Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 14
Epidemiology of Isolated Impaired Glucose Tolerance Among Adults Aged Above 50 Years in Rural China
Authors Tian X, Li Y , Liu J , Lin Q, Yang Q, Tu J, Wang J , Li J, Ning X
Received 21 July 2021
Accepted for publication 4 September 2021
Published 16 September 2021 Volume 2021:14 Pages 4067—4078
DOI https://doi.org/10.2147/DMSO.S330470
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Xiaobing Tian,1,* Yan Li,2,3,* Jie Liu,1,2,4,5,* Qiuxing Lin,1,2,4,5 Qiaoxia Yang,6 Jun Tu,1,2,4,5 Jinghua Wang,1,2,4,5 Jidong Li,2,7 Xianjia Ning1,2,4,5
1Department of Neurology, Tianjin Medical University General Hospital, Tianjin, People’s Republic of China; 2Center of Clinical Epidemiology & Evidence-Based Medicine, The Jizhou People’s Hospital, Tianjin, People’s Republic of China; 3Department of Anesthesiology, The Jizhou People’s Hospital, Tianjin, People’s Republic of China; 4Laboratory of Epidemiology, Tianjin Neurological Institute, Tianjin, People’s Republic of China; 5Tianjin Neurological Institute, Key Laboratory of Post-Neuroinjury Neuro-Repair and Regeneration in Central Nervous System, Ministry of Education and Tianjin City, Tianjin, People’s Republic of China; 6Department of Cardiology, Tianjin Medical University General Hospital, Tianjin, People’s Republic of China; 7Department of Neurosurgery, The Jizhou People’s Hospital, Tianjin, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xianjia Ning; Jidong Li Email [email protected]; [email protected]
Introduction: Isolated impaired glucose tolerance (i–IGT) is a subtype of prediabetes in which an individual demonstrates elevated 2-h post-glucose load glucose levels but normal fasting plasma glucose levels. However, few studies have explored the prevalence and risk factors of i–IGT among adults in rural China. Thus, we aimed to explore the prevalence and risk factors of i–IGT among adults ≥ 50 years old in a low-income, rural population in China.
Materials and Methods: Individuals aged ≥ 50 years with normal fasting plasma glucose levels were included in the final analysis. Fasting and 2-h venous blood samples were collected to assess the selected parameter measurements.
Results: A total of 2175 individuals were included in this study. The i–IGT prevalence was 22.9% and significantly higher among females than among males (P< 0.05). Older age [odds ratio (OR), 1.606; 95% confidence interval (CI), 1.101– 2.342; P=0.014), hypertension (OR, 1.554; 95% CI, 1.152– 2.019; P=0.004), and central obesity (OR, 1.395; 95% CI, 1.099– 1.771; P=0.006) were associated with i–IGT. Moreover, white blood cell (OR, 1.089; 95% CI, 1.009– 1.175; P=0.029), high-sensitivity C-reactive protein (OR, 1.049; 95% CI, 1.020– 1.078; P=0.001), serum uric acid (OR, 1.0003; 95% CI, 1.001– 1.004; P=0.001), triglyceride (OR, 1.540; 95% CI, 1.105– 2.147; P=0.011), and alanine aminotransferase (OR, 1.012; 95% CI, 1.004– 1.021; P=0.004) levels were also linked to i–IGT in the analyzed population.
Conclusion: Health promotion education and a standardized approach to managing body weight, BP, and lipid and uric acid levels would benefit this low-income population in rural China for reducing the risk of cardiovascular disease.
Keywords: impaired glucose tolerance, prevalence, risk factors, normal fasting plasma glucose, epidemiology
Introduction
Isolated impaired glucose tolerance (i–IGT) is a subtype of prediabetes that manifests as elevated 2-h post- glucose load plasma glucose (2-h PG) levels in individuals who also exhibit normal fasting plasma glucose (FPG) levels. Prediabetic individuals are of interest to clinicians because they have an increased risk of developing type 2 diabetes.1 A nationally representative survey carried out in 2013 showed that the prevalence of diabetes and prediabetes in China was 10.9% and 35.7%, respectively; thus, approximately 200.4 million males and 187.7 million females were estimated to have prediabetes in China.2 Prediabetes increases the risk of hyperglycemia, cardiovascular disease, cognitive impairment, and dementia.3–5 Moreover, young people with i–IGT have a 2-fold to 32-fold higher risk of developing hypertension and metabolic syndrome.6
Previous studies have shown the estimated prevalence of i–IGT to be between 6.2% and 9.3% worldwide.7–9 However, in rural areas of Korea, the i–IGT prevalence was 3.7% for adults ≥20 years old.10 By comparison, a nationally representative survey conducted in China in 2010 demonstrated that the prevalence of i–IGT was 11.0% in males and 10.9% in females,11 with a higher prevalence among rural residents than among urban ones.2 However, this study lacks information on i–IGT prevalence among low-income adults in China. Numerous studies have shown that the conventional risk factors, which include older age, family history of diabetes, hypertension, obesity, and hypertriglyceridemia, are also associated with i–IGT development.6,12–14 Although 2-h PG levels rise sharply with advancing age,15 few studies have reported the factors associated with i–IGT development among low-income, poorly educated populations, and particularly the elderly. In China, more than 50% of the population resides in rural areas; detecting the prevalence and associated factors of i–IGT in this sub-population is crucial in reducing the burden of diabetes in China. Thus, this study aimed to explore the prevalence and risk factors of i–IGT among adults ≥50 years old in a low-income, poorly educated population in rural China.
Materials and Methods
Study Population
This population-based, cross-sectional study was performed between April and June 2019. The project was designed as previously described.16 Briefly, all participants were recruited from the Atherosclerosis Cohort of Tianjin Brain Study, which was conducted in 2014. All residents aged 45 years were qualified to be recruited from 18 administrative villages in Yangjinzhuang Town, Jizhou District, Tianjin, China. Residents aged <45 years were not included in this study because many of them were working outside Tianjin. In 2019, we recruited all the survivors of the Atherosclerosis Cohort aged >50 years to detect the prevalence and risk factors of i–IGT; we excluded those with cancer, severe psychiatric disturbances, hepatic failure, and serious renal disease. The educational attainment and income of the residents in this area were relatively low. The average number of education years was 4.75 years in 1991 and 5.26 years in 2011. The per capita disposable income was less than 1600 US dollars in 2014.17
The present study was approved by the Ethics Committee of Tianjin Medical University General Hospital, and a written consent form was provided by each participant. This study was conducted in accordance with the Declaration of Helsinki.
Data Collection
A standard questionnaire was used to collect information regarding educational attainment, personal and family income, previous medical history, and lifestyle risk factors. The participants were interviewed face-to-face by professional researchers. Demographic information, such as sex and date of birth, was obtained from existing records.
Lifestyle risk factors included cigarette smoking, alcohol consumption, physical exercise, sleep duration, and snoring. Cigarette smoking was defined as smoking at least 1 cigarette/day for ≥1 year; participants were categorized as non-smokers, former smokers (those who had stopped smoking for ≥6 months), and current smokers. Alcohol consumption was defined as drinking at least 500 g of alcohol/week for ≥1 year; participants were categorized as non-drinkers, former drinkers (temperance for ≥6 months), and current drinkers. Physical exercise was defined as participation in a moderate or vigorous activity for ≥30 min/day on at least 3 days each week. Sleep durations and snoring habits were based on self-reports.
Measurements
Anthropometric measurements, including height, weight, waist circumference, blood pressure (BP), and heart rate, were performed by epidemiological researchers. Height and weight assessments were conducted with the participants wearing light clothing without hats or shoes; both measurements were determined using an appropriately prepared ultrasonic instrument with the participant in a fully vertical position. Waist circumference was measured using a non-stretch ruler along the horizontal plane at the midpoint between the top of the iliac crest and the bottom of the costal margin in the midaxillary line. Systolic (SBP) and diastolic (DBP) BPs were obtained using a sphygmomanometer. After 5 minutes of quiet rest, participants were asked to expose their arms to facilitate BP and heart rate measurements; the measurements were repeated 5 min later, and the average was reported.
Participants were required to follow a 10-h overnight fast before undergoing oral glucose tolerance tests (OGTTs). They were also required to provide venous blood samples for the determination of levels of FPG, total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), high-sensitivity C-reactive protein (hs-CRP), serum uric acid (SUA), white blood cells (WBCs), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin (TBIL). Then, 75-g glucose (for 75-g OGTT) was administered to the participants, none of whom had a medical history of diabetes. Additional blood samples were obtained 120 min after the first glucose administration to measure the 2-h PG levels. All blood samples were immediately delivered to Guangzhou KingMed Diagnostics Group, an independent clinical laboratory, for analysis.
Definitions
Body mass index (BMI) was calculated as the participant’s body mass (kg) divided by the height squared (m2). According to the BMI, participants were categorized as normal weight (<24.0 kg/m2), overweight (24.0–27.9 kg/m2), or obese (≥28 kg/m2).18 Central obesity was defined as a waist circumference >90 cm for men and >85 cm for women.19 Hypertension was defined as a SBP ≥140 mmHg, DBP ≥90 mmHg, or taking antihypertension medications.
Normal glucose tolerance was defined as an FPG level <5.6 mmol/L and a 2-h PG level <7.8 mmol/L. The 3 subtypes of prediabetes were defined as follows: isolated impaired fasting glucose (i–IFG; 5.6 mmol/L ≤ FPG < 7.0 mmol/L and 2-h PG < 7.8 mmol/L), i–IGT (FPG < 5.6 mmol/L and 7.8 mmol/L ≤ 2-h PG < 11.1 mmol/L), and combined IFG and IGT (IFG + IGT; 5.6 mmol/L ≤ FPG < 7.0 mmol/L and 7.8 mmol/L ≤ 2- h PG < 11.1 mmol/L).11 Diabetes was defined as a FPG level ≥7.0 mmol/L, a 2-h PG level ≥11.1 mmol/L, a previous history of diagnosed diabetes, or use of antihyperglycemia drugs.20
Statistical Analyses
Continuous variables (age, years of formal education, BMI, SBP, DBP, heart rate, WBC counts, and levels of FPG, 2-h PG, hs-CRP, SUA, TC, TG, HDL-C, LDL-C, ALT, AST, and TBIL) are presented as means with standard deviations; between-group comparisons were made using Student’s t-tests. Categorical variables (age group, education group, income group, smoking status, alcohol consumption status, number of participants engaged in physical activity, and hypertensive status) are presented as numbers with frequencies, and between-group comparisons were made using chi-squared tests. Binary logistic regression analyses were used to evaluate the associated factors in the univariate and multivariate analyses. The relationships are presented as unadjusted odds ratios (ORs) and 95% confidence intervals (CIs) in the univariate analysis and adjusted OR (95% CI) in the multivariate analysis. Two-tailed P-values <0.05 were considered statistically significant. SPSS for Windows (version 22.0; IBM, Armonk, NY, USA) was used for all statistical analyses.
Results
A total of 3130 individuals were recruited into the study. Four hundred eighty-three did not receive OGTTs due to a history of diabetes or other health reasons, and 472 had FPG levels >6.1 mmol/L. Thus, 2175 individuals were included in the final analysis.
Demographic and Clinical Characteristics
The 2175 included participants [1172 (53.9%) females] had a mean age of 64.46 years and a mean of 5.53 years of formal education; more than half of the participants had annual incomes of <2000 yuan. The mean BMI (25.45 kg/m2), SBP (149.03 mmHg), DBP (84.66 mmHg), and heart rate (72.81 bpm) were determined. Further, 74.6% of the participants had hypertension, and 64.6% were overweight or obese. The mean FPG and 2-h PG levels were 5.26 mmol/L and 6.75 mmol/L, respectively; SUA, ALT, and AST levels were 298.08 µmmol/L, 18.08 U/L, and 20.83 U/L, respectively (Table 1).
 |
Table 1 Demographic and Clinical Characteristics of All Participants |
I-IGT Prevalence by Demographic Characteristics
Table 2 shows the prevalence of i–IGT by demographic characteristics of the participants in this study. The overall prevalence of i–IGT was 22.9%, although it was significantly higher in females (24.7%) than in males (20.8%; P=0.034). The prevalence of i–IGT increased with increasing participant age (P<0.001), and there was a higher prevalence of i–IGT among the less-educated (P<0.001) and lower-income (P=0.026) participants.
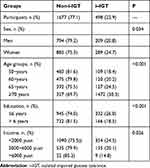 |
Table 2 The Prevalence of Isolated Impaired Glucose by Demographic Characteristics |
Factors Associated with I-IGT in the Univariate Analysis
Female sex, old age, low education level, and low income were associated with i–IGT development (all, P<0.05; Table 3). Participants with hypertension and obesity were also more likely to develop i–IGT (both, P<0.001). Further, individuals were more likely to develop i–IGT if they were current drinkers and underwent physical exercise (both, P<0.05).
 |
Table 3 The Associated Factors of I–IGT for All Participants in the Univariate Analysis |
Participants with i–IGT had higher heart rates, WBC counts, and levels of hs-CRP, SUA, and ALT than those without i–IGT (all, P<0.05). Furthermore, the levels of TC, TG, and LDL-C were significantly higher among participants with i–IGT than among those without i–IGT, but there was an inverse trend for HDL-C levels (all, P<0.05).
Multivariate Analysis of the Factors Associated with I-IGT
In the multivariate analysis, older age, hypertension, central obesity, high WBC counts, and elevated levels of hs-CRP, SUA, TG, and ALT were independent factors associated with i–IGT among adults ≥50 years old. When using the ≤60-year-old age group as a reference group, the prevalence of i–IGT was 60.6% higher among individuals who were ≥70 years old (OR, 1.606; 95% CI, 1.101–2.342; P=0.014). Participants with hypertension had 55.4% higher prevalence of i–IGT than those with normal BP (OR, 1.554; 95% CI, 1.152–2.095; P=0.004). The prevalence of i–IGT was also 39.5% higher among individuals with central obesity compared to individuals without central obesity (OR, 1.395; 95% CI, 1.099–1.771; P=0.006). The prevalence of i–IGT increased with each 1 unit increase in the WBC count (8.9%; OR, 1.089; 95% CI, 1.009–1.175; P=0.029), hs-CRP level (4.9%; OR, 1.049; 95% CI, 1.020–1.078; P=0.001), SUA level (0.3%; OR, 1.0003; 95% CI, 1.001–1.004; P=0.001), TG level (54%; OR, 1.540; 95% CI, 1.105–2.147; P=0.011), and ALT level (1.2%; OR, 1.012; 95% CI, 1.004–1.021; P=0.004) (Table 4).
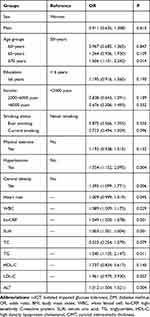 |
Table 4 The Associated Factors of I–IGT in the Multivariate Analysis |
Discussion
In this population-based, cross-sectional study, we explored the i–IGT prevalence and associated factors among adults ≥50 years old in a low-income population in rural China. The overall prevalence of i–IGT was 22.9%, and it was significantly higher among females than males and in low-income, poorly educated individuals. Old age, hypertension, central obesity, and elevated levels of WBCs, hs-CRP, SUA, TG, and ALT were associated with i–IGT.
Due to population growth, ageing, and dietary transitions, China has witnessed a sharp increase in the prevalence of abnormal glucose regulation.21,22 In the current study population, the overall prevalence of i–IGT was 22.9% and much higher than that reported in other studies. For example, in southern Germany, 35–59‐year‐old individuals were reported to have an i–IGT prevalence of 6.3%.9 Further, a national, cross-sectional survey conducted in Spain demonstrated an i–IGT prevalence of 9.2% for individuals ≥18 years old.7 Consistent with this, a cross-sectional study performed in Shanghai Pudong New District showed that the i–IGT prevalence was 9.2% for urban residents of China.14 In Hubei Province, central China, the prevalence of i–IGT was estimated to be 13.9% among Han ≥40 years old.23 Thus, in this low-income, low education level population, the i–IGT prevalence was much higher than that in urban China. Differences in age, income, and education level of the present study’s target population, as well as study design differences, may partly explain the high prevalence of i–IGT in our study population compared with the other study populations. However, the high i–IGT prevalence also indicates that OGTTs are essential for the early identification of individuals with abnormal glucose regulation in the target population.
Previous studies have revealed that older age is associated with an increased risk of diabetes.7,11,24 For example, a 12-year, prospective study with participants aged 40–69 years showed that older age was independently associated with progression to diabetes (hazard ratio, 1.02; 95% CI, 1.02–1.03).25 Furthermore, the Tehran Lipid and Glucose Study indicated that advanced age (≥40 years old) was an important predictor of i–IGT in adults with normal FPG levels.12 In the present low-income population, we concluded that older age was an independent risk factor of i–IGT among adults ≥50 years old. Insulin secretion decreases by approximately 0.7% per year as people age; however, this decline in β-cell function is accelerated approximately 2-fold in people with IGT,26 partly explaining the effect of age on plasma glucose levels.
A positive association between hypertension and diabetes has also been reported in numerous studies.27–29 In a meta-analysis of prospective studies involving 4.1 million adults, each 20 mmHg-elevation in SBP and 10 mmHg-elevation in DBP increased the risk of new-onset diabetes by 58% and 52%, respectively, when compared with normal BPs.30 A population-based survey conducted in Beijing demonstrated that 14–28-year-old individuals with hypertension had a 368% higher risk of i–IGT than those with normal BP (<130/85 mmHg).6 Similarly, hypertension was an independent risk factor of i–IGT in the target population of the present study. Diabetes and hypertension are closely related because of their similar risk factors, such as endothelial dysfunction, vascular inflammation, advanced glycation end-products, and oxidative stress.31,32
In the present survey, we concluded that central obesity was an independent risk factor of i–IGT in the target population. Many studies have shown that central obesity is positively associated with diabetes.33–35 A rural cohort study conducted in the Henan Province of China indicated that central obesity increased the risk of diabetes by 138% (OR, 2.38; 95% CI, 2.11–2.70).36 Further, a population-based, cross-sectional study of adults with normal BMIs in the Jilin Province of China showed a significant association between central obesity and diabetes.37 Resistin is an adipocyte-derived cytokine that causes insulin resistance and glucose intolerance in mice.38 Increased resistin expression in abdominal fat compared with thigh fat may explain the increased risk of i–IGT associated with central obesity.39
Type 2 diabetes presents as a form of inflammatory disease.40 Previously, a prospective study in China revealed a U-shaped association between WBC counts and the incidence of diabetes in adults between 18 and 59 years old.41 However, other studies indicated that WBC counts are an independent risk factor for the development of diabetes even when the counts are within the normal range.42,43 Moreover, a 6‐year follow‐up study indicated that elevated WBC counts are associated with impaired fasting glycemia risk in Chinese adults.44 The increase in WBC counts and the activation of WBCs play a key role in diabetes-induced organ damage and increase in free fatty acid levels caused by microcirculation dysfunction.45,46 In addition, in patients with type 2 diabetes and coronary heart disease, higher WBC counts are predictors of atrial fibrillation, all-cause death, and hospitalization for acute myocardial infarction.47 In the present study, we demonstrated that elevated WBC counts are an independent risk factor of i–IGT. The elevated WBC counts in patients with i–IGT further suggest a role for chronic inflammation in the progression of i–IGT.
Hs-CRP, produced by the liver, is a nonspecific marker of acute phase, systemic inflammation, and previous studies have shown an association between hs-CRP levels and diabetes.48,49 For example, in the Jackson Heart Study, participants with hs-CRP levels in the third tertile demonstrated a higher risk of type 2 diabetes when compared with those in the first tertile of hs-CRP levels (HR 2.07; 95% CI, 1.67–2.56).50 The present study shows that an elevated hs-CRP level is an independent risk factor of i–IGT in the target population. Because hs-CRP levels predict changes in insulin secretion and insulin sensitivity,51 hs-CRP levels may also predict i–IGT development.
SUA is a purine metabolite that can cause hyperuricemia, gout, and renal failure, and high levels of SUA are associated with an increased risk of IGT and diabetes.52,53 A prospective study in Shanghai has revealed that SUA levels are strongly associated with the incidence of diabetes in middle-aged and elderly adults.54 Moreover, the Beijing Health Management Cohort has shown that persistent hyperuricemia results in a 75% increase in the risk of diabetes (risk ratio, 1.75; 95% CI, 1.47–2.08).55 Similarly, we found that SUA levels represent an independent i–IGT risk factor in our study population. High SUA levels can cause inflammation and oxidative stress,56 which can partially mediate insulin resistance,57 leading to i–IGT and diabetes.
Previous studies have shown that TG levels are also associated with a higher risk of diabetes in Chinese adults.11,58,59 Furthermore, a study performed in Beijing demonstrated that hypertriglyceridemia increases the risk of i–IGT by 192% compared with normal TG levels (OR, 2.92; 95% CI, 1.27–6.73; P=0.012).6 In the present study’s target population, we found that the prevalence of i–IGT increased by 54% for each 1 mmol/L-increase in TG levels. Hence, strict lipid management appears to be essential for this population.
ALT, found in a variety of cells, is most abundant in hepatocytes. Previous studies have revealed that ALT levels are positively associated with diabetes risk.60,61 Further, a prospective study in China showed that ALT levels are positively associated with the incidence of diabetes (hazard ratio, 1.12; 95% CI, 1.02–1.22) after adjusting for nonalcoholic fatty liver disease and other covariates.62 In the present study, we concluded that high ALT levels are an independent risk factor of i–IGT. High ALT levels decrease hepatic insulin sensitivity and predict the incidence of type 2 diabetes,63 possibly explaining the link between ALT levels and i–IGT development.
This study had several limitations. First, the nature of cross-sectional studies prevents the identification of causal relationships. Second, this study involved only people living in the rural areas of Tianjin, China; therefore, our findings cannot be generalized to other populations. Third, since we did not measure insulin levels, we could not evaluate insulin resistance or insulin secretion. Fourth, residents aged <50 years were excluded from this study because there were large proportions of them living outside the area due to work. This may impact the assessment of i–IGT status among younger individuals. Our future studies will evaluate this. Finally, our reliance on self-reported sleep durations may have influenced the accurate assessment of any association between sleep duration and i–IGT.
Conclusion
In this cross-sectional study conducted in rural China, the overall prevalence of i–IGT was 22.9% among adults ≥50 years old; the i–IGT prevalence was significantly higher among females and individuals with low incomes and low levels of education. The conventional risk factors, including older age, hypertension, central obesity, and elevated TG and SUA levels, were independently associated with a high risk of i–IGT development. The results suggest that promoting health education and standardizing the management of body weight, BP, lipid levels, and uric acid levels is essential for this low-income population in rural China.
Abbreviations
i-IGT, isolated impaired glucose tolerance; OR, odds ratio; CI, confidence interval; 2-h PG, 2-h plasma glucose; FPG, fasting plasma glucose; BPs, blood pressures; SBP, systolic blood pressure; DBP, diastolic blood pressure; OGTTs, oral glucose tolerance tests; TC, total cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein; SUA, serum uric acid; WBCs, white blood cells; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBIL, total bilirubin; BMI, body mass index.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding authors on reasonable request.
Ethics Approval and Consent to Participate
The study was approved by the medical research ethics committee at Tianjin Medical University General Hospital; written informed consent was obtained from each participant during recruitment.
Acknowledgments
We thank all participants of the Tianjin Brain Study, and local medical care professionals for their valuable contributions.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declare no competing financial interests and no conflicts of interest for this work.
References
1. Morris DH, Khunti K, Achana F, et al. Progression rates from HbA1c 6.0-6.4% and other prediabetes definitions to type 2 diabetes: a meta-analysis. Diabetologia. 2013;56(7):1489–1493. doi:10.1007/s00125-013-2902-4
2. Wang L, Gao P, Zhang M, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA. 2017;317(24):2515–2523. doi:10.1001/jama.2017.7596
3. Rao Kondapally Seshasai S, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364(9):829–841.
4. Huang Y, Cai X, Mai W, Li M, Hu Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta-analysis. BMJ. 2016;355:i5953. doi:10.1136/bmj.i5953
5. Xue M, Xu W, Ou YN, et al. Diabetes mellitus and risks of cognitive impairment and dementia: a systematic review and meta-analysis of 144 prospective studies. Ageing Res Rev. 2019;55:100944. doi:10.1016/j.arr.2019.100944
6. Li Y, Feng D, Esangbedo IC, et al. Insulin resistance, beta-cell function, adipokine profiles and cardiometabolic risk factors among Chinese youth with isolated impaired fasting glucose versus impaired glucose tolerance: the BCAMS study. BMJ Open Diabetes Res Care. 2020;8(1):e000724. doi:10.1136/bmjdrc-2019-000724
7. Soriguer F, Goday A, Bosch-Comas A, et al. Prevalence of diabetes mellitus and impaired glucose regulation in Spain: the [email protected]. Study Diabetologia. 2012;55(1):88–93. doi:10.1007/s00125-011-2336-9
8. Wilmot EG, Edwardson CL, Biddle SJ, et al. Prevalence of diabetes and impaired glucose metabolism in younger ‘at risk’ UK adults: insights from the STAND programme of research. Diabet Med. 2013;30(6):671–675. doi:10.1111/dme.12173
9. Meisinger C, Strassburger K, Heier M, et al. Prevalence of undiagnosed diabetes and impaired glucose regulation in 35–59-year-old individuals in Southern Germany: the KORA F4 Study. Diabet Med. 2010;27(3):360–362. doi:10.1111/j.1464-5491.2009.02905.x
10. Lee JE, Jung SC, Jung GH, et al. Prevalence of diabetes mellitus and prediabetes in dalseong-gun, Daegu City, Korea. Diabetes Metab J. 2011;35(3):255–263. doi:10.4093/dmj.2011.35.3.255
11. Yang W, Lu J, Weng J, et al. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362(12):1090–1101. doi:10.1056/NEJMoa0908292
12. Rambod M, Hosseinpanah F, Ardakani EM, Padyab M, Azizi F. Fine-tuning of prediction of isolated impaired glucose tolerance: a quantitative clinical prediction model. Diabetes Res Clin Pract. 2009;83(1):61–68. doi:10.1016/j.diabres.2008.09.040
13. Zhang F, Wan Q, Cao H, et al. Identical anthropometric characteristics of impaired fasting glucose combined with impaired glucose tolerance and newly diagnosed type 2 diabetes: anthropometric indicators to predict hyperglycaemia in a community-based prospective cohort study in southwest China. BMJ Open. 2018;8(5):e019735. doi:10.1136/bmjopen-2017-019735
14. Qian Q, Li X, Huang X, et al. Glucose metabolism among residents in Shanghai: natural outcome of a 5-year follow-up study. J Endocrinol Invest. 2012;35(5):453–458. doi:10.3275/7854
15. Metter EJ, Windham BG, Maggio M, et al. Glucose and insulin measurements from the oral glucose tolerance test and mortality prediction. Diabetes Care. 2008;31(5):1026–1030. doi:10.2337/dc07-2102
16. Qi D, Liu J, Wang C, et al. Sex-specific differences in the prevalence of and risk factors for hyperuricemia among a low-income population in China: a cross-sectional study. Postgrad Med. 2020;132(6):559–567. doi:10.1080/00325481.2020.1761133
17. Center CNHDR. China National Health Accounts Report 2018. 2019.
18. Zhou BF. Effect of body mass index on all-cause mortality and incidence of cardiovascular diseases–report for meta-analysis of prospective studies open optimal cut-off points of body mass index in Chinese adults. Biomed Environ Sci. 2002;15(3):245–252.
19. Tian Y, Jiang C, Wang M, et al. BMI, leisure-time physical activity, and physical fitness in adults in China: results from a series of national surveys, 2000-14. Lancet Diabetes Endocrinol. 2016;4(6):487–497. doi:10.1016/S2213-8587(16)00081-4
20. Chinese Diabetes Society. Guidelines for the prevention and treatment of type 2 diabetes in China (2017 Edition). Chin J Diabetes. 2018;10(1):4–67. doi:10.3760/cma.j.issn.1674-5809.2014.07.004
21. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387(10027):1513–1530. doi:10.1016/S0140-6736(16)00618-8
22. He Y, Li Y, Yang X, et al. The dietary transition and its association with cardiometabolic mortality among Chinese adults, 1982–2012: a cross-sectional population-based study. Lancet Diabetes Endocrinol. 2019;7(7):540–548. doi:10.1016/S2213-8587(19)30152-4
23. Hu X, Zhang Q, Zeng TS, et al. Not performing an OGTT results in underdiagnosis, inadequate risk assessment and probable cost increases of (pre)diabetes in Han Chinese over 40 years: a population-based prospective cohort study. Endocr Connect. 2018;7(12):1507–1517. doi:10.1530/EC-18-0372
24. Liu L, Zhou C, Du H, et al. The prevalences of impaired fasting glucose and diabetes mellitus in working age men of North China: Anshan Worker Health Survey. Sci Rep. 2014;4:4835. doi:10.1038/srep04835
25. Han SJ, Kim HJ, Kim DJ, Lee KW, Cho NH. Incidence and predictors of type 2 diabetes among Koreans: a 12-year follow up of the Korean Genome and Epidemiology Study. Diabetes Res Clin Pract. 2017;123:173–180. doi:10.1016/j.diabres.2016.10.004
26. Szoke E, Shrayyef MZ, Messing S, et al. Effect of aging on glucose homeostasis: accelerated deterioration of beta-cell function in individuals with impaired glucose tolerance. Diabetes Care. 2008;31(3):539–543. doi:10.2337/dc07-1443
27. Tsimihodimos V, Gonzalez-Villalpando C, Meigs JB, Ferrannini E. Hypertension and Diabetes Mellitus: coprediction and Time Trajectories. Hypertension. 2018;71(3):422–428. doi:10.1161/HYPERTENSIONAHA.117.10546
28. Tatsumi Y, Ohkubo T. Hypertension with diabetes mellitus: significance from an epidemiological perspective for Japanese. Hypertens Res. 2017;40(9):795–806. doi:10.1038/hr.2017.67
29. Kim MJ, Lim NK, Choi SJ, Park HY. Hypertension is an independent risk factor for type 2 diabetes: the Korean genome and epidemiology study. Hypertens Res. 2015;38(11):783–789. doi:10.1038/hr.2015.72
30. Emdin CA, Anderson SG, Woodward M, Rahimi K. Usual blood pressure and risk of new-onset diabetes: evidence from 4.1 million adults and a meta-analysis of prospective studies. J Am Coll Cardiol. 2015;66(14):1552–1562. doi:10.1016/j.jacc.2015.07.059
31. Libianto R, Batu D, MacIsaac RJ, Cooper ME, Ekinci EI. Pathophysiological links between diabetes and blood pressure. Can J Cardiol. 2018;34(5):585–594. doi:10.1016/j.cjca.2018.01.010
32. Petrie JR, Guzik TJ, Touyz RM. Diabetes, Hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol. 2018;34(5):575–584. doi:10.1016/j.cjca.2017.12.005
33. Feng Y, Yang Y, Ma X, et al. Prevalence of diabetes among Han, Manchu and Korean ethnicities in the Mudanjiang area of China: a cross-sectional survey. BMC Public Health. 2012;12:23. doi:10.1186/1471-2458-12-23
34. Ohnishi H, Saitoh S, Takagi S, et al. Incidence of type 2 diabetes in individuals with central obesity in a rural Japanese population: the Tanno and Sobetsu study. Diabetes Care. 2006;29(5):1128–1129. doi:10.2337/dc06-0222
35. Hartaigh B, Jiang CQ, Bosch JA, et al. Independent and combined associations of abdominal obesity and seated resting heart rate with type 2 diabetes among older Chinese: the Guangzhou Biobank Cohort Study. Diabetes Metab Res Rev. 2011;27(3):298–306. doi:10.1002/dmrr.1178
36. Abdulai T, Li Y, Zhang H, et al. Prevalence of impaired fasting glucose, type 2 diabetes and associated risk factors in undiagnosed Chinese rural population: the Henan Rural Cohort Study. BMJ Open. 2019;9(8):e029628. doi:10.1136/bmjopen-2019-029628
37. Zhang P, Wang R, Gao C, et al. Prevalence of central obesity among adults with normal BMI and its association with metabolic diseases in Northeast China. PLoS One. 2016;11(7):e0160402. doi:10.1371/journal.pone.0160402
38. Steppan CM, Bailey ST, Bhat S, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409(6818):307–312. doi:10.1038/35053000
39. McTernan CL, McTernan PG, Harte AL, Levick PL, Barnett AH, Kumar S. Resistin, central obesity, and type 2 diabetes. Lancet. 2002;359(9300):46–47. doi:10.1016/S0140-6736(02)07281-1
40. Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11(2):98–107. doi:10.1038/nri2925
41. Du X, Zhu B, Hu G, et al. U-shape association between white blood cell count and the risk of diabetes in young Chinese adults. Diabet Med. 2009;26(10):955–960. doi:10.1111/j.1464-5491.2009.02801.x
42. Twig G, Afek A, Shamiss A, et al. White blood cells count and incidence of type 2 diabetes in young men. Diabetes Care. 2013;36(2):276–282. doi:10.2337/dc11-2298
43. Zhang H, Yang Z, Zhang W, et al. White blood cell subtypes and risk of type 2 diabetes. J Diabetes Complications. 2017;31(1):31–37. doi:10.1016/j.jdiacomp.2016.10.029
44. Zang X, Meng X, Wang Y, et al. Six-year follow-up study on the association between white blood cell count and fasting blood glucose level in Chinese adults: a community-based health examination survey. Diabetes Metab Res Rev. 2019;35(4):e3125. doi:10.1002/dmrr.3125
45. Azekoshi Y, Yasu T, Watanabe S, et al. Free fatty acid causes leukocyte activation and resultant endothelial dysfunction through enhanced angiotensin II production in mononuclear and polymorphonuclear cells. Hypertension. 2010;56(1):136–142. doi:10.1161/HYPERTENSIONAHA.110.153056
46. Wolf D, Ley K. Immunity and Inflammation in Atherosclerosis. Circ Res. 2019;124(2):315–327. doi:10.1161/CIRCRESAHA.118.313591
47. Kawabe A, Yasu T, Morimoto T, et al. WBC count predicts heart failure in diabetes and coronary artery disease patients: a retrospective cohort study. ESC Heart Fail. 2021. doi:10.1002/ehf2.13513
48. Wang C, Yatsuya H, Tamakoshi K, et al. Positive association between high-sensitivity C-reactive protein and incidence of type 2 diabetes mellitus in Japanese workers: 6-year follow-up. Diabetes Metab Res Rev. 2013;29(5):398–405. doi:10.1002/dmrr.2406
49. Parrinello CM, Lutsey PL, Ballantyne CM, Folsom AR, Pankow JS, Selvin E. Six-year change in high-sensitivity C-reactive protein and risk of diabetes, cardiovascular disease, and mortality. Am Heart J. 2015;170(2):380–389. doi:10.1016/j.ahj.2015.04.017
50. Effoe VS, Correa A, Chen H, Lacy ME, Bertoni AG. High-Sensitivity C-Reactive protein is associated with incident type 2 diabetes among African Americans: the Jackson heart study. Diabetes Care. 2015;38(9):1694–1700. doi:10.2337/dc15-0221
51. Fizelova M, Jauhiainen R, Kangas AJ, et al. Differential associations of inflammatory markers with insulin sensitivity and secretion: the prospective METSIM Study. J Clin Endocrinol Metab. 2017;102(9):3600–3609. doi:10.1210/jc.2017-01057
52. Juraschek SP, McAdams-Demarco M, Miller ER, et al. Temporal relationship between uric acid concentration and risk of diabetes in a community-based study population. Am J Epidemiol. 2014;179(6):684–691. doi:10.1093/aje/kwt320
53. Bombelli M, Quarti-Trevano F, Tadic M, et al. Uric acid and risk of new-onset metabolic syndrome, impaired fasting glucose and diabetes mellitus in a general Italian population: data from the Pressioni Arteriose Monitorate E Loro Associazioni study. J Hypertens. 2018;36(7):1492–1498. doi:10.1097/HJH.0000000000001721
54. Wang T, Bi Y, Xu M, et al. Serum uric acid associates with the incidence of type 2 diabetes in a prospective cohort of middle-aged and elderly Chinese. Endocrine. 2011;40(1):109–116. doi:10.1007/s12020-011-9449-2
55. Liu J, Tao L, Zhao Z, et al. Two-year changes in hyperuricemia and risk of diabetes: a five-year prospective cohort study. J Diabetes Res. 2018;2018:6905720. doi:10.1155/2018/6905720
56. Sautin YY, Nakagawa T, Zharikov S, Johnson RJ. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol. 2007;293(2):C584–596. doi:10.1152/ajpcell.00600.2006
57. Furukawa S, Fujita T, Shimabukuro M, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114(12):1752–1761. doi:10.1172/JCI21625
58. Lin D, Qi Y, Huang C, et al. Associations of lipid parameters with insulin resistance and diabetes: a population-based study. Clin Nutr. 2018;37(4):1423–1429. doi:10.1016/j.clnu.2017.06.018
59. Guerrero-Romero F, Rodríguez-Moran M. Hypertriglyceridemia is associated with development of metabolic glucose disorders, irrespective of glucose and insulin levels: a 15-year follow-up study. Eur J Intern Med. 2014;25(3):265–269. doi:10.1016/j.ejim.2014.01.015
60. Wang YL, Koh WP, Yuan JM, Pan A. Association between liver enzymes and incident type 2 diabetes in Singapore Chinese men and women. BMJ Open Diabetes Res Care. 2016;4(1):e000296. doi:10.1136/bmjdrc-2016-000296
61. De Silva N, Borges MC, Hingorani AD, et al. Liver function and risk of type 2 diabetes: bidirectional Mendelian randomization study. Diabetes. 2019;68(8):1681–1691.
62. Shen X, Cai J, Gao J, et al. Nonalcoholic fatty liver disease and risk of diabetes: a prospective study in China. Endocr Pract. 2018;24(9):823–832. doi:10.4158/EP-2018-0098
63. Vozarova B, Stefan N, Lindsay RS, et al. High alanine aminotransferase is associated with decreased hepatic insulin sensitivity and predicts the development of type 2 diabetes. Diabetes. 2002;51(6):1889–1895. doi:10.2337/diabetes.51.6.1889
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
