Back to Journals » Infection and Drug Resistance » Volume 16
Epidemiology and Risk Factors of Community-Associated Bloodstream Infections in Zhejiang Province, China, 2017–2020
Authors An R , Ou Y , Pang L, Yuan Y , Li Q, Xu H, Sheng B
Received 3 December 2022
Accepted for publication 4 March 2023
Published 18 March 2023 Volume 2023:16 Pages 1579—1590
DOI https://doi.org/10.2147/IDR.S400108
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Rongcheng An,1 Yingwei Ou,1 Lingxiao Pang,1 Yongsheng Yuan,1 Qian Li,1 Hao Xu,2 Bin Sheng1
1Emergency and Critical Care Center, Department of Emergency Medicine, Zhejiang Provincial People’s Hospital (Affiliated People’s Hospital, Hangzhou Medical College), Hangzhou, People’s Republic of China; 2Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, the First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, People’s Republic of China
Correspondence: Bin Sheng, Emergency and Critical Care Center, Department of Emergency Medicine, Zhejiang Provincial People’s Hospital (Affiliated People’s Hospital, Hangzhou Medical College), Hangzhou, People’s Republic of China, Tel +86 571 85893793, Email [email protected]
Purpose: Community-associated bloodstream infection (CA-BSI) is increasing in many community settings. However, the clinical significance and epidemiology of CA-BSI present in hospital admissions in China are not well established. In this work, we identified the risk factors in outpatients presenting with CA-BSI, and investigate the role of procalcitonin (PCT) and hypersensitive C-reactive protein (CRP) in diagnosing different types of the pathogen in patients with acute CA-BSI.
Methods: A retrospective study enrolling 219 outpatients with CA-BSI from The Zhejiang People’s Hospital from January 2017 to December 2020 was performed. Susceptibility of the isolates obtained from these patients was examined. Subjecting receiver operating characteristic curves (ROC) were constructed to analyze the specificity and sensitivity of PCT, CRP, and WBC in determining infections caused by different bacterial genera. Risk factors for CA-BSI in the emergency setting were analyzed using essential information and simple identification of other pathogenic bacterial species through rapidly tested biomarkers.
Results: A total of 219 patients were included in the selection criteria, of which 103 were infected with Gram-positive bacteria (G+) and 116 with Gram-negative bacteria (G-). The PCT was significantly higher in the GN-BSI group than in the GP-BSI group, while no significant difference was observed between the two groups for CRP. Subjecting ROC curves were constructed to analyze WBC, CRP, and PCT, and the area under the curve (AUC) of the PCT in this model was 0.6661, with sensitivity = 0.798 and specificity = 0.489.
Conclusion: The PCT between the GP-BSI group and the GN-BSI group was significantly different. By combining the knowledge of clinicians and the clinical signs of patients, PCT should be utilized as a supplementary approach to initially determine pathogens and direct medication in the early stages of clinical practice.
Keywords: community-associated bloodstream infections, PCT, gram-negative, gram-positive, diagnostic predictions
Introduction
Bloodstream infection (BSI) is usually caused by an endogenous or exogenous pathogen invading the host’s bloodstream system, stimulating a systemic inflammatory response, and causing bloodstream infection. A local inflammatory response can lead to further infection and cause systemic inflammatory response syndrome (SIRS), sepsis, septic shock and even death. Community acquired bloodstream infection (CA-BSI) is a type of bloodstream infection that is acquired within 48 hours of the patient’s admission from the community. Typical diseases acquired in the community are pneumonia, abdominal infection, urinary tract infection, meningitis, infective endocarditis and skin and soft tissue infection. Although the mortality rate from sepsis has improved in the last decade, it is still higher than 15%.1–3 The emergency department (ED), as the primary department in the hospital receiving patients with acute and critical illnesses, needs to quickly identify and first precisely treat patients presenting with acute fever and suspected BSIs.4,5 It is also necessary to rapidly assess the condition through several laboratory tests or scoring scales and to make clinical decisions.6 In the past, much attention has been paid to the focal issues of timely diagnosis, risk assessment and prognostic evaluation of sepsis. Various relevant disease scores are also used to assess or predict mortality in patients with sepsis in EDs and acute intensive care units.
When a patient presents to the ED with an acute fever, there is usually an inflammatory response. In contrast, infection or sepsis can occur when patients present with severe infections. In previous guidelines for the management of sepsis, it has been suggested that some nonspecific biomarkers may be necessary to establish sepsis’s prognosis.7 Usually, blood culture is the gold standard for diagnosing bloodstream infections. Although blood cultures take time to grow and test in the clinic, they are still the best way to identify bacteria in the blood, as they are less likely to be contaminated or produce false positives.8 Therefore, it does not provide quick guidance in treating fever or severe infections in patients who present to the ED.9,10
Leukocytes (WBC) and their sorting cells, as traditional anti-inflammatory cells involved in the inflammatory response, have long been used to assess a patient’s infection status. Procalcitonin (PCT), a precursor of calcitonin, has likewise been shown to be an important reference marker for infection.11 Hypersensitive C-reactive protein (CRP) is an acute-phase reactive protein that rapidly reflects the severity of inflammation. In previous studies, CRP and PCT concentrations have been correlated with the prognosis of patients with sepsis.12 In a mate analysis, PCT was found to be more effective in diagnosing bacterial infection than CRP and IL-6.13 These indicators can be monitored to treat the patient’s infection properly.
We noticed that there were a few studies have demonstrated the epidemiology and risk factors of CA-BSI in China.14–17 We therefore retrospectively evaluated the risk factors among ED patients in 2017–2020 at Zhejiang Provincial People’s Hospital. Our work provides a simple prediction of the pathogenic genus of patients with suspected CA-BSI by using some simple tests in EDs so that the appropriate antibiotics can be selected for rapid and accurate treatment of the patient in the EDs.
Methods
Case Definition and Enrollment
In this retrospective clinical study, we recorded data on all patients with emergency fever and blood cultures through the Department of Emergency Medicine, Zhejiang Provincial People’s Hospital from January 2017 to December 2020. Inclusion criteria: 1. age > 16 years; 2. fever ≥ 37.3°C; 3. blood cultures on both sides; 4. meeting diagnostic criteria for sepsis. Exclusion criteria: 1. previous haematological disorders, autoimmune diseases, or tumour-related diseases; 2. treatment on anti-infective drugs before emergency admission; 3. emergency transfer patients. The research flow chart of this study is shown in Figure 1.
 |
Figure 1 The flow chart for the selection of data for the study is as follows. |
The study was approved by the Institutional Review Board/Independent Ethics Committee of Zhejiang Provincial People’s Hospital, and all data are anonymized without the need to sign informed consent. Patient privacy and data confidentiality are maintained following the Declaration of Helsinki.
Data Collection
The data included in this item are age, gender, underlying illness, days in the hospital, days with fever, season of stay, blood culture results, WBC count, CRP, and PCT. The blood culture results are those taken at the first emergency visit of the emergency patient; the complete blood count, CRP, and PCT results are those taken before the first visit of the emergency patient without antibiotics and those taken on the second and third day after the emergency hospitalization.
Statistical Analyses
Statistical analyses were performed using SPSS version 26.0 (SPSS, Chicago, IL, USA). Continuous variables were expressed as mean (standard deviation) or median (interquartile range) expressions and analyzed comparatively by t-test or Wilcoxon test. Categorical variables were expressed as frequencies and percentages and analysed using the chi-square test or Fisher’s exact test. The Loess method was utilized to create a fitting curve, which illustrated the varying pattern of inflammatory indicators. Relevant variables were subjected to subject operating characteristic curve analysis to determine the early predictive value of Gram-negative bloodstream infections; all tests had both conditions, and p < 0.05 was considered statistically significant. A statistical comparison of AUCs was conducted using DeLong’s test.
Patient and Public Involvement
Patients or the public were not involved in our research’s design, conduct, reporting, or dissemination plans.
Results
The Basic Profile of All Febrile Patients and Positive Blood Cultures
The data from this study on positive blood cultures for CA-BSI are listed in Table 1. A total of 219 patients with positive blood cultures were enrolled, including 103 patients with Gram-positive bloodstream infections and 116 patients with Gram-negative bloodstream infections. As can be observed in Table 1, there was no statistical difference between the age and gender of the patients infected with Gram-negative and Gram-positive bacteria. The mean age of onset for all patients with bloodstream infections was 67.05 years. For CA-BSI, there were no statistically significant differences between seasons, but the distribution of patients was observed to be more frequent in summer and autumn. The mean number of hospital days for all patients was 12 days, with shorter hospital days for GN-BSI compared to the GP-BSI group (p < 0.001), which was statistically significant. The short duration of the fever may be related to the prompt anti-infective and antipyretic treatment after emergency consultation. The number of GN-BSI and GP-BSI was similar in patients with bloodstream infection combined with hypertension and diabetes and was not statistically significant.
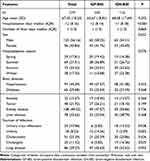 |
Table 1 Basic Profile and Prognosis of Patients with Emergency Fever |
The proportion of patients with combined liver disease and renal disease in the GN-BSI group was not statistically different from that in the GP-BSI group (p = 0.368; p = 0.726; respectively); meanwhile, bloodstream infections leading to shock accounted for 22.37% of patients, with GN-BSI causing significantly more shock than GP-BSI, but not statistically significant. Initial infections in the GN-BSI group were primarily found in urinary tract infections and biliary tract infections (p = 0.038; p = 0.025; respectively.) The GP-BSI group mainly was associated with pulmonary infections and inflammatory gallbladder disease, but there was no statistically significant difference between the two groups (divided into p = 0.093; p = 0.426;). A total of 18 patients developed bloodstream infections in uremia, 15 in the GP-BSI group and 3 in the GN-BSI group, with a statistically significant comparison between the two groups (p = 0.003).
Pathogenic Distribution and Characteristics
Table 2 and Figure 2 show that a total of 219 patients with positive blood cultures were counted in this study, of which 116 were of negative genera, accounting for 52.97% of all positive blood culture patients, and 103 were of positive genera, accounting for 47.03% of all positive blood culture patients. Among the Gram-negative genera, Escherichia coli (57, 49.14%) and Klebsiella pneumoniae (31, 26.72%) were predominant. Gram-positive bacteria were dominated by Staphylococcus aureus (25, 24.27%) and Staphylococcus epidermidis (24, 23.30%). Figure 3 suggests that Enterobacter spp. infections accounted for a high proportion of all blood culture-positive patients (90, 41%), followed by coagulase-negative Staphylococcus and other genera (52, 24%). Gram-negative Enterobacter spp. had a high susceptibility to imipenem (94.03%) and some resistance to quinolones, second and third-generation cephalosporins (19.12–22.86%). Coagulase-negative staphylococci were more common among the gram-positive bacteria, with high susceptibility to vancomycin, linezolid, quinupristin/da, and high resistance to penicillin, oxacillin, erythromycin, ciprofloxacin and levofloxacin (46–93.33%). The next most susceptible genus, S. aureus, had high susceptibility to vancomycin, linezolid, and quinupristin/da while having high resistance to penicillin G and second- and third-generation cephalosporins (Table 3).
 |
Table 2 Distribution and Composition Ratio of Pathogenic Bacteria in Blood Cultures (n = 219) |
 |
Table 3 Resistance of Coagulase-Negative Staphylococci/Enterobacteriaceae to Commonly Used Antimicrobials |
 |
Figure 2 Blood culture distribution of pathogenic bacteria by species. |
 |
Figure 3 Blood culture distribution of Enterobacteriaceae, coagulase-negative Staphylococcus, Staphylococcus aureus, and other pathogens. |
Laboratory Characteristics of Patients with CA-BSI
Table 4 and Table 5 show that in all patients with positive blood cultures and a CRP test taken on the first day, for the GN-BSI group, the CRP values were significantly higher than for the GP-BSI group but not statistically different (p=0.095). In contrast, there was no difference between the GN-BSI group and the GP-BSI group for patients with a WBC test left on the first day (p = 0.097). After these patients were admitted to the emergency department, they were empirically treated with anti-infective therapy, and a total of 122 CRP values were counted on the second day. CRP values were similar between the two groups of patients in this group and were not statistically significantly different (p = 0.814). After the patients were admitted, both groups had identical peak CRP values and were not statistically significant. A total of 166 patients had positive blood cultures, and PCT tests were left on the first day, with the GN-BIS group being significantly higher than patients in the GP-BIS group (p = 0.001). However, in the 77 patients who left for PCT testing on the second day, the results were similar in the GN-BIS and GP-BIS groups and were not statistically significant (p = 0.680). As seen in Figure 4, the inflammatory markers in patients with CA-BSI, whether CRP or PCT, peaked on the second day and gradually decreased on the empirical administration of antibiotics following admission.
 |
Table 4 Comparison of Gram-Negative and Gram-Positive Bacteria CRP and WBC |
 |
Table 5 An Analysis of the PCT Value of Gram-Negative and Gram-Positive Bacteria |
 |
Figure 4 The variation of PCT and CRP over time. |
Multi-Factor Regression Analysis
A multifactorial regression equation was constructed combining gender, age, days in the hospital and inflammatory factors (Table 6). The results showed that men were at a higher risk of developing infections compared to women. While older age was associated with a higher risk of developing bloodstream infections, statistical significance did not emerge. Although gender, days in hospital and age were not statistically significant in the predictiveness of GN-BSI, this may be related to the small sample size of subjects included in this project. In contrast, for inflammatory indicators, the higher the WBC, the higher the risk of Gram-negative bloodstream infection, which was statistically significant (OR = 0.956, 95% CI 0.915–0.998, p < 0.05). The higher the calcitoninogen, the higher the risk of Gram-negative bloodstream infection, which was statistically significant (OR = 1.020, 95% CI 1.006–1.035, p < 0.05). An increased WBC count is not only an indication of Gram-positive bacteria, it could also be a sign of Gram-negative bacteria. It is proposed that a higher PCT value is more indicative of a negative bacterial presence.
 |
Table 6 Multi-Factor Regression Analysis Exploring Predictors of Early Identification of GN-BSI |
ROC Curves of Relevant Indicators and Models as Markers of GN-BSI
The corresponding ROC curves were constructed to further confirm the predictive value of the indicators we explored. As shown in Figure 5 and Table 7, PCT showed a higher ability to identify different pathogen types than CRP and WBC. The PCT had a higher area under the curve (AUC) (0.6661, 95% CI: 0.593–0.739), sensitivity = 0.798 and specificity = 0.489, with p < 0.0001 being statistically significant.
 |
Table 7 Predicted ROC Value for GN-BSI |
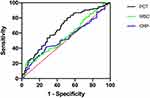 |
Figure 5 The predicted ROC curve for GN-BSI. |
Discussion
Sepsis is still one of the diseases with a high mortality rate worldwide. Previous studies have also suggested that bloodstream infections caused by Gram-negative bacteria are more prone to more severe sepsis or even septic shock than Gram-positive bacteria,18–20 which also leads to a higher mortality rate. Therefore, it is important to quickly identify the source of infection in patients with CA-BSI, determine the type of pathogen, making early diagnosis and early treatment, and thus reduce patient mortality.21
WBC, CRP and PCT are all readily available blood tests, and PCT has good sensitivity for identifying Gram-negative bloodstream infections to some extent. This is an excellent diagnostic and therapeutic aid for patients and clinicians who present to the ED with suspected CA-BSI. Blood cultures are not a quick guide to the condition of patients with bloodstream infections, or sepsis is seen in the ED, but WBC, CRP and PCT can be performed quickly and accurately in the ED to reflect the patient’s inflammatory status.
Our study found that most CA-BSI comes from primary infections in the bloodstream from the lungs, urinary system and gallbladder, where Gram-negative bacteria also predominate. However, most patients with uremic syndrome complicated by bloodstream infections had predominantly positive organisms. These patients are primarily dialysis-related catheter-associated infections, the same as most of the national and international researchers who have mainly studied.22 Most patients with pulmonary diseases associated with bloodstream infections are triggered by the pulmonary route of infection into the bloodstream. Most patients are considered to have predominantly community-acquired pneumonia as their first disease, with K. pneumoniae and Staphylococcus predominating. Most of the patients with CA-BSI had one or even more co-morbidities. Preliminary statistical analysis shows that the average age of patients with CA-BSI is around 67 years, with a predominance of male patients over female patients. Statistically, CA-BSI did not significantly correlate with the season but occurred more often in summer and autumn. Therefore, older men with more previous illnesses are at a higher risk of CA-BSI.
In the EDs, CRP and PCT are the more readily available tests. In this study, we found that CRP showed varying degrees of elevation in both the GN-BSI and GP-BSI groups, while mostly peaking on the second day. However, the comparison between the two groups was not statistically significant due to the small volume of patients and the lack of second-day CRP results in some patients. However, in our study, we found that CRP, either on the first day or at the peak, did not show statistically significant differences between the GN-BSI and GP-BSI groups, which is consistent with the study by Bassetti and Liu HH et al23,24 Additionally, the construction of working characteristic curves for the subjects showed that CRP and WBC did not show significant sensitivity and specificity (p > 0.05). The curves of inflammatory indicators over time could also indicate that the decrease in inflammatory indicators was more pronounced in the patients after they were treated by emergency consultation and prompt selection of the appropriate anti-infective treatment, most of them based on the empirical selection of carbapenem antibiotics that are more sensitive to Gram-negative bacteria.
PCT is used as an important biomarker for infectious diseases and even sepsis, which can be obtained quickly in an emergency and guide treatment. It is also used as an important indicator for the rapid diagnosis of sepsis and even septic shock.25 PCT was first identified in thyroid tumour cell cultures and is a peptide hormone. And in earlier reports, it was also suggested that PCT is altered to varying degrees in infectious and non-infectious diseases.26 The level of PCT in the blood is proportional to the severity of the infection.27 Therefore, PCT is also used as a common test to determine the infectious disease. The value of PCT in the diagnosis of nosocomial GNBSI has long been demonstrated in previous clinical studies of nosocomial bloodstream infections. The use of PCT for the rapid diagnosis of CA-BSI, where the common causative organisms are different from those of nosocomial infections, was also part of this project. The results of this study showed that PCT was diagnostic for GN-BSI in 219 patients collected, with the first day of PCT results in all emergency admissions indicating significantly higher negative bacterial infections compared to positive bacterial infections (p = 0.001), and the construction of subject working characteristic curves indicated an AUC of 0.6661 (95% CI 0.593 to 0.739) with a sensitivity of 0.798 and specificity of 0.489 (p < 0.0001). And it was also shown in some studies that the GN-BSI group had a higher level of PCT than the GP-BSI group, which is consistent with our findings.11,23,24 Gram-negative bacteria stimulate the body to produce an inflammatory response through their endotoxins, which can also induce stimulation of different concentrations of PCT, so that PCT can identify different bacterial types of infection.28 However, the value of PCT as a biomarker for the diagnosis of sepsis or even septic shock alone is still being investigated and remains controversial in some studies.29,30 In certain individuals with impaired liver function, the production of CRP may be disrupted, thus making PCT a more reliable measure for the diagnosis of all-inflammatory infections.31 In sepsis 3.0, PCT is also recommended only as a biomarker for the prognosis of sepsis, not for diagnosis. And in this study, although PCT proved to be of high diagnostic significance in Gram-negative spp., it could not be used as one of the only indicators for the diagnosis of CA-BSI or sepsis and should be considered in conjunction with the patient’s clinical presentation and other scores.
It has also been shown in previous studies that indicators such as platelets and mean platelet volume in routine blood tests can also identify bloodstream infections caused by different pathogenic bacteria. However, Manzoni et al suggested that platelets are not used as a marker for the specific diagnosis of bloodstream infections.32 However, studies in recent years have shown that a stronger inflammatory response can inhibit platelet production and stimulate varying degrees of platelet activation and increased reactivity, thereby causing changes in platelet parameters. In one study, it was shown that the identification of different bacterial types by changes in indicators such as platelets may have some sensitivity and specificity.18 This also provides the theoretical basis for the Centre’s subsequent research.
This is a single-center retrospective study and the results are subject to varying degrees of bias, including data volume, and may require a multi-center, multi-sample prospective study for further confirmation and statistical discovery of additional and more sensitive indicators for the rapid identification of patients with CA-BSI to guide clinical management.
Conclusions
Owing to the limited sample size and single-center nature of this study, the results must be interpreted with caution. PCT, WBC and CRP were significantly altered in all patients with bloodstream infections; PCT was significantly different between the GP-BSI and GN-BSI groups. PCT can be used for early differential diagnosis of community-associated Gram-negative and Gram-positive bloodstream infections with good sensitivity. PCT is an accurate indicator for CA-BSI, yet it is not specific. PCT should be employed as a supplementary tool in clinical practice, in conjunction with the expertise of clinicians and the clinical signs of patients, to make an initial assessment of the pathogenic bacteria and direct the use of medications in the early stages of treatment. Once complete blood culture and drug sensitivity results have been obtained, the treatment plan can be adjusted accordingly.
Ethics Approval
This project is a retrospective study and does not involve the privacy of the subjects. The Ethics Committee of Zhejiang Provincial People’s Hospital has approved this project under the Ethics Approval No. 2021QT369.
Acknowledgments
This work was supported by the Zhejiang Provincial Natural Science Foundation of China (LQ20H200003).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the global burden of disease study. Lancet. 2020;395(10219):200–211. doi:10.1016/S0140-6736(19)32989-7
2. Paoli CJ, Reynolds MA, Sinha M, Gitlin M, Crouser E. Epidemiology and costs of sepsis in the United States—an analysis based on timing of diagnosis and severity level. Crit Care Med. 2018;46(12):1889–1897. PMID: 00003246-201812000-00001.
3. Kaukonen K-M, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA. 2014;311(13):1308.
4. Chen C, Xu H, Liu R, et al. Emergence of neonatal sepsis caused by MCR-9- and NDM-1-co-producing Enterobacter hormaechei in China. Front Cell Infect Microbiol. 2022;12:879409. PMID: 35601097. PMCID: PMC9120612.
5. Zheng B, Chen Y, Violetta L, Xiao Y, Li L. Bloodstream infections caused by Entero-bacteriaceae in China. Lancet Infect Dis. 2019;19(8):810–811. PMID: 31345454.
6. Yan S, Zhang G. Predictive performance of critical illness scores and procalcitonin in sepsis caused by different gram-stain bacteria. Clinics. 2021;76:e2610–e. PMID: 34133658.
7. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017;45(3):486–552. PMID: 00003246-201703000-00015.
8. Zheng B, Yu X, Xu H, et al. Complete genome sequencing and genomic characterization of two Escherichia coli strains co-producing MCR-1 and NDM-1 from bloodstream infection. Sci Rep. 2017;7(1):17885. PMID: 29263349. PMCID: PMC5738369.
9. Vincent J-L, Moreno R. Clinical review: scoring systems in the critically ill. Crit Care. 2010;14(2):207. PMID: 20392287.
10. Minne L, Abu-Hanna A, de Jonge E. Evaluation of SOFA-based models for predicting mortality in the ICU: a systematic review. Crit Care. 2009;12(6):R161–R. PMID: 19091120. doi:10.1186/cc7160
11. Gai L, Yan B-Q. Research on the diagnostic effect of PCT level in serum on patients with sepsis due to different pathogenic causes. Eur Rev Med Pharmacol Sci. 2018;22:4238–4242.
12. Cui N, Zhang H, Chen Z, Yu Z. Prognostic significance of PCT and CRP evaluation for adult ICU patients with sepsis and septic shock: retrospective analysis of 59 cases. J Int Medl Res. 2019;47(4):1573–1579.
13. Wu CW, Wu JY, Chen CK, et al. Does procalcitonin, C-reactive protein, or interleukin-6 test have a role in the diagnosis of severe infection in patients with febrile neutropenia? A systematic review and meta-analysis. Support Care Cancer. 2015;23(10):2863–2872. PMID: 25701436.
14. Wang X, Liu Q, Zhang H, et al. Molecular characteristics of community-associated staphylococcus aureus isolates from pediatric patients with bloodstream infections between 2012 and 2017 in Shanghai, China. Front Microbiol. 2018;9:1211. PMID: 29928269. PMCID: PMC5997952.
15. Hu Q, Cheng H, Yuan W, et al. Panton-Valentine leukocidin (PVL)-positive health care-associated methicillin-resistant Staphylococcus aureus isolates are associated with skin and soft tissue infections and colonized mainly by infective PVL-encoding bacteriophages. J Clin Microbiol. 2015;53(1):67–72. PMID: 25339405. PMCID: PMC4290966.
16. Wang LJ, Du XQ, Nyirimigabo E, Shou ST. Concurrent infectious mononucleosis and community-associated methicillin-resistant Staphylococcus aureus bacteremia. Am J Emerg Med. 2014;32(4):393e5–6. PMID: 24268847.
17. Zheng B, Xu H, Yu X, et al. Low prevalence of MCR-1-producing Klebsiella pneumoniae in bloodstream infections in China. Clin Microbiol Infect. 2018;24(2):205–206. PMID: 28811245.
18. Gao Q, Li Z, Mo X, Wu Y, Zhou H, Peng J. Combined procalcitonin and hemogram parameters contribute to early differential diagnosis of gram-negative/gram-positive bloodstream infections. J Clin Lab Anal. 2021;6:e23927. PMID: 34363413.
19. Abe R, Oda S, Sadahiro T, et al. Gram-negative bacteremia induces greater magnitude of inflammatory response than gram-positive bacteremia. Crit Care. 2010;14(2):R27. PMID: 20202204. PMCID: PMC2887127.
20. Xu XJ, Luo ZB, Xia T, et al. Comparison of interleukin-6, interleukin-10, procalcitonin and C-reactive protein in identifying high-risk febrile illness in pediatric cancer patients: a prospective observational study. Cytokine. 2019;116:1–6. PMID: 30684912.
21. Vanker N, Ipp H. The use of the full blood count and differential parameters to assess immune activation levels in asymptomatic, untreated HIV infection. S Afr Med J. 2013;104(1):45–48. PMID: 24388088.
22. Murea M, James KM, Russell GB, et al. Risk of catheter-related bloodstream infection in elderly patients on hemodialysis. Clin J Am Soc Nephrol. 2014;9(4):764–770. PMID: 24651074. PMCID: PMC3974362.
23. Liu HH, Zhang MW, Guo JB, Li J, Su L. Procalcitonin and C-reactive protein in early diagnosis of sepsis caused by either gram-negative or gram-positive bacteria. Ir J Med Sci. 2017;186(1):207–212. PMID: 27139197.
24. Bassetti M, Russo A, Righi E, et al. Role of procalcitonin in predicting etiology in bacteremic patients: report from a large single-center experience. J Infect Public Health. 2020;13(1):40–45. PMID: 31248812.
25. Uzzan B, Cohen R, Nicolas P, Cucherat M, Perret GY. Procalcitonin as a diagnostic test for sepsis in critically ill adults and after surgery or trauma: a systematic review and meta-analysis. Crit Care Med. 2006;34(7):1996–2003. PMID: 16715031.
26. Cohn B. Can procalcitonin differentiate sepsis from systemic inflammatory response syndrome without infection? Ann Emerg Med. 2014;63(5):631–632. PMID: 24439716.
27. Zhang Y, La M, Sun J, et al. Diagnostic value and prognostic significance of procalcitonin combined with C-reactive protein in patients with bacterial bloodstream infection. Comput Math Methods Med. 2022;2022:6989229. PMID: 35991149. PMCID: PMC9388258.
28. Surbatovic M, Popovic N, Vojvodic D, et al. Cytokine profile in severe Gram-positive and Gram-negative abdominal sepsis. Sci Rep. 2015;5:11355. PMID: 26079127. PMCID: PMC4468818.
29. Jekarl DW, Lee S, Kim M, Kim Y, Woo SH, Lee WJ. Procalcitonin as a prognostic marker for sepsis based on SEPSIS-3. J Clin Lab Anal. 2019;33(9):e22996. PMID: 31420921. PMCID: PMC6868407.
30. Liu Y, Hou JH, Li Q, Chen KJ, Wang SN, Wang JM. Biomarkers for diagnosis of sepsis in patients with systemic inflammatory response syndrome: a systematic review and meta-analysis. Springerplus. 2016;5(1):2091. PMID: 28028489. PMCID: PMC5153391.
31. Lin KH, Wang FL, Wu MS, et al. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection in patients with liver cirrhosis: a systematic review and meta-analysis. Diagn Microbiol Infect Dis. 2014;80(1):72–78. PubMed PMID: 24974271.
32. Guida JD, Kunig AM, Leef KH, McKenzie SE, Paul DA. Platelet count and sepsis in very low birth weight neonates: is there an organism-specific response? Pediatrics. 2003;111(6 Pt 1):1411–1415. PMID: 12777561.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
