takes more than 12 hours.1 The delay in the access to health infrastructure increases the risk of death or sequalae, given that medical attention over 6 hours after snakebite is classified as severe with a subsequent negative prognosis.35 Indeed, Isbiter et al,36 reported the association between the administration of antivenoms after 2 hours of the snakebite and the occurrence of low effectiveness for the coagulopathy in snake envenoming in Australia, while Otero et al,37 reported acute renal failure or central nervous system hemorrhages after 2 hours envenomation by Bothrops, in Colombia.
Regarding average by department, the figures reached 133 cases (CI 95% CI 121–145), with 13 departments above the average. The departments with the highest number of cases were Antioquia, 647 (CI 95% 588–706), Norte de Santander, 279 (CI 95% 231–327), Bolivar, 277 (CI 95% 243–311), Cordoba, 256 (CI 95% 208–305), Cesar, 250 (CI 95% 202–299) and Meta, 243 (CI 95% 222–264) (Figure 2). Among the municipalities in Antioquia reporting the largest number of cases are Turbo, Chigorodó, Amalfi, Apartadó, San Pedro de Urabá, Zaragoza, Taraza, Necoclí, El Bagre, Andes, San Roque, Ciudad Bolívar, Cáceres, Mutata, Yolombo and Valdivia, representing 51% of the accidents in Antioquia.38
 |
Figure 2 Snakebite average. Average number of snakebites by department with 95% confidence interval for the period 2008–2020. The red dot line indicates the annual departmental average. |
Population Incidence
When calculating the population incidence rate per 100,000 inhabitants, it was found that the national rate was 9.5 (CI 95% 8.75–10.27), which was higher than the previous report by Leon-Nuñez et al6 in Colombia for the period 2008–2016 (7.0 and 9.3 per 100,000 inhabitants) and Chippaux2 for the period 2009–2014 (8.69 per 100,000 inhabitants). The difference may be associated with a significant rise in snakebite envenomation (11,6%) during the period 2017–2020 in comparison to the period 2008–2016 analyzed by Leon-Nuñez et al.6
Twenty departments showed higher rates than the national average and 4 reported over 50 cases per 100,000 inhabitants. The highest rates were reported in Vaupés, 116.1 (CI 95% 102.7–129.6), Guaviare, 79.2 (CI 95% 69.1–89.3), Amazonas, 65.7 (CI 95% 53.1–78.3) and Arauca, 52.2 (CI 95% 47.3–57.1). The departments with the lowest rated were Cundinamarca, 2.33 (CI 95% 2.1–2.6), Valle, 2.0 (CI 95% 1.73–2.31), San Andrés, 1.41 (CI 95% 0.0–3.5) and Bogotá 0.12 (CI 95% 0.0 −0.31). The localities of Vaupés with the highest number of reports were Mitú, Pacoa and Cururu, and in the department of Guaviare, San José Del Guaviare, El Retorno, Miraflores and Calamar (Figure 3). These findings are coherent with the results by Leon-Nuñez et al6 in Colombia for the period 2008–2016, where the departments with the higher incidence were Vaupes, Guaviare, Amazonas, Vichada and Arauca. The incidence of snakebite in these regions is more than 5 times higher (> 54 per 100,000 inhabitants) than in the rest of the country,34 which could be attributed to their highly diverse snake fauna, being the least populated areas in Colombia and a large part of their population being located in rural dispersed areas with a significant number of ethnic communities, especially indigenous.30,39
 |
Figure 3 Average number of snakebites by department with 95% confidence interval (2008–2020 period). The red dot line indicates the national rate per 100,000 inhabitants. |
Severity of Snakebite Envenomation
Regarding the severity of snakebite envenomation, 2008–2020 figures indicate that about 59.3% of accidents were mild, 31.5% were moderate and 6.2%, were severe (Figure 4). All percentages were slightly below the previous report by Leon-Nuñez et al6 who reported 61.5%, 32.2%, and 6.3% for mild, moderate and severe cases respectively. The deaths and sequelae rate derived from this type of accident ranged between 0.04% and 7.6%, and between 5% and 9% respectively, being limb loss the worst sequel occurring in the 6.3% of the accidents.28,34,40–42 These findings were very similar to the reported by Leon-Nuñez et al6 for the period 2008–2016 in Colombia. The decrease in the severity of the accidents may be associated with an increase in the antivenom provided to treat each classified envenomation, as explained below. On the other hand, the figures for deaths and sequelae found in this study were consistent with those published in several retrospective and prospective studies and research carried out in Colombia.17,35
All snakebites classified at any level of severity should receive medical attention either in hospitals, clinics, or emergency services. Despite this, only 58.5% of injured patients receive medical care including hospitalization, which is below the national goal of 100% according to the official guidelines.26,28,34,40–43 Hospitals should provide specific treatment according to the level of severity. Considering the neutralizing capacity of the differente antivenoms, produced by the official manufacturer (National Health Institute) or imported, the official guideline on the management of envenomation stipulate that 8, 11 and 19 vials should be used to treat mild, moderate and severe snake envenomation respectively.43
Characterization of the Affected Population
According to the epidemiological reports consulted, in the last 13 years 76.7% ± 0.3% of the snakebite envenomation occurred in rural areas. In these areas, the highest risk was linked to agricultural activities, with an average of 41.3% ± 0.3%. Regarding the affiliation to the General Health Insurance System (SGSSS, according to its initials in Spanish), epidemiological reports showed that 73.5% ± 2.1% of those affected belonged to the subsidized system (public) and 11.9% ± 2.1% were not affiliated to the SGSSS. The number of accidents in people who do not have any type of affiliation to the health system has decreased in the last decade, which coincides with the increase in accidents in the number of people in the health system subsidized by the government. Understanding the health system affiliation status of people affected by snakebites allows the comprehension of the impact on the public health system and the affected population, especially when they have access to antivenoms with a high cost-effectiveness impact.44
The high accident rate in rural areas is related to the majority of their inhabitants living in scattered rural areas of agricultural activity in plantations of products such as banana or plantain, and cattle activities such as livestock.45 Likewise, many disperse groups of indigenous and afro communities, inhabit these territories.39,46 These two ethnic groups are commonly affected by snakebite accidents, representing 18% of the accidents (>790 individuals) per year.21,28,34,40–42,45,47–51 Most of the population inhabiting rural areas is not affiliated to the SGSSS or belongs to the public regime,52 explaining thus the high rate of the population affected by snakebites not affiliated to the SGSSS or affiliated to the public system.
Antivenom Usage
Regarding antivenom usage, it was found that only 74.4% ± 1.7% of injured people received it (3323 patients). Regarding the people not receiving antivenoms (25.6%), 8% of the victims (357 patients) did not receive the specific treatment due to the lack of clinical manifestations (accidents classified as dry bites), while in 18% of the total cases, despite the detection of clinical manifestations and symptoms of envenoming, the specific treatment was not administered. Considering the classification of the severity of the accident, the SIVIGILA figures showed that an average of 26.4%, 12.5%, and 10.5% of the mild, moderate and severe classified accidents respectively did not receive antivenoms (Table 1).28,34,40–42,51 A decrease in the number of patients that did not receive any antivenom was detected in the figures from Leon-Nuñez et al6 for the period 2008–2016 in Colombia. Despite this decrease, the fact that envenomed patients still not receiving the specific treatment is especially concerning, and place people at a high risk of sequels or death, as supported by Otero et al.37 After using antivenoms, adverse reactions were reported in 7.5% of the individuals.28,34,40–42 According to the official epidemiological reports of the INS, in 2015 and 2016, an average of 4.5 vials of antivenom per patient were used. This data is obtained by dividing the total number of vials (used in a time period) by the number of patients who received treatment.41,42 Although this figure gives direct evidence of the number of vials spent by the patients, it cannot be used to calculate the number of vials needed to guarantee access to the specific treatment as explained below. Indeed, according to Otero et al,53 the average of vials used per patient should be above 7 vials.
 |
Table 1 Percentage of Snakebites Patients That Despite Presenting Clinical Manifestations Did Not Receive Antivenom Therapy |
Need for Antivenom and Production Figures
In Colombia, there are two GMP authorized manufacturers producing second-generation whole IgG antivenoms, in two dfifferent pharmaceutical presentations — liquid (by the public manufacturer) and solid (by the private manufacturer). Both manufacturers are allowed to produce polyvalent antivenoms against Bothrops asper, Lachesis spp (by cross-reactivity with B. asper), and Crotalus durissus cummanensis; and a polyvalent antivenom against Micrurus species.
There are different methods to estimate the vials needed to attend the number of accidents per year, one in such a way that consumption is covered (direct demand) and the other where access to antivenoms is guaranteed. In Colombia, we identified 3 different methods, used by the government and different authors, to calculate the need for antivenom. Since the antivenom manufactured by the official producer (National Health Institute) is the most potent and common treatment available, we used these antivenoms as a reference to calculate antivenom figures. If we consider the antivenom produced by the private manufacturer, the number of vials surpasses the reported figures when applying methods 2 and 3, since the neutralizing capacity is lower (25 mg of B. asper, 20 mg Lachesis spp and 10 mg of C. durissus). The neutralizing potency of the imported antivenom from Mexico is similar to the antivenom by the private manufacturer.
Method 1: According to INS information (and some authors in Colombia),54 we found that the calculation of the needed number of antivenom vials in Colombia is obtained from the direct result of the multiplication of the number of accidents of the previous year and the theoretical average consumption of vials, which is assumed to be 4.5 vials per patient according to the epidemiological reports.41,42 In this sense, Colombia needed around 25,380 vials in 2020 (data derived from the multiplication of the accidents from 2019 and the average of used vials 5640 x 4.5). This data would be useful only to know the direct consumption or expenditure of vials but does not consider variables such as accident severity, historical maximum consumption in a time period, and underreporting, among others, making these figures unreal and insufficient to determine the actual number of vials needed per year in Colombia.
Method 2: Another perspective is to estimate the number of vials needed to attend a snakebite according to the severity of the accidents and statistics on snake envenomation of the previous year of estimation and multiply it by the number of vials needed to attend each level of severity. If we follow this approach, the number of vials needed in 2020 was 50,021 vials (24,641 vials more when compared to method 1). In this case, the number of vials is obtained considering the number of accidents by severity occurred on 2019 (2916 mild accidents, 1794 moderate accidents and 367 severe accidents) and the usage of 8, 11 and 19 vials to attend mild, moderate and severe accidents respectively, according to the national guidelines on the management of envenomations.26 The number of vials used by severity corresponds to the antivenoms manufactured by National Health Institute and imported.
Method 3: In a closer and more realistic estimation carried out by researchers from the University of Antioquia and based on the availability to guarantee antivenom access,53 it is estimated that in Colombia, with the current accident figures, the number of vials needed in 2020 was 54,440 vials (29,060 vials more when compared to method 1) should be available on the market every year to guarantee not only access to this type of essential drugs, but to satisfy the demand for availability of antivenom. This figure is obtained from an equation that includes variables such as underreporting, average use of vials per patient, maximum number of accidents in the last decade, expected consumption of vials and current consumption of vials.53
When we compare the figures related to the need for antivenoms with those of antivenom production in Colombia during the last 7 years (2014–2020), a deficit is detected when applying all methods, specially 2 and 3. During this period of time, INVIMA has released and apporbed an average of 21,104 vials per year (including anticoral vials) with an average of 11 batches per year from the local antivenom producers (Table 2). The official manufacturer releases an average of 13,103 vials per year, while the private one releases an average of 8001 vials per year, in both cases including polyvalent antiophidic and anticoral vials. The year of highest production of antivenoms in Colombia was 2016 with 38,578 vials (with 27,065 and 11,513 vials released by the public and private manufacturers respectively). However, when 2020 figures were analyzed, they dropped to 19,196 vials (with 0 vials issued by the private manufacturer). Figure 5 shows the historical and irregular issue of batches released each year, per producer. A third-party collaborator in antivenom production comes from Mexico, producing approximately 10,000 vials per year. Based on the second and third method and considering all the available suppliers of antivenom in Colombia (Table 2), between 2014 and 2020, the antivenom availability in the market, has not been guaranteed (Figure 5). Linking the last 5 years of total domestic antivenom production by year with the annual snakebite envenomation figures, we observed a tendency characterized by the decrease of antivenom production and increase in snakebites (Figure 6).
 |
Table 2 Sources and Availability of Antivenoms in Colombia Based on the Released Batches by the INVIMA in the Period 2014–2020 |
 |
Figure 6 Antivenom production vs Snakebite envenomation in Colombia. Levels of domestic antivenom production in Colombia vs reported snakebite envenomation cases by year. |
Gomez et al used method number 1 to calculate the number of vials needed, stating that Colombia needs 30,000 vials per year. However, as seen above, this is only to cover the direct consumption, and the number of vials needed in Colombia should be calculated using methods 2 or 3, which consider more variables than method 1 and provide more accurate figures of needed antivenom to guarantee their access.54 Scheske et al55 indicate that one of the key issues to guaranteeing snakebite treatment is the limited access to antivenom due to its low production, inadequate distribution chains, and the need for specific antivenoms for each region. Methods 2 and 3 consider not only the direct consumption but also variables such as the average of vials used per patient by severity (method 2), underreporting, maximum value of accidents in the last decade, expected consumption of vials and current consumption of vials (method 3). When compared to method 1, the application of methods 2 and 3 results in a higher number of vials needed, increasing 97.1% and 114.5% respectively the numbers of vials required. It is important to consider that accessibility depends also on the distribution strategies in rural dispersed areas, as stated by Gomez et al.54
Gomez et al54 indicated that the private manufacturer used to produce an average of 38,108 vials per year (for the period 2008–2016), and employed this figure to argue that Colombia produces enough antivenom to cover the need. Decree 1782 of 2014 states that “The commercialization of all biological treatment batches is subjected to the approval issued by the regulatory authority (INVIMA)” and according to official figures by the INVIMA, the private producer released an average above 8001 vials (see Table 2). In fact, during the last two years of the analyzed period in this study (2019–2020), the private manufacturer did not introduce a single vial (0 vials) into the market (Figure 5). The difference between the production figures indicated by Gomez et al54 and the introduced vials approved by the INVIMA (30,107 vials) could be explained by the private manufacturer exportation of antivenoms to Perú and Ecuador, as stated by Gomez et al.54
Policies to promote a constant and sufficient production are needed especially when both producers have very irregular manufacturing rates, as seen in Figure 5. This study showed a clear and systematic shortage of antivenoms during the last 14 years, based on the official manufacturing figures in Colombia and the current need to guarantee access to antivenoms. This result is coherent with the toxicological emergency guidelines of the Ministry of Health of Colombia and the WHO technical documents for handling snakebite envenomation, recommending that every snakebite envenomation must be treated with antivenom.26,56,57 The claim of shortage of antivenoms is based on: 1) the different and current Health Emergency Declarations in the national territory due to stock-outs of different types of antivenoms (resolutions 2413 of 2008, 002206 of 2009, 2397 and 2672 of 2010, 1300, 1301, 1302 of 2014, 1241 of 2015, 1478 of 2016, 1209 of 2017 and resolution 1487 of 2018), 2) the inclusion of antivenoms into lists of vital medicine not available in Colombia, 3) the figures of vials production versus the demand for availability of antivenom. Both periods of shortage (2004–2010 and 2014–2020) and the constant Health Emergency Declarations, indicate that Colombia does not have an antivenom policy leading to a stable and constant availability of antivenoms to meet current needs.
According to decree 481 of 2004 (issued by the Health Ministry of Colombia), the definition of vital medicines not available includes those medicines essential, indispensable and irreplaceable to safeguard life or relieve any suffering patient or group of patients. During the last 7 years (2014–2020), antivenoms in Colombia (antilonomic, antiophidic, antielapidic-anticoral, antiscorpionic and antiarachnidic) were classified into the category of vital medicines not available by the INVIMA (only polyvalent antiophidic antivenom was excluded as vital and not available at the end of 2020). Currently, in Colombia, there is no local production of antilonomic, antiscorpionic or antiarachnidic antivenoms.
Antivenom Distribution
Concerning the distribution chain of antivenoms, it was found that there is no available programs or distribution network in Colombia to guarantee antivenom availability in the areas of greatest need. The Colombian state left the acquisition of antivenoms in the hands of hospitals, which acquired antivenoms by calling the National Health Institute directly or through local distributors of medicines that act as a third party in the distribution and supply chain, which increases the final cost of the product. In some cases, the Ministry has sent antivenoms to some territorial entities to support emergencies.
It is also observed that Colombia does not have a clear and defined policy for the distribution of antivenoms in the most affected areas. The current method of passive acquisition of antivenoms by health centers causes the concentration of antivenoms in populated centers (large and intermediate cities) and their unavailability in the most affected areas, as reported by other authors.53 Additionally, there is no cold chain for the distribution of liquid antivenoms, which makes it more difficult in Colombia to acquire them in some areas where, in addition to the lack of health infrastructure, there is a lack of electrical infrastructure. These two factors, together with the variable levels of production, generate limited access to and availability of antivenoms, despite having a decent snakebite report system, according to different reports.6,58
Limitations
Among the limitations of this study, it is noteworthy that snakebite envenomation epidemiological reports delivered by the INS lack uniformity in the registration and analysis of the information. Likewise, the information consulted does not consider events per patient, number of vials used per patient, patients receiving them according to the degree of poisoning, adverse reactions, and complications or sequelae resulting from accidents (loss of limbs, surgical interventions, etc.). Despite current toxicological guides in Colombia clearly describing the snakebite envenomation classification, the severity is sometimes misclassified by the health personnel. The analysis presented in this study was carried out with statistical aggregates that must be interpreted with caution to avoid ecological fallacy (associations in aggregate populations may be different from those found in the individual plane). In addition, it is important to bear in mind that many snakebite envenomations occur in indigenous populations or to inhabitants of dispersed rural areas that do not attend health centers, and therefore, they are not registered with SIVIGILA. Also, it should be considered that these ethnic communities usually treat snakebites with therapeutic practices other than antivenom, sometime before its administration.37,59 During the timeline of this study in these communities, 7.3% used nature herbs, 8.3%, potions and 6.9%, prayers.21,28,34,40–42,59 Calculating underreporting in Colombia is an issue that exceeds any technical and scientific capacity, however, some approaches have been made indicating that the underreporting can be 43%.37
Conclusion
Snakebites in Colombia have continuously grown during the last years affecting all departments in Colombia. Conditions like increased registration and reporting of the snakebites, expansion of urban areas to rural lands, and growth of agricultural activity in rural areas (expansion of agricultural frontier) may be the three main factors associated with the rise of snakebites in Colombia. Multiple efforts should be done to guarantee access to antivenoms: 1. There should be a higher production and better quality of antivenoms manufactured in Colombia. Despite the figures on local antivenom production, the annual production rates do not cover the number of vials needed to guarantee access to antivenoms. 2. A better estimation method to calculate the need for antivenom in Colombia should be used. Methods 2 and 3 presented in this study should be considered by the local public health authorities to estimate the number of vials needed in Colombia annually to guarantee access to antivenoms. 3. A production-distribution model linking public and private laboratories should be implemented in Colombia to guarantee antivenoms access, considering it as the main aim of antivenom production. To overcome the continuous antivenom shortage in Colombia, new production strategies must be linked with distribution schemes to ensure that antivenoms reach the most affected populations, guaranteeing access and tackling the issue of 74.4% of the victims receiving treatment, as seen today, where even severe patients are not receiving antivenom.
Data Sharing Statement
All data generated or analyzed during this study are included in this article.
Ethics Approval
The study was approved by the Bioethical Committee for Human Research of the Universidad de Antioquia minute No: 224 - 2022.
Acknowledgments
The authors are grateful to Juan Jose Zuluaga and all Tech Innovation Group staff.
Funding
This research was funded by the Universidad de Antioquia (UdeA) through the Project CIQF-289 financed by the Ministerio de Ciencia Tecnología e Innovación (Code 111577757691) and the Comité para el Desarrollo de la Investigación CONADI, Universidad Cooperativa de Colombia.
Disclosure
The authors declare that they have no competing interests.
References
1. Longbottom J, Shearer FM, Devine M, et al. Vulnerability to snakebite envenoming: a global mapping of hotspots. Lancet. 2018;392(10148):673–684. doi:10.1016/S0140-6736(18)31224-8
2. Chippaux J-P. Snakebite envenomation turns again into a neglected tropical disease! J Venom Anim Toxins Incl Trop Dis. 2017;23(1):38. doi:10.1186/s40409-017-0127-6
3. World Health Organization. Snakebite envenoming; 2018.
4. Temprano GA, Aprea P, Dokmetjian JC. The public production of antivenoms in the Americas, a key favtor to accesibility. Rev Panam Salud Publica. 2017;41:1. doi:10.26633/RPSP.2017.109
5. de la Hoz F, Duran ME, García OE, et al. 1. Snakebite, Public Health Surveillance Protocol. Instituto Nacional de Salud; 2017.
6. León-Núñez LJ, Camero-Ramos G, Gutiérrez JM. Epidemiology of snakebites in Colombia (2008–2016). Rev Salud Publica. 2020;22(3):1–5. doi:10.15446/rsap.v22n3.87005
7. World Health Organization. Guidelines for the production, control and regulation of snake antivenoms immunoglobulins (WHO); 2018.
8. Arce V, Rojas E, Ownby CL, Rojas G, Gutierrez JM. Preclinical assessment of the ability of polyvalent (Crotalinae) and anticoral (Elapidae) antivenoms produced in Costa Rica to neutralize the venoms of North American snakes. Toxicon. 2003;41(7):851–860. doi:10.1016/S0041-0101(03)00043-6
9. Gutierrez JM, Rojas E, Quesada L, et al. Pan-African polyspecific antivenom produced by caprylic acid purification of horse IgG: an alternative to the antivenom crisis in Africa. Trans R Soc Trop Med Hyg. 2005;99(6):468–475. doi:10.1016/j.trstmh.2004.09.014
10. Otero R, Nunez V, Osorio RG, Gutierrez JM, Giraldo CA, Posada LE. Ability of six Latin American antivenoms to neutralize the venom of mapana equis (Bothrops atrox) from Antioquia and Choco (Colombia). Toxicon. 1995;33(6):809–815. doi:10.1016/0041-0101(95)00009-B
11. Rojas E, Quesada L, Arce V, Lomonte B, Rojas G, Gutierrez JM. Neutralization of four Peruvian Bothrops sp. snake venoms by polyvalent antivenoms produced in Peru and Costa Rica: preclinical assessment. Acta Trop. 2005;93(1):85–95. doi:10.1016/j.actatropica.2004.09.008
12. Saravia P, Rojas E, Escalante T, et al. The venom of Bothrops asper from Guatemala: toxic activities and neutralization by antivenoms. Toxicon. 2001;39(2–3):401–405. doi:10.1016/S0041-0101(00)00122-7
13. Otero R, Gutierrez JM, Nunez V, et al. A randomized double-blind clinical trial of two antivenoms in patients bitten by Bothrops atrox in Colombia. The Regional Group on Antivenom Therapy Research (REGATHER). Trans R Soc Trop Med Hyg. 1996;90(6):696–700. doi:10.1016/S0035-9203(96)90442-3
14. Otero R, Gutierrez JM, Rojas G, et al. A randomized blinded clinical trial of two antivenoms, prepared by caprylic acid or ammonium sulphate fractionation of IgG, in Bothrops and Porthidium snake bites in Colombia: correlation between safety and biochemical characteristics of antivenoms. Toxicon. 1999;37(6):895–908. doi:10.1016/S0041-0101(98)00220-7
15. Otero R, Leon G, Gutierrez JM, et al. Efficacy and safety of two whole IgG polyvalent antivenoms, refined by caprylic acid fractionation with or without beta-propiolactone, in the treatment of Bothrops asper bites in Colombia. Trans R Soc Trop Med Hyg. 2006;100(12):1173–1182. doi:10.1016/j.trstmh.2006.01.006
16. Otero-Patino R, Cardoso JL, Higashi HG, et al. A randomized, blinded, comparative trial of one pepsin-digested and two whole IgG antivenoms for Bothrops snake bites in Uraba, Colombia. The Regional Group on Antivenom Therapy Research (REGATHER). Am J Trop Med Hyg. 1998;58(2):183–189. doi:10.4269/ajtmh.1998.58.183
17. Otero-Patino R, Segura A, Herrera M, et al. Comparative study of the efficacy and safety of two polyvalent, caprylic acid fractionated [IgG and F(ab’)2] antivenoms, in Bothrops asper bites in Colombia. Toxicon. 2012;59(2):344–355. doi:10.1016/j.toxicon.2011.11.017
18. Alfaro-Chinchilla A, Á Segura, Gómez A, et al. Expanding the neutralization scope of the Central American antivenom (PoliVal-ICP) to include the venom of Crotalus durissus pifanorum. J Proteomics. 2021;246:104315. doi:10.1016/j.jprot.2021.104315
19. Quintana-Castillo JC, Vargas LJ, Segura C, Estrada-Gómez S, Bueno-Sánchez JC, Alarcón JC. Characterization of the Venom of C. d. cumanesis of Colombia: proteomic analysis and antivenomic study. Toxins. 2018;10(2):85. doi:10.3390/toxins10020085
20. Saravia P, Rojas E, Arce V, et al. Geographic and ontogenic variability in the venom of the neotropical rattlesnake Crotalus durissus: pathophysiological and therapeutic implications. Rev Biol Trop. 2002;50(1):337–346.
21. LJ León-Núñez. Snakebite final reportr in Colombia, 2014. Insitituo Nacional de Salud; 2014: 1–28.
22. Gutiérrez JM. Improving antivenom availability and accessibility: science, technology, and beyond. Toxicon. 2012;60(4):676–687. doi:10.1016/j.toxicon.2012.02.008
23. Johnston CI, Ryan NM, O’Leary MA, Brown SG, Isbister GK. Australian taipan (Oxyuranus spp.) envenoming: clinical effects and potential benefits of early antivenom therapy - Australian Snakebite Project (ASP-25). Clin Toxicol. 2017;55(2):115–122. doi:10.1080/15563650.2016.1250903
24. Wakefield J. Ecologic studies revisited. Annu Rev Public Health. 2008;29(1):75–90. doi:10.1146/annurev.publhealth.29.020907.090821
25. Morgenstern H. Ecologic studies in epidemiology: concepts, principles, and methods. Annu Rev Public Health. 1995;16(1):61–81. doi:10.1146/annurev.pu.16.050195.000425
26. Walteros D, Andrea P. Snakebite, Public Health Surveillance Protocol. Insitituo Nacional de Salud; 2014: 1–21.
27. Dettori JR, Norvell DC. The anatomy of data. Glob Spine J. 2018;8(3):311–313. doi:10.1177/2192568217746998
28. Rojas-Bárcenas AM. Snakebite final reportr in Colombia, 2017. Insitituo Nacional de Salud; 2017: 1–33.
29. Chippaux J-P. Incidence and mortality due to snakebite in the Americas. PLoS Negl Trop Dis. 2017;11(6):e0005662. doi:10.1371/journal.pntd.0005662
30. 1 Agricultural National Census, 2014.Departamento Administrativo Nacional de Estadística; 2015: 1–60.
31. Dolab JA, de Roodt AR, de Titto EH, et al. Epidemiology of snakebite and use of antivenom in Argentina. Trans R Soc Trop Med Hyg. 2014;108(5):269–276. doi:10.1093/trstmh/tru038
32. González-Andrade F, Chippaux JP. Snake bite envenomation in Ecuador. Trans R Soc Trop Med Hyg. 2010;104(9):588–591. doi:10.1016/j.trstmh.2010.05.006
33. Soto E, José J. Natioanl Bank, board of directors report, March 2018. 2018.
34. León Nuñez LJ. Snakebite final reportr in Colombia, 2019. Insitituo Nacional de Salud; 2019: 1–33.
35. Otero-Patino R. Epidemiological, clinical and therapeutic aspects of Bothrops asper bites. Toxicon. 2009;54(7):998–1011. doi:10.1016/j.toxicon.2009.07.001
36. Isbister GK, Duffull SB, Brown SG. Failure of antivenom to improve recovery in Australian snakebite coagulopathy. QJM. 2009;102(8):563–568. doi:10.1093/qjmed/hcp081
37. Otero R, Gutiérrez J, Beatriz mesa M, et al. Complications of Bothrops, Porthidium, and Bothriechis snakebites in Colombia. A clinical and epidemiological study of 39 cases attended in a university hospital. Toxicon. 2002;40(8):1107–1114. doi:10.1016/s0041-0101(02)00104-6
38. SIVIGILA Vycesp. Data from: vigilancia Rutinaria Por Municipios Y Corregimientos Departamentales Hasta Periodo Epidemiológico Xiii Sivigila 2020. Vigilancia rutinaria. Portal SIVIGILA - Ministerio de Salud; 2020.
39. DANE. Population National Census, 2018. 2018. Available from: https://www.dane.gov.co/index.php/estadisticas-por-tema/demografia-y-poblacion/censo-nacional-de-poblacion-y-vivenda-2018.
40. Snakebite final reportr in Colombia, 2018.Insitituo Nacional de Salud; 2018: 1–33.
41. Snakebite final reportr in Colombia, 2015. Insitituo Nacional de Salud; 2015: 1–30.
42. Snakebite final reportr in Colombia, 2016. Insitituo Nacional de Salud; 2016: 1–33.
43. Snakebite, Public Health Surveillance Protocol. Insitituo Nacional de Salud; 2014: 27.
44. Quintana-Castillo JC, Estrada-Gomez S, Cardona-Arias JA. Economic Evaluations of Interventions for Snakebites: a Systematic Review. Clinicoecon Outcomes Res. 2020;12:547–554. doi:10.2147/CEOR.S259426
45. Visión Urabá, biodiversidad y servicios ecosistémicos como base para el desarrollo, la sostenibilidad y el bienestar. Informe final de consultoría CPS 164_303PS. (Instituto de Investigación de Recursos Biológicos Alexander von Humboldt); 2014: 1–98.
46. DANE. Censo Nacional de Poblacion y Vivienda 2018 - Grupos etnicos. Available from: https://www.dane.gov.co/index.php/estadisticas-por-tema/demografia-y-poblacion/grupos-etnicos/informacion-tecnica.
47. Snakebite final reportr in Colombia, 2012. Insitituo Nacional de Salud; 2012: 1–22.
48. Snakebite final reportr in Colombia, 2013. Insitituo Nacional de Salud; 2013: 1–32.
49. Snakebite final reportr in Colombia, 2010. Insitituo Nacional de Salud; 2010: 1–22.
50. Snakebite final reportr in Colombia, 2011.Insitituo Nacional de Salud; 2011: 1–24.
51. Snakebite final reportr in Colombia, 2020.Insitituo Nacional de Salud; 2020: 1–3.
52. Ayala-Garcia J. Health in Colombia. Higher coverage but less access. In: Documento de trabajo sobre Economía Regional Banco de la Republica. 2014:204.
53. Otero R, Callejas ME, Gutiérrez J, et al. Real needs od antivenoms in Colombia. Products and market. Rev Epidemiol Antioquia. 2002;12(1):49–59.
54. Gomez JPG, Gomez ML. Antivenoms in Colombia: production and distribution analysis with recommendations to improve the production network. Biosalud. 2017;16:2.
55. Scheske L, Ruitenberg J, Bissumbhar BB. Needs and availability of snake antivenoms: relevance and application of international guidelines. Int J Health Policy Manag. 2015;4(7):447–457. doi:10.15171/ijhpm.2015.75
56. Ayerbe SR. Accident by venemous animlas and toxic plants. In: Guia para el manejo de urgencias toxicológicas. Imprenta Nacional de Colombia; 2008:277–308.
57. World Health Organization. Guidelines for the management of snake-bites; 2010.
58. Gutiérrez JM. Snakebite poisoning in Latin America and the Caribbean: An integral view from a regional perspective. Bol Malariol Salud Ambient. 2011;51:1–16.
59. Otero R, Fonnegra R, Jiménez SL, et al. Snakebites and ethnobotany in the northwest region of Colombia: part I: traditional use of plants. J Ethnopharmacol. 2000;71(3):493–504. doi:10.1016/s0378-8741(00)00243-9
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


