Back to Journals » Clinical Optometry » Volume 11
Epidemiology and molecular diagnosis of acute conjunctivitis in patients attending Hamadan, west Iran ophthalmology clinics 2016–2017
Authors Johari Moghadam MM, Mohamad Yari M , Azizi Jalilian F, Amini R, Bazzazi N
Received 10 June 2019
Accepted for publication 29 August 2019
Published 15 October 2019 Volume 2019:11 Pages 105—111
DOI https://doi.org/10.2147/OPTO.S217722
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Mr Simon Berry
Mohamad Mehdi Johari Moghadam,1 Milad Mohamad Yari,2 Farid Azizi Jalilian,3 Razieh Amini,4 Nooshin Bazzazi5
1Student Research Committee, Hamadan University of Medical Sciences, Hamadan, Iran; 2Student Research Committee, Ilam University of Medical Sciences, Ilam, Iran; 3Department of Virology, Faculty of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran; 4Department of Molecular Medicineand Genetics, Faculty of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran; 5Department of Ophthalmology, Faculty of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran
Correspondence: Farid Azizi Jalilian
Department of Virology, Faculty of Medicine, Hamadan University of Medical Sciences, Shahid Fahmideh Street, Hamadan 6517838678, Iran
Tel +98 813 838 0462
Fax +98 813 838 0462
Email [email protected]
Razieh Amini
Department of Molecular Medicine and Genetics, Faculty of Medicine, Hamadan University of Medical Sciences, Shahid Fahmideh Street, Hamadan 6517838678, Iran
Tel +98 813 838 0572
Fax +98 813 838 0208
Email [email protected]
Background: Viruses are considered the most common cause of infectious conjunctivitis. PCR has been approved as the best standard method to diagnose viral conjunctivitis. This study was conducted to investigate epidemiological patterns of conjunctivitis in Hamadan, west Iran. In addition, the frequency of the most important cause of infectious conjunctivitis diagnosed by PCR and its seasonal variations and association with certain socioeconomic and health factors were studied.
Methods: In this cross-sectional study, 125 patients with suspected viral conjunctivitis or keratoconjunctivitis in Hamadan, west Iran from July 2016 to June 2017 were examined for the presence of herpes simplex virus 1 (HSV1), HSV2, varicella-zoster virus (VZV), adenovirus. and Chlamydia trachomatis using multiplex real-time PCR.
Results: Adenoviruses were the most prevalent pathogens (94.4%). HSV1 was found in two (1.6%) patients. HSV2, VZV, and C. trachomatis were not seen in any patients. There was no difference in acquisition of conjunctivitis between men and women. A total of 55 (44%) patients attended the clinics in summer.
Conclusion: This study demonstrated that adenoviruses were a much more common viral cause of conjunctivitis in the studied region compared to findings in other regions. In addition, the acquisition rate of eye infection is expected to decrease dramatically in this region through control of adenoviruses. Demographic variables ie, age, sex, and income level, were not significantly associated with acquisition of viral infection.
Keywords: epidemiology, conjunctivitis, virus, polymerase chain reaction
Introduction
Worldwide, conjunctivitis is a common eye disease and caused by infection with viruses, bacteria, or in some cases chlamydia. Viruses are considered the most common cause of infectious conjunctivitis.1,2 Symptoms of viral conjunctivitis include redness, swelling, tearing, and irritation, which often last for 1–3 weeks. Because no efficient antiviral treatment has yet been offered for conjunctivitis, this infection is mainly managed by symptomatic treatment.3 If vision-threatening complications occur, topical corticosteroids represent a drug of choice.4 Despite standard treatments, such as cold compresses, artificial tears, and topical vasoconstrictors, patients continue to suffer from great pain until complete healing of the disease.5 Although viral conjunctivitis is a self-limiting disease, it is associated with high morbidity, due to the likelihood of contagion and its symptoms.6
Adenovirus and herpes simplex virus (HSV) are common viral causes of keratoconjunctivitis for which a rapid laboratory diagnosis is often very helpful.7 HSV is considered the leading infectious cause of corneal blindness in developed countries.8 Also, chlamydia is a cause of conjunctivitis that can result in focal corneal scarring, chronic inclusion conjunctivitis, and neovascularization, probably leading to pneumonitis and otitis media in neonates.9 Severity of conjunctivitis can be mild to severely disabling. Ocular adenovirus infections take place all over the world in sporadic and epidemic forms. They are spread via direct contact, droplet, fecal–oral transmission, and contact with unchlorinated or unsatisfactory chlorinated water.7
Adenoviruses are the cause of 15%–70% of eye infections.10 A diagnosis of adenoviral conjunctivitis is usually based on history, symptoms, and clinical evidence. Patients usually complain of foreign-body sensation, pruritus, photophobia, epiphora, and blurred vision. Chemosis, bulbar conjunctival redness, tarsal follicular reaction, petechial, or even subconjunctival hemorrhage are also some other signs of viral conjunctivitis.11 Infiltration of immune cells and occurrence of focal inflammatory lesions in the subepithelial layer is one of the complications of viral conjunctivitis.4
PCR, direct immunofluorescence, and rapid antigen-detection immunoassays may be used to diagnose conjunctivitis. Viral cell cultures of the conjunctival specimen may help confirm the presence of adenovirus with immunofluorescence, but are less frequently conducted, due to the requirement of elaborate equipment and trained laboratory staff, as well as significant delays in obtaining results. Much evidence has proved that PCR is the best standard method to diagnose viral conjunctivitis.12 This technique has been demonstrated to be more sensitive and accurate and less time-consuming than cell culture. In a study at Johns Hopkins Hospital, conjunctivitis specimens of 307 staff were taken and examined by PCR. Only 22 (7%) patients were infected.13 Sambursky et al reported that among 50 patients with suspected acute conjunctivitis, 31 were found to be positive for adenovirus by PCR.2
In Iran, little research has been conducted on this issue, because ocular viral diseases are treated on the basis of clinical appearance.14 Because different types of conjunctivitis cannot be diagnosed or especially differentiated by clinical approaches15 and no specific treatment for conjunctivitis has yet been offered, early diagnosis can greatly contribute to accelerating the process of recovery. Considering that identifying the causative agents of conjunctivitis in a specific geographic region can help select appropriate treatment protocols and that few studies have yet been conducted on the etiology of this disease in Hamadan, west Iran, this study was conducted to investigate epidemiological patterns of conjunctivitis in this region. In addition, the frequency of viral conjunctivitis (due to adenoviruses, HSV1, HSV2, varicella zoster virus [VZV], and Chlamydia trachomatis) diagnosed by PCR and seasonal variations and association with certain socioeconomic and health factors were studied. The findings of such a study can help health-care systems manage and plan for controlling and preventing conjunctivitis, as well as selecting appropriate treatment strategies for this disease.
Methods
Data collection
In this cross-sectional nonrandomized study, 125 patients with suspected viral conjunctivitis or keratoconjunctivitis attending ophthalmology clinics in Hamedan from July 2016 to June 2017 were enrolled after providing written informed consent to participate in the study and filling out a demographic questionnaire. Data on age, sex, education, income, place of residence (rural/urban), underlying diseases (eg, diabetes, autoimmune diseases), previous acquisition of conjunctivitis, history of animal husbandry, history of taking ocular drugs, history of wearing lenses, and reasons for referral to the doctor were gathered. This study was approved by the Research Ethics Committee of Hamadan University of Medical Sciences with code (IR.UMSHA.REC.1394.80). Ethical considerations, such as keeping personal information private, were observed during conduction of this study. This study was conducted in accordance with the Declaration of Helsinki. The initial diagnosis of viral conjunctivitis was made by clinical examinations conducted by an ophthalmologist, observation of conjunctival erythema, and patients’ complaining of itching, burning, and watery eyes in one or both eyes. Then, samples of lower conjunctival discharge were taken by a doctor using a sterile swab. A total of 125 conjunctival swabs were collected and then placed in 1 mL viral transport media.
Nucleic acid extraction
Specimens were extracted using a column-based High Pure viral nucleic acid kit (Roche). Briefly, samples in viral transport medium were centrifuged at 13,000 g for 10 minutes. Then, 200 μL sediments were added to 400 μL lysis buffer, vortexed, and incubated at 72°C for 10 minutes. Subsequently, the mixture was spiked with 5 μL IC. After mixing, the solution was centrifuged through a filter tube at 13,000 rpm for 1 minute. In the next step, 500 μL inhibitor removal buffer was added to the filter tube and again centrifuged. After the column had been washed twice with the appropriate buffer, the genome was eluted with 50 μL elution buffer. Extracted DNA purity was verified based on its absorbance at 260 and 280 nm wavelengths with NanoDrop and then stored at −70°C until later use.
Real-time PCR
The samples were used for adenovirus PCR analysis. We used multiplex real-time PCR, which was able to detect adenovirus, HSV1, HSV2, VZV, and C. trachomatis. The assay was performed at a private laboratory with a real-time LightCycler (Roche). Each assay was divided into two tubes, one for C. trachomatis and adenovirus and the other for HSV1, HSV2, and VZV, based on the manufacturer's guidelines.
Reactions were set up and performed according to the manufacturer’s instructions. Briefly, each tube of 25 µL real-time polymerase chain reaction (PCR) contained 1 µL enzymes, 1.5 µL primer/probe mix, 12.5 µL buffer and 10 µL extracted DNA. Real-time PCR was performed using the LightCycler and multiplex eye-infection kit (Fast Track Diagnostics) to amplify viral and bacterial targets at 42°C for 15 minutes, followed by 94°C for 3 minutes, and finally 40 cycles for 8 seconds at 94°C and 60°C for 34 seconds. The presence of specific viral sequences in the reaction was represented by an increase in fluorescence from the relevant dual-labeled probes and reported as cycle-threshold valus bya real-time thermocycler.
Data analysis
Data were analyzed by Fisher’s exact test with Freeman–Halton extension in SPSS version 22. P<0.05 was considered significant.
Results
During the study, a total of 125 conjunctival swabs were collected from patients who attended ophthalmology clinics with symptoms of conjunctivitis. Of 125 specimens, 118 (94.5%) were positive for adenovirus and two (1.6%) for HSV1. There were no specimens positive for HSV2, VZV, or C. trachomatis. In five specimens, none of the investigated pathogens was detected.
The patients’ age range was 3–77 years. Most patients with conjunctivitis were 30–40 years old, and only two were aged <5 years. A total of 69 (55.2%) patients were female, and 106 (84.8%) lived in urban areas. In sum, 46 (36.8%) patients did not volunteer to divulge their income level and 43 (34.4%) had low (<IRR 6,000,000 per month) income. Twelve (9.6%) patients were keeping domestic animals, eight (6.4%) suffered from diabetes, and only one had autoimmune disease, while 117 (93.6%) did not take any medication for conjunctivitis, 105 (84%) had no history of eye diseases, 122 (97.6%) had no history of wearing contact lenses and 101 (80.8%) were not taking any ocular drug. Also, 78 (62.4%) patients attended the ophthalmology clinics due to eye redness (Table 1).
 |
Table 1 Studied demographic variables |
This study was performed within 1 year, and 55 (44%) patients attended the clinics in summer (Figure 1). According to Freeman–Halton test results, income, diabetes and ocular drug taking were not significantly associated with adenovirus prevalence (P=0.809, 0.871, and 0.652, respectively)
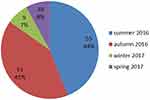 |
Figure 1 Frequency of conjunctivitis cases in different seasons. |
Discussion
Real-time PCR was used to study the prevalence of viral conjunctivitis in 125 patients attending ophthalmology clinics in Hamedan. Previous similar studies had not considered such patient demographics, but we decided to study associations of occupation, income, and medical history with this disease as well. In our study, adenoviruses were the most prevalent pathogen in patients with conjunctivitis: 94.4% had adenovirus in their specimens of lower conjunctival discharge. This study was first to investigate viral conjunctivitis and associated demographic characteristics in a city in west Iran. Two studies had already been conducted in central Iran. One of these was conducted on 100 patients with eye redness and conjunctivitis symptoms attending a university-affiliated hospital in Tehran, 16 of whom were positive for adenovirus on PCR.16 The other study investigated the prevalence rate of viral causes among patients attending hospitals across Tehran. To achieve this purpose, the researchers took samples from 150 suspected patients using sterile swabs and examined them using PCR. They found that 14.6% of the patients were positive for adenovirus and 3.3% for HSV1.14 Both studies found that patient sex was not associated with acquisition of conjunctivitis, although aging and increased incidence of the disease were closely associated. Despite consistency in evidence on higher prevalence of adenoviruses among the causes of conjunctivitis, the prevalence of adenoviruses in the current study was found to be much higher than reported by studies conducted in central Iran. In this study, a high rate of occurrence of adenovirus during summer and fall was almost the same, perhaps showing an epidemic of adenovirus.
It seems that the main reason for high prevalence of this viral agent among the patients was lack of health care and diagnostic facilities in suburban and rural areas, as well as the high prevalence of animal husbandry and agriculture in west Iran compared to other regions of the country. Given that viral infection is associated with environmental factors, such as hand contact, early detection of pathogens has a significant effect in reducing the spread of conjunctivitis and promoting eye health. On the other hand, adenoviruses are usually spread from contaminated ophthalmic instruments, such as eyedrops shared by patients and the tonometer used to measure ocular pressure. Nosocomial-spread inpatients may even necessitate temporary closure of an ophthalmic unit, and outpatient clinics may be the cause of community epidemics. Tese factors together with low personal hygiene may play a role in the high prevalence of ocular adenovirus infections.
Several similar studies have been conducted in different countries. Pinto et al conducted a study in a hospital in Brazil and reported that 59% of patients were infected with adenovirus, comparable to global and regional prevalence.17 A 5-year study in Turkey investigated 488 patients with suspected conjunctivitis and found adenovirus DNA in 43.6% of these patients on PCR.18A study in Tokyo, Japan demonstrated that among 189 patients with viral conjunctivitis, 82% carried adenovirus nucleic acid.19 A study conducted on patients attending an ophthalmology center in Karachi, Pakistan found that 75% of 388 conjunctivitis specimens had adenovirus, 2% had HSV, and 5% had C. trachomatis in culture media.20
An epidemiological study in Saudi Arabia investigated 65 patients with adenoviral keratoconjunctivitis with clinical complications of sudden redness, irritation, tearing, and eye pain, and found seven adenovirus serotypes to be the causes of keratoconjunctivitis. This study found no significant association between patient sex and acquisition of this infection.21 The findings of the cited studies and the current study indicate that adenoviruses are the most common viral causes of conjunctivitis, while a study on 18 patients with suspected conjunctivitis in Egypt using real-time PCR indicated that none of these patients carried adenovirus and 17 carried enterovirus.22
Although the prevalence of adenoviral conjunctivitis was 94.4% in the present study, we cannot consider this figure to represent an epidemic, because sampling in this study was conducted within 1 year, while an epidemic is restricted to a short period. An ideal method to diagnose pathogenic agents of eye infections should be able to diagnose the agents before the patients leave the clinic.23 Due to the high cost of real-time PCR, it does not seem to be practical in Iran.
In this study, one of the demographic variables studied was income level, and 34.4% of patients had low (<IRR 6,000,000 per month) incomes. There was no statistically significant relationship between conjunctivitis and other studied variables, such as drug taking or suffering from diabetes. In the light of ever-increasing prevalence of adenoviral conjunctivitis, especially in summer, appropriate and early diagnosis can play an important role in reducing the spread of this disease.
Two specimens containing HSV1 were isolated from the patients. A study estimated the incidence rates of HSV eye disease ranged from approximately four to 13 new cases per 100,000 population.24 Chlamydia is the most frequent identifiable cause of neonatal conjunctivitis in many countries, and its incidence is still on the rise on an annual basis.9 Human HSV3, also known as VZV, is a main pediatric infection that presents with a vesicular rash referred to as chickenpox.25 Despite the fact that no cases of C. trachomatis or VZV were found in this study on PCR, these two pathogens are considered the main agents of causing infection in infants.
No significant association was observed between demographic variables (age, sex, education, income, place of residence [urban/rural], suffering from underlying diseases [eg, diabetes, autoimmune diseases], previous acquisition of conjunctivitis, history of animal husbandry, history of taking ocular drugs, wearing lenses, and reasons for referral to the doctor) and the acquisition of conjunctivitis (P>0.05). However, the highest prevalence of disease was seen in summer (44%), followed by autumn (41%), which agrees with other studies in different regions of the world. In a study in Turkey, 52% of samples were diagnosed in summer.18 In Matsui et al, increased incidence of disease was observed in spring and summer, with the highest incidence observed in September.19 In addition, Sohrabi et al reported that 63% of adenoviral infections occurred in summer, while HSV infection and seasonal variations were not significantly associated.14
The increased prevalence of conjunctivitis in summer and autumn is likely to be related to increased frequency of certain activities, such as swimming and walking outside the city during warm seasons, while in winter, because of cold weather and decreased frequency of activities that are associated with increased risk of contagion, significant decrease in the prevalence of the disease has been reported. This finding indicates that contagion has a significant role in the increased prevalence of the disease.26 Considering that most patients with conjunctivitis are diagnosed and treated with clinical symptoms, the prevalence of pathogenic agents is not estimated. Study of prevalence of the disease's etiological agents using a rapid laboratory method can offer valuable information about the most common pathogenic agents to health-care providers, in order to prevent and treat diseases. Adenoviruses were found to be the most common cause of conjunctivitis in the studied region, as with other regions of the world.
Conclusion
This study demonstrated that adenoviruses were a much more common viral cause of conjunctivitis in the studied region than found in other regions. In addition, the acquisition rate of eye infection is expected to decrease dramatically in this region through control of adenoviruses. Demographic variables, such as age, sex, and income, were not significantly associated with acquisition of viral conjunctivitis. Most cases of conjunctivitis were reported to occur in summer and autumn. On the other hand, this study revealed that multiplex PCR has the potential to replace several diagnostic tests, with subsequent cost savings. This test also reduces the risk of misdiagnosis by clinicians. In light of the high prevalence of adenoviral conjunctivitis in the studied region, additional studies should investigate common viral genomes in west Iran, including Hamadan, so that health-care systems can be assisted in preventing and treating viral infections.
Acknowledgment
This study was derived from a research project approved by the research and technology deputy of Hamadan University of Medical Sciences (project 9405132558). Hereby, we gratefully thank this deputy and Dr Kavyani, Dr Malekifar, Dr Janahmad, Dr Lotfi, Dr Basirinia, Kobra Heidarzadi, Javad Shekarchi, Masoomeh Jalilian, Atefeh Morshedi, Mansour Amraei, and all people who helped us conduct it.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Li J, Yang Y, Lin C, et al. Etiology of acute conjunctivitis due to coxsackievirus A24 variant, human adenovirus, herpes simplex virus, and Chlamydia in Beijing, China. Jpn J Infect Dis. 2014;67(5):349–355.
2. Sambursky RP, Fram N, Cohen EJ. The prevalence of adenoviral conjunctivitis at the wills eye hospital emergency room. Optometry. 2007;78(5):236–239. doi:10.1016/j.optm.2006.11.012
3. Visscher KL, Hutnik CML, Thomas M. Evidence-based treatment of acute infective conjunctivitis: breaking the cycle of antibiotic prescribing. Can Fam Physician. 2009;55(11):1071–1075.
4. Gonzalez-Lopez JJ, Morcillo-Laiz R, Munoz-Negrete FJ. Adenoviral keratoconjunctivitis: an update. Arch Soc Esp Oftalmol. 2013;88(3):108–115. doi:10.1016/j.oftal.2012.07.007
5. Shiuey Y, Ambati BK, Adamis AP. A randomized, double-masked trial of topical ketorolac versus artificial tears for treatment of viral conjunctivitis. Ophthalmology. 2000;107(8):1512–1517. doi:10.1016/s0161-6420(00)00177-9
6. Lyra AFV, Bastos LC, Lima RCS, Maranhão LVL, Arantes TE. Artificial tears alone versus 0.45% ketorolac tromethamine with artificial tears for the treatment of acute viral conjunctivitis. Arq Bras Oftalmol. 2014;77(2):99–102.
7. El-Aal AMA, El Sayed M, Foad MF, et al. Adeno and herpes simplex viral infection in keratoconjunctivitis a comparison between two diagnostic laboratory methods. Int J Curr Microbiol App Sci. 2015;4(12):807–814.
8. Liesegang TJ, Melton LJ
9. Rours IG, Hammerschlag MR, Ott A, et al. Chlamydia trachomatis as a cause of neonatal conjunctivitis in Dutch infants. Pediatrics. 2008;121(2):321–326. doi:10.1542/peds.2007-0153
10. Jhanji V, Chan TC, Li EY, Agarwal K, Vajpayee RB. Adenoviral keratoconjunctivitis. Surv Ophthalmol. 2015;60(5):435–443. doi:10.1016/j.survophthal.2015.04.001
11. Trinavarat A, Atchaneeyasakul LO. Treatment of epidemic keratoconjunctivitis with 2% povidone-iodine: a pilot study. J Ocul Pharmacol Ther. 2012;28(1):53–58. doi:10.1089/jop.2011.0082
12. Wölfel R, Pfeffer M, Essbauer S, Nerkelun S, Dobler G. Evaluation of sampling technique and transport media for the diagnostics of adenoviral eye infections. Adenovirus sampling and transport. Graefes Arch Clin Exp Ophthalmol. 2006;244(11):1497–1504. doi:10.1007/s00417-006-0283-9
13. Kuo IC, Espinosa C, Forman M, Valsamakis A. A polymerase chain reaction-based algorithm to detect and prevent transmission of adenoviral conjunctivitis in hospital employees. Am J Ophthalmol. 2016;163:38–44. doi:10.1016/j.ajo.2015.12.007
14. Sohrabi M, Goodarzi Z, Saberfar E, Lashini H. The prevalence of viral conjunctivitis in patients who referred to eye specialist hospitals in Tehran, Iran. Iran J Ophthalmol. 2014;26(1):29–32.
15. Schnurr D, Dondero ME. Two new candidate adenovirus serotypes. Intervirology. 1993;36(2):79–83. doi:10.1159/000150325
16. Shamsi-Shahrabadi M, Mousavi E, Monavari SHR, Ataei-Pirkooh A, Bakhtiari P. Incidence of adenoviral conjunctivitis in patients referred to the Iran university affiliated hospital. Iran J Virol. 2009;3(2):7–11. doi:10.21859/isv.3.2.7
17. Pinto RD, Lira RP, Arieta CE, Castro RS, Bonon SH. The prevalence of adenoviral conjunctivitis at the clinical hospital of the State University of Campinas, Brazil. Clinics (Sao Paulo). 2015;70(11):748–750. doi:10.6061/clinics/2015(11)06
18. Erdin BN, Pas SD, Durak I, Schutten M, Sayıner AA. A 5-year study of adenoviruses causing conjunctivitis in Izmir, Turkey. J Med Virol. 2015;87(3):472–477. doi:10.1002/jmv.24071
19. Matsui K, Shimizu H, Yoshida A, Ngaoka E, Nishio O, Okuda K. Monitoring of adenovirus from conjunctival scrapings in Japan during 2005–2006. J Med Virol. 2008;80(6):997–1003. doi:10.1002/jmv.21175
20. Woodland RM, Darougar S, Thaker U, et al. Causes of conjunctivitis and keratoconjunctivitis in Karachi, Pakistan. Trans R Soc Trop Med Hyg. 1992;86(3):317–320. doi:10.1016/0035-9203(92)90328-a
21. Tabbara KF, Omar M, Hammouda E, et al. Molecular epidemiology of adenoviral keratoconjunctivitis in Saudi Arabia. Mol Vis. 2010;16:2132–2136.
22. Ayoub EA, Shafik CF, Gaynor AM, et al. A molecular investigative approach to an outbreak of acute hemorrhagic conjunctivitis in Egypt, October 2010. Virol J. 2013;10:96. doi:10.1186/1743-422X-10-96
23. Gordon YJ. Rapid diagnostic tests for infectious ocular disease. Int Ophthalmol Clin. 1993;33(1):153–161.
24. Young RC, Hodge DO, Liesegang TJ, Baratz KH. Incidence, recurrence, and outcomes of herpes simplex virus eye disease in Olmsted County, Minnesota, 1976–2007: the effect of oral antiviral prophylaxis. Arch Ophthalmol. 2010;128(9):1178–1183. doi:10.1001/archophthalmol.2010.187
25. Hambleton S. Chickenpox. Curr Opin Infect Dis. 2005;18(3):235–240.
26. Azari AA, Barney NP. Conjunctivitis: a systematic review of diagnosis and treatment. JAMA. 2013;310(16):1721–1729. doi:10.1001/jama.2013.280318
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
