Back to Journals » Infection and Drug Resistance » Volume 12
Epidemiology Of Human Pulmonary Infection With Nontuberculous Mycobacteria In Southeast China: A Prospective Surveillance Study
Authors Lin S, Wei S , Zhao Y, Lin J, Pang Y
Received 19 July 2019
Accepted for publication 30 October 2019
Published 12 November 2019 Volume 2019:12 Pages 3515—3521
DOI https://doi.org/10.2147/IDR.S223828
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Shufang Lin,1,2 Shuzhen Wei,1,2 Yong Zhao,1,2 Jian Lin,1,2 Yu Pang3
1Institute of Tuberculosis Control and Prevention, Fujian Center for Disease Control and Prevention, Fuzhou, People’s Republic of China; 2Fujian Provincial Key Laboratory of Zoonosis Research, Fujian Center for Disease Control and Prevention, Fuzhou, People’s Republic of China; 3National Clinical Laboratory on Tuberculosis, Beijing Key Laboratory for Drug-Resistant Tuberculosis Research, Beijing Chest Hospital, Capital Medical University, Beijing Tuberculosis and Thoracic Tumor Institute, Beijing, People’s Republic of China
Correspondence: Shufang Lin
Institute of Tuberculosis Control and Prevention, Fujian Center for Disease Control and Prevention, No. 76, Jintai Road, Gulou District, Fuzhou, Fujian 350001, People’s Republic of China
Tel/Fax +86 591 8343 1464
Email [email protected]
Yu Pang
Beijing Chest Hospital, Capital Medical University, No. 97, Machang, Tongzhou District, Beijing 101149, People’s Republic of China
Tel/Fax +86 10 8950 9359
Email [email protected]
Background: China is facing a great challenge of pulmonary nontuberculous mycobacteria (NTM) infections. This primary objective of this study was to assess the prevalence of NTM isolates among patients with presumptive TB in Fujian.
Methods: The mycobacterial isolates were collected from the tuberculosis survey from Fujian Province conducted between July 1, 2010 and June 30, 2011.
Results: From July 1, 2010 to June 30, 2011, 1425 isolates were included in the final analysis, of which 60 (4.2%) were identified as NTM species. M. intracellulare was the most frequently isolated NTM in Fujian, accounting for 68.3% of all NTM isolates. Compared with patients aged <45 years, patients aged 45–59 were more likely to have NTM infections. The education level of patients had an impact on the distribution of NTM infections. Illiterate patients had significantly higher odds of having NTM compared to literate patients. Patients with a previous TB episode had higher NTM risk as compared to those without previous TB episodes.
Conclusion: In conclusion, the predominant NTM is M. intracellulare among patients with presumptive TB in Fujian. In addition, elderly patients, those with a previous TB episode and illiterate patients have higher NTM risk.
Keywords: nontuberculous mycobacteria, prevalence, risk factor
Introduction
Although tuberculosis (MTB) remains a major public health concern worldwide,1 the increasing incidence of nontuberculous mycobacteria (NTM) calls for considerably more attention for environmental opportunistic pathogens.2 Evidence collected over the past decades has confirmed that the NTM are normal inhabitants of a variety of environmental habits, including soil and water sources.3,4 The living habit overlap between NTM and humans, therefore, becomes a major determinant in the acquisition of the disease. NTM can affect multiple organs of the human body, whereas they primarily affect the lungs.5 Pulmonary diseases due to NTM infections frequently occur in individuals with chronic airway diseases, relative immunodeficiency and advanced age.6
Unlike tuberculosis, the evidence of a person-to-person transmission of NTM is still controversial.7,8 These environmental microorganisms are more likely to be transmitted to humans from nonbiological exposures.7 As a consequence, the geographic diversity of NTM species in the environmental niche has a profound impact on the NTM prevalence of pulmonary NTM diseases.2,9 Previous reports have demonstrated that Mycobacterium avium complex (MAC) was the most frequently isolated species in Australia, Southern Africa, South America and Eastern Asian,2,10 whilst M. kansasii was the most frequently encountered species in Europe.2 The changing frequency of NTM species and their different susceptibility profiles make it difficult to formulate appropriate treatment without the results of species identification and drug susceptibility testing. Therefore, there is an urgent need to investigate the distributions of NTM species by regions, which will provide important hints for the clinical management of patients affected by NTM.
Although great progress has been achieved in TB control, China is facing a great challenge of pulmonary NTM infections.9,11 Recent national population-based data indicate that the prevalence of NTM among all mycobacterial isolates has increased from 11.1% to 22.9%.12 Notably, NTM prevalence varies greatly across China, and eastern and southern regions are considered as the “hotspots” of this disease compared with elsewhere, reflecting the potential more suitable environment for NTM habitation in these regions.9 However, a few studies have reported the prevalence of NTM among these NTM-endemic settings,13––15 which are limited in scope and reflect the prevalence of NTM only in one particular state or hospital. To address this concern, we carried out this study on the basis of the strains collected from a tuberculosis survey from Fujian Province, located in the coastal region of southeast China. This primary objective of this study was to assess the prevalence of NTM isolates among patients with presumptive TB in Fujian. We also aimed to identify factors associated with the isolation of NTM.
Methods
Ethics
The survey protocol was approved by the Ethical Committee of the Fujian Province Center for Disease Control (CDC). All human subjects were adults. Each enrolled patient provided signed informed consent forms prior to study enrolment.
Sampling Method
The Fujian CDC was responsible for the implementation of this survey. The cluster-randomized sampling method endorsed by the World Health Organization was used to calculate the sample size of smear-positive patients with presumptive TB.16 It was estimated that the required sample size of new smear-positive patients was 40 and that of previously treated smear-positive patients was 20. Thirty counties were randomly selected from the overall 80 counties. The smear-positive patients were consecutively recruited in this study. Each enrolled patient was interviewed by a survey administrator using the questionnaire, which included demographic and clinical characteristics.
Laboratory Method
Three sputum samples (night, spot and morning) were collected from each patient for smear microscopy, and two sputum samples were used for mycobacterial culture. The simple method was conducted to yield culture results according to a previous report.17 Briefly, the specimens were digested with an equal quantity of 4% NaOH for 15 mins. Then, 0.1 mL of the decontaminated specimen was inoculated into the acidic Löwenstein–Jensen (L–J) medium. The culture was observed weekly to record the growth of mycobacteria and continued for 8 weeks before they can be identified as negative. Positive cultures were sent to the Fujian Province TB Reference Laboratory to perform conventional drug susceptibility testing and bacterial species identification.
The proportional method on the L–J medium was used to determine the drug susceptibility of positive cultures as previously reported.16 The concentration of each drug tested was as follows: 0.2 μg/mL for isoniazid, 40 μg/mL for rifampin, 4 μg/mL for streptomycin, 2 μg/mL for ethambutol, 30 μg/mL for kanamycin, and 2 μg/mL for ofloxacin. In addition, mycobacterium species identification was conducted by growth test on a medium containing 500 mg/mL of p-nitrobenzoic acid (PNB) and 5 mg/mL of 2-thiophenecarboxylic acid hydrazide (TCH), respectively.
The NTM isolates identified by the conventional method were further identified in species level with multiple target sequencing, including 16S rRNA, hsp65, rpoB, and 16S-23S rRNA internal transcribed spacer (ITS) sequence.12 The DNA sequences were aligned with the homologous sequences of the reference mycobacteria strains via multiple sequence alignments.
Statistical Analysis
All collected data were entered using Epi Data 3.02 software (EpiData Association, Odense, Denmark). Two operators entered the data separately to ensure accuracy. Chi-square test was used for comparing the prevalence of NTM among different regions. Factors associated with NTM infections were analysed with univariate and multivariable logistic regression models. Multivariable models were built by using forward stepwise logistic regression procedures (with inclusion if P<0.1). All statistical analysis was performed using SPSS 17.0 software (SPSS Inc, Chicago, USA). Differences were declared as significant if the P value was less than 0.05.
Definitions
The definition of NTM infection met the criteria established by the American Thoracic Society (ATS) in 2007,18 including chest radiograph and clinical symptoms suggestive of pulmonary mycobacterial diseases, and isolation of the same NTM species from two different sputum specimens. The new pulmonary TB patient is defined as a patient who has never received TB drugs or has received them for less than 1 month. The previously treated pulmonary TB patient is defined as a patient who has received TB drugs for more than 1 month.16 Multidrug-resistant tuberculosis was defined as tuberculosis with resistance to both isoniazid and rifampin. Extensively drug-resistant tuberculosis was defined as MDR-TB resistant with additional resistance to ofloxacin and kanamycin.
Results
Between July 1, 2010 and June 30, 2011, 1,579 smear-positive TB patients were enrolled, including 1,223 new cases and 356 previously treated patients. Of these, 36 (2.3%) were excluded for culture contamination, 61 (3.9%) were culture-negative, 30 (1.9%) were subculture failure, and 27 were missing information in the questionnaire. Using the conventional biochemical method, 60 (4.2%, 60/1425) isolates were primarily identified as NTM species, and the other 1365 (95.8%, 1365/1425) belonged to MTBC.
Incidence And Proportion Of Drug-Resistant TB Cases
In this survey, the proportion of new and previously treated TB patients resistant to at least one of the first-line drugs were 16.8% and 34.3%, respectively. SM resistance was the most frequent drug resistance for new cases (10.1%), whereas the highest proportion was noted in RIF resistance for previously treated cases (24.2%). With regard to MDR-TB, 3.2% of new TB patients and 15.9% of previously treated TB patients were affected by MDR-TB, respectively (Table 1). In addition, among those with MDR-TB, only one previously treated case had XDR-TB (0.4%).
 |
Table 1 Resistance To First- And Second-Line Antituberculosis Drugs |
Prevalence Of Nontuberculous Mycobacteria
Among 1425 smear-positive cases, 60 (4.2%) were infected with NTM. Molecular species identification revealed that 60 isolates belonged to six different NTM species. M. intracellulare was the most frequently isolated NTM in Fujian, accounting for 68.3% (41/60) of all NTM isolates. As the second prevalent species, 6 isolates (6/60, 10.0%) were identified as M. abscessus, followed by M. avium (4/60, 6.7%), M. kansasii (4/60, 6.7%), M. massiliense (3/60, 5.0%) and M. fortuitum (2/60, 3.3%) (Figure 1).
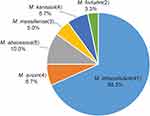 |
Figure 1 Distribution of NTM species in Fujian Province. |
Risk Factors Associated With NTM Infections
Tables 2 and 3 summarize patient characteristics of pulmonary NTM patients compared with those of pulmonary TB. The distribution of NTM differed among different age groups. When setting patients aged <45 years as the control group, patients aged 45–59 [adjusted odds ratio (AOR): 6.16, 95% confidence interval (CI): 2.66–14.30] and ≥60 years (AOR: 4.20, 95% CI: 1.68–10.52) were more likely to have NTM infections. In addition, the education level of patients had an impact on the distribution of NTM infections. Illiterate patients had significantly higher odds of having NTM compared to literate patients (AOR: 1.93, 95% CI: 1.09–3.42). Patients with a previous TB episode had higher NTM risk as compared to those without previous TB episodes (AOR: 3.03, 95% CI: 1.78–5.16). In contrast, we found that several surveyed factors had no influence on the distribution of NTM infection, including sex, ethnicity, job, and resident area (P>0.05).
 |
Table 2 General Characteristics Of Patients Infected With MTB And NTM Enrolled In This Study |
 |
Table 3 Multivariate Analysis Of Risk Factors For NTM Infections In Fujian |
Discussion
Despite halving in the prevalence of tuberculosis over the past decades in China, the emerging epidemic of drug-resistant tuberculosis remains a major threat to the control of tuberculosis in this country.16,19 In this study, we ascertained that 3.2% of new TB patients were affected by MDR-TB, which is comparable to those of surrounding eastern regions, including Zhejiang (4.4%) and Shanghai (3.9%), while are lower than the national level (5.7%) and those of northern (8.6% in Jilin) and western regions (7.3% in Inner Mongolia).20 The low prevalence of MDR-TB reflects the effective intervention strategies for timely detection and proper management of MDR-TB patients in Fujian. On one hand, since 2007, the scenarios start from the scale-up of in vitro drug susceptibility testing (DST) in the prefectures of Fujian, which makes the individual DST more universally accessible. The early diagnosis of drug-resistant TB, especially MDR-TB, is essential to reduce the delay of initiation of appropriate treatment for individuals infected with MDR-TB, thereby preventing transmission of MDR-TB due to reduced duration of infectivity of index patients in the community. On the other hand, TB is curable in almost all non-MDR-TB patients if treated adequately.21 Directly observed therapy (DOT) ensures that the patient is treated with the correct dose of medication within a complete treatment course.22 This strategy was implemented by the CDC system through its local public health clinics in 2001 in Fujian. The sufficient financial support from the local government improves the quality of patient management. Given that the implementation of the DOT strategy plays an important role in the prevention of drug resistance, we hypothesize that the low prevalence of MDR-TB may be contributed to the successful management of active TB cases in Fujian.
A recent nationwide study revealed that the prevalence of NTM was 22.9% among all mycobacterial isolates in China.19 In contrast, only 4.2% of smear-positive patients were affected by NTM in Fujian. Similar results are reported by Shao and colleagues, which demonstrated that the overall proportion of NTM isolates from whole specimens was 3.4% in another province of Eastern China.15 There are several plausible explanations for the obvious difference in the proportion of NTM isolates among the latter studies. First, the nationwide study included all people aged 15 years or older and a local resident in the survey to assess the TB prevalence in China. Although the abnormal chest radiograph was used as the primary criterion for the selection of individuals suspected of having TB, it is difficult to distinguish between colonizers and causative pathogens for NTM isolates,23 which may serve as an important explanation for the increased prevalence of NTM isolates. Second, the operators collected the sputum specimens from individuals with abnormal chest radiograph intensively in the former survey, which could result in laboratory technicians becoming overloaded. Poor quality of mycobacterial culture may result from inadequate laboratory staffing, thus leading to a high proportion of NTM contamination. Consistent with our hypothesis, a high rate of NTM isolates from the national survey belonged to hypovirulent NTM species rather than the frequently observed virulent NTM species (data not shown).Taken together, our data indicate that the prevalence of NTM infections may be overestimated due to the inclusion of potential contamination and colonization. Further epidemiological study on NTM diseases is urgently required to confirm the prevalence of NTM disease in China.
In agreement with a recent cross-section study from China, the predominant NTM of Fujian was M. intracellulare, accounting for 68.3% of the NTM isolates tested.9 Besides, M. intracellulare was the most frequently isolated strain in South Africa and Australia.2 In contrast, another member of Mycobacterium avium complex, M. avium, was the dominant strain in North and South America.2 On the one hand, the geographic diversity in the prevalence of NTM may reflect the NTM species distribution in the local environment across regions. On the other hand, M. avium is an opportunistic infection typically associated with advanced immune suppression, such as AIDS.24 Considering that China has a low-level HIV epidemic, M. intracellulare exceeds M. avium as the predominant causative agent of pulmonary NTM disease.
There is strong evidence that pulmonary NTM infection more frequently affects elderly patients.25,26 Similarly, our results demonstrated that the patients aged ≥65 years are at high risk of NTM infections in this population. The age‐associated decreased immunity against mycobacteria infection in elderly persons is considered as the most important explanation for this observation.27 Alternatively, we hypothesize that the advanced age among elderly patients may increase environmental exposure to NTM, thereby resulting in a high incidence of NTM infections. In addition, a systematic meta-analysis by Simons et al found that pulmonary NTM disease in Asia had a relatively high percentage of patients with a history of TB.10 In this study, we found that patients with a previous TB episode had higher NTM risk. This observation may reflect that these individuals may be associated with a higher susceptibility to mycobacterial infection. Further study will be necessary to address the shared mechanisms of the immune response against mycobacteria infection across mycobacteria species. Notably, another risk factor associated with NTM infections was illiterate patients. As an explanation for the relatively high incidence of NTM among this population, it could be speculated that illiterate patients are more prone to experiencing poor economic status, which could negatively influence their nutritional status, and impair the immune responses against challenges with mycobacteria.28 Hence, a relatively higher number of NTM isolates were identified among illiterate patients. Additionally, it could reflect a higher frequency of exposure to environmental NTM, including nonsterile water and contaminated food. Management of NTM disease should, therefore, promote education toward patients at high risk to modify the environment and personal habits to reduce environmental exposure to NTM.
There are several obvious limitations to our study. First, the survey was conducted 7 years ago. The prevalence of NTM might be changed in the past decade. Second, despite the enrolment of a large number of smear-positive patients, the small sample size of NTM may limit the overall significance of our study conclusion. Third, the prevalence of NTM varies widely by geographic area depending on endemicity of TB and environment promoting the growth of NTM. Hence, our conclusion is not generalized outside the study area. Fourth, M. intracellulare was identified as the most predominant NTM species in Fujian, while the exact reason for this preponderance remains unknown at this stage. Fifth, although increasing evidence has demonstrated the diagnostic performance of molecular tests to distinguish between MTB and NTM, this study did not collect information on this aspect because of limited laboratory capability in Fujian. Finally, because of being outside the scope of the National Tuberculosis Programme, the clinical outcomes of these patients with NTM were not collected in this study.
In conclusion, our data demonstrate that the prevalence of NTM is 4.2% among patients with presumptive TB in Fujian, and the predominant NTM is M. intracellulare. In addition, elderly patients, those with a previous TB episode and illiterate patients have higher NTM risk. Further management of NTM disease should, therefore, promote education toward patients at high risk to modify the environment and personal habits to reduce environmental exposure to NTM.
Acknowledgments
The study was supported by the Health Department of Fujian Province, the National Natural Science Foundation of China (81361138019) and Swedish Research Council (540-2013-8797). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We are grateful to the contributions of all of the participants who participated in this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. Global Tuberculosis Report 2018. 2018.
2. Hoefsloot W, van Ingen J, Andrejak C, et al. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: an NTM-NET collaborative study. Eur Respir J. 2013;42(6):1604–1613. doi:10.1183/09031936.00149212
3. Aksamit TR, Philley JV, Griffith DE. Nontuberculous mycobacterial (NTM) lung disease: the top ten essentials. Respir Med. 2014;108(3):417–425. doi:10.1016/j.rmed.2013.09.014
4. Falkinham JO
5. Johnson MM, Odell JA. Nontuberculous mycobacterial pulmonary infections. J Thorac Dis. 2014;6(3):210–220. doi:10.3978/j.issn.2072-1439.2013.12.24
6. Cassidy PM, Hedberg K, Saulson A, McNelly E, Winthrop KL. Nontuberculous mycobacterial disease prevalence and risk factors: a changing epidemiology. Clin Infect Dis. 2009;49(12):e124–129. doi:10.1086/599195
7. Raju RM, Raju SM, Zhao Y, Rubin EJ. Leveraging advances in tuberculosis diagnosis and treatment to address nontuberculous mycobacterial disease. Emerg Infect Dis. 2016;22(3):365–369. doi:10.3201/eid2203.151643
8. Bryant JM, Grogono DM, Greaves D, et al. Whole-genome sequencing to identify transmission of Mycobacterium abscessus between patients with cystic fibrosis: a retrospective cohort study. Lancet. 2013;381(9877):1551–1560. doi:10.1016/S0140-6736(13)60632-7
9. Pang Y, Tan Y, Chen J, et al. Diversity of nontuberculous mycobacteria in eastern and southern China: a cross-sectional study. Eur Respir J. 2017;49(3). doi:10.1183/13993003.01429-2016
10. Simons S, van Ingen J, Hsueh PR, et al. Nontuberculous mycobacteria in respiratory tract infections, eastern Asia. Emerg Infect Dis. 2011;17(3):343–349. doi:10.3201/eid170310060
11. Pang Y, Zheng H, Tan Y, Song Y, Zhao Y. In vitro activity of bedaquiline against nontuberculous mycobacteria in China. Antimicrob Agents Chemother. 2017;61(5). doi:10.1128/AAC.02627-16
12. Zhang Z, Pang Y, Wang Y, Cohen C, Zhao Y, Liu C. Differences in risk factors and drug susceptibility between Mycobacterium avium and Mycobacterium intracellulare lung diseases in China. Int J Antimicrob Agents. 2015;45(5):491–495. doi:10.1016/j.ijantimicag.2015.01.012
13. Jing H, Wang H, Wang Y, et al. Prevalence of nontuberculous mycobacteria infection, China, 2004-2009. Emerg Infect Dis. 2012;18(3):527–528. doi:10.3201/eid1803.110175
14. Wu J, Zhang Y, Li J, et al. Increase in nontuberculous mycobacteria isolated in Shanghai, China: results from a population-based study. PLoS One. 2014;9(10):e109736. doi:10.1371/journal.pone.0109736
15. Shao Y, Chen C, Song H, et al. The epidemiology and geographic distribution of nontuberculous mycobacteria clinical isolates from sputum samples in the eastern region of China. PLoS Negl Trop Dis. 2015;9(3):e0003623. doi:10.1371/journal.pntd.0003623
16. Zhao Y, Xu S, Wang L, et al. National survey of drug-resistant tuberculosis in China. N Engl J Med. 2012;366(23):2161–2170. doi:10.1056/NEJMoa1108789
17. Pang Y, Xia H, Zhang Z, et al. Multicenter evaluation of genechip for detection of multidrug-resistant Mycobacterium tuberculosis. J Clin Microbiol. 2013;51(6):1707–1713. doi:10.1128/JCM.03436-12
18. Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416. doi:10.1164/rccm.200604-571ST
19. Wang L, Zhang H, Ruan Y, et al. Tuberculosis prevalence in China, 1990–2010; a longitudinal analysis of national survey data. Lancet. 2014;383(9934):2057–2064. doi:10.1016/S0140-6736(13)62639-2
20. Yang X, Yuan Y, Pang Y, et al. The burden of MDR/XDR tuberculosis in coastal plains population of China. PLoS One. 2015;10(2):e0117361. doi:10.1371/journal.pone.0117361
21. Kumaresan J, Smith I, Arnold V, Evans P. The global TB drug facility: innovative global procurement. Int J Tuberc Lung Dis. 2004;8(1):130–138.
22. Volmink J, Matchaba P, Garner P. Directly observed therapy and treatment adherence. Lancet. 2000;355(9212):1345–1350. doi:10.1016/S0140-6736(00)02124-3
23. Chien JY, Lai CC, Sheng WH, Yu CJ, Hsueh PR. Pulmonary infection and colonization with nontuberculous mycobacteria, Taiwan, 2000-2012. Emerg Infect Dis. 2014;20(8):1382–1385. doi:10.3201/eid2008.131673
24. Nardi G, Drago L, De Vecchi E, Rosina M, Gismondo MR. Gastrointestinal and respiratory Mycobacterium avium colonization and development of disseminated infection in HIV-positive patients. Clin Microbiol Infect. 1999;5(3):176–177. doi:10.1111/j.1469-0691.1999.tb00534.x
25. Tortoli E. Clinical manifestations of nontuberculous mycobacteria infections. Clin Microbiol Infect. 2009;15(10):906–910. doi:10.1111/j.1469-0691.2009.03014.x
26. Tan Y, Su B, Shu W, et al. Epidemiology of pulmonary disease due to nontuberculous mycobacteria in Southern China, 2013–2016. BMC Pulm Med. 2018;18(1):168. doi:10.1186/s12890-018-0728-z
27. Solana R, Pawelec G, Tarazona R. Aging and innate immunity. Immunity. 2006;24(5):491–494. doi:10.1016/j.immuni.2006.05.003
28. Chandra RK. Nutrition and the immune system from birth to old age. Eur J Clin Nutr. 2002;56(Suppl 3):S73–76. doi:10.1038/sj.ejcn.1601492
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
