Back to Journals » Research and Reports in Tropical Medicine » Volume 6
Epidemiology and disease burden of Buruli ulcer: a review
Authors Röltgen K, Pluschke G
Received 6 July 2015
Accepted for publication 2 September 2015
Published 16 November 2015 Volume 2015:6 Pages 59—73
DOI https://doi.org/10.2147/RRTM.S62026
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Thomas Unnasch
Katharina Röltgen,1,2 Gerd Pluschke1,2
1Molecular Immunology, Swiss Tropical and Public Health Institute, 2University of Basel, Basel, Switzerland
Abstract: Buruli ulcer (BU) is a neglected tropical skin disease caused by Mycobacterium ulcerans. Infection foci occur mainly in remote, rural areas of Central and West Africa, but also in Australia and Papua New Guinea. In addition, infections caused by M. ulcerans strains of a different lineage are sporadically reported from scattered foci in Asia and the Americas. While in the past decade more than 42,000 BU cases have been reported worldwide, an assessment of the actual global disease burden is complicated by the remoteness of affected populations and a lack of data on the incidence of BU in a number of countries, from which cases have been historically reported. Moreover, as BU patients present with diverse clinical manifestations ranging from relatively unspecific nodules, plaques, or edema to necrotic, ulcerative lesions, differential diagnosis is manifold and thus clinical misclassification may occur. Since to date reservoirs and transmission pathways of M. ulcerans remain equivocal, early diagnosis and treatment of patients are key determinants to control the disease. Particularly in view of the apparent decline in BU incidence in regions of West Africa, awareness and knowledge of BU in endemic regions must be retained to ensure a continuous monitoring and control. An integrated approach for the control of tropical skin diseases should be considered to cope with this difficult task. This review article aims at providing an overview of the current global burden of BU and summarizes the state of knowledge on the various epidemiological aspects of this enigmatic disease.
Keywords: neglected tropical disease, chronic skin ulcers, Mycobacterium ulcerans, geographically confined infection foci
Introduction
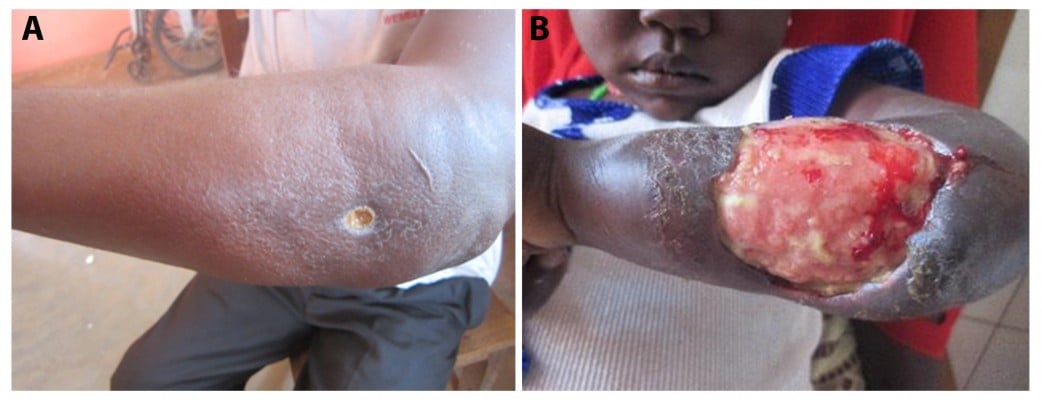
Chronic skin ulcers consistent with Mycobacterium ulcerans disease (Figure 1) were first described in The Mengo Hospital Notes (Kampala, Uganda) in 1897 by the British physician Albert Cook.1 However, it was only in 1948 when MacCallum and Tolhurst were able to isolate the etiologic organism of these “unusual skin ulcers” in patients from the Bairnsdale District in Australia, a mycobacterium later referred to as M. ulcerans.2 In the Democratic Republic of the Congo (then the Belgian Congo), chronic ulcers caused by mycobacteria were reported in 170 patients in the 1940s and 1950s.3 Infections occurred largely in a geographically restricted area situated between two rivers, exemplifying two of the main characteristics of the disease – the highly focal occurrence and the association with water bodies. Large numbers of cases were reported from the Buruli County near the river Nile in Uganda in the early 1960s,4 giving rise to the official designation Buruli ulcer (BU) for the disease. In the following decades, cases of BU caused by the two main human pathogenic M. ulcerans lineages have been reported from 34 countries mainly with tropical and subtropical climates (Angola, Australia, Benin, Brazil, Burkina Faso, Cameroon, Central African Republic, People’s Republic of China, Congo, Côte d’Ivoire, Democratic Republic of the Congo, Equatorial Guinea, French Guiana, Gabon, Ghana, Guinea, Indonesia, Japan, Kenya, Republic of Kiribati, Liberia, Malawi, Malaysia, Mexico, Nigeria, Papua New Guinea [PNG], Peru, Senegal, Sierra Leone, South Sudan, Sri Lanka, Suriname, Togo, and Uganda). While only single, sporadic BU cases have been reported from regions, where the ancestral lineage of M. ulcerans is prevalent, strains of the classical lineage account for infection foci in Africa and Australia with often very high incidences. Since the 1980s, such highly endemic areas were identified in several West African countries, including Benin,5 Côte d’Ivoire,6,7 Ghana,8 and Cameroon9 as well as in Australia.10,11 Surveys for BU in the endemic African countries revealed vast underreporting.7–9 Patients tend to report late or not at all to the formal health system for many reasons, including limited access to health facilities, stigmatization, and traditional beliefs, prompting them to seek treatment with traditional healers.
  | Figure 1 Presentation of BU lesions. |
In 1998, the World Health Organization (WHO) established the Global Buruli Ulcer Initiative in order to raise awareness and to coordinate global BU control and multidisciplinary research efforts.12 Today, cases of BU are actively reported from 13 countries in Africa (Benin, Cameroon, Côte d’Ivoire, Democratic Republic of the Congo, Gabon, Ghana, Guinea, Nigeria, and Togo), the Americas (French Guiana), Asia (Japan), and the Western Pacific (Australia and PNG).13 While more than 42,000 BU cases have been reported worldwide by 20 countries in the last 10 years (Table 1), case numbers seem to be declining in countries actively reporting BU with a total of 2,251 cases reported by the WHO in 2014 (Figures 2 and 3).13 However, the actual worldwide disease burden is still not clear, since it can be assumed that BU continues to exist in many of the previously reporting countries and that there may be underreporting by some of the countries reporting to the WHO. Reasons for under- or non-reporting of BU are manifold and include lack of awareness or resources (health systems overburdened by other more prevalent diseases), limited access to the remote populations affected, and political instability in certain countries.
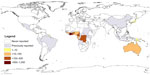  | Figure 2 Worldwide distribution of BU. |
  | Figure 3 Number of BU cases reported worldwide. |
The current downward trend in case numbers in several BU endemic African countries may in part be due to the establishment of effective national BU control programs. In this regard, it has been hypothesized that humans with active BU lesions that are not treated appropriately may play a role in transmission by shedding the bacteria into the environment.14–16 Transmission may thus be reduced by active case search followed by adequate treatment with the WHO-recommended antibiotic combination therapy of daily rifampicin and streptomycin for 8 weeks.17,18 The mechanism by which M. ulcerans is transmitted from the environment to humans thereby remains inconclusive.
Methods
We conducted an extensive review of the literature from all years by searching PubMed for the term “Buruli ulcer” or “Mycobacterium ulcerans”. All titles (N=~980) and available abstracts (N=~750) were screened for their epidemiological relevance. A total of 310 publications were selected for full-text review and the reference lists of included papers were perused for additional articles/webpages of interest. Due to the multitude of identified publications for certain endemic regions and epidemiological aspects, a selection of representative references had to be made that was mainly based on the scale/comprehensiveness of the studies.
Genomic diversity, ecology, and virulence of M. ulcerans
The occurrence of BU is often perceived as mysterious, mainly due to incomplete knowledge regarding reservoirs and transmission pathways of M. ulcerans. Detailed genomic investigations on the origin and evolution of this enigmatic pathogen have at least provided some clarifications. Genetic analyses indicate that M. ulcerans has diverged from the ubiquitous waterborne organism Mycobacterium marinum19 perhaps around a million years ago.20 While M. marinum usually infects fish and frogs and only occasionally causes granulomatous skin lesions in humans,21 emergence of the new, more pathogenic species M. ulcerans from M. marinum was coined primarily by the adoption of a virulence plasmid (pMUM). pMUM was shown to carry genes encoding the enzymatic machinery required for the synthesis of a unique macrocyclic polyketide toxin referred to as mycolactone22 that proved to be a key player in the pathogenesis of BU. Mycolactone has cytotoxic and immunosuppressive activities leading to the formation of chronic ulcerative skin lesions23 and the killing of infiltrating immune cells before they reach the active center of the lesions. In the course of the evolution of M. ulcerans, acquisition and expansion of insertion sequence (IS) elements (IS2404 and IS2606) have caused inactivation of genes and extensive loss of DNA.24 This became evident from a comparison of the whole-genome sequences of M. marinum strain M, comprising a 6.6 Mb circular chromosome with 5,424 coding sequences and 65 inactivated genes25 and the African M. ulcerans strain Agy99, consisting of two circular replicons with a size of 5.8 Mb, 4,241 coding sequences and a total of 771 pseudogenes.24 These genomic signatures (acquisition of foreign DNA, proliferation of IS elements, extensive gene loss through pseudogene formation, and genome downsizing) are indicative of an adaptation of M. ulcerans to a protected niche environment, where genes once needed for survival under diverse conditions are no longer required.24,26,27 However, it is not clear, to which environmental niche M. ulcerans is adapting to and by which mechanism the pathogen is transmitted. An adaptation within the aquatic ecosystem appears very likely, considering the origin of M. ulcerans as well as the association of BU infection foci with stagnant water bodies or river basins. Deletion or inactivation of genes required for pigment biosynthesis and anaerobiosis indicate that M. ulcerans is adapting to a dark and oxygen-poor ecological niche. Rather than free-living, it may persist in the environment as a commensal, associated with other protective organisms such as amoebae.28–30 However, detection of M. ulcerans-specific DNA sequences in many biotic components of aquatic ecosystems, such as plants, snails, fish, or insects, has been also interpreted as indication for the ubiquitous presence of this pathogen in these habitats. Furthermore, mycolactone-producing mycobacteria have been isolated from different fish and frog species presenting with mycobacterial infections. While these isolates have initially been given distinct species names, such as M. marinum,31 Mycobacterium pseudoshottsii,32 and Mycobacterium liflandii,33 genetic analyses suggest that all mycolactone-producing mycobacteria are derived from a common ancestor and should be considered a single (M. ulcerans) species.27,34
A detailed analysis of M. ulcerans human isolates of diverse geographical origin based on comparative genomic hybridization analysis revealed extensive large sequence polymorphisms, enabling a differentiation of M. ulcerans clinical isolates into two principal lineages. These lineages were designated “ancestral” in reference to M. ulcerans strains from Asia (People’s Republic of China and Japan) and the Americas (Mexico, French Guiana, and Suriname) being closely related to M. marinum, and “classical”, with most BU cases worldwide – namely those from Africa, Australia, and PNG – being caused by isolates belonging to this lineage.
A comparative whole-genome sequencing analysis including strains isolated from infected fish and frogs have suggested the presence of at least three different lineages within the species M. ulcerans:27 lineage 1, including frog and fish isolates of worldwide origin, but also a human isolate from French Guiana; lineage 2 represented by a human isolate from Japan; and lineage 3 comprising human isolates from Africa and Australia. In view of the broad host range of M. ulcerans including humans, other mammals35–39 and ectotherms, the different lineages appear to have adapted to distinct niche environments and may be considered as ecovars.
Different patterns of BU case distribution with highly endemic foci in Africa and Australia associated with the classical lineage as compared with only scattered, sporadic cases in Asia and the Americas associated with the ancestral lineage support the idea of the development of distinct M. ulcerans ecovars. The ancestral M. ulcerans lineage may thus be adapted to an ecological niche, from which strains only occasionally infect humans. In contrast, strains of the classical lineage may be present in an environmental reservoir that is associated with a higher risk for humans to contract an M. ulcerans infection. The pathology caused by American and Asian ancestral lineage strains40,41 may be as severe as the one observed in Africans and Australians infected by classical lineage strains.
Geographical distribution and disease burden of BU
Ancestral lineage
Americas (French Guiana, Suriname, Peru, Brazil, and Mexico)
There is only sparse information on the prevalence and geographical distribution of BU in the Americas. Published reports on the occurrence of M. ulcerans infections are limited41–44,47,48 and only very few cases of BU have been reported to the WHO. While fewer than ten cases per country have been confirmed in Suriname, Mexico, Peru, and Brazil in the past 50 years,42 >250 cases have been notified between 1969 and 2014 in French Guiana, which borders Brazil to the east and south, and Suriname to the west. Most inhabitants of French Guiana reside along a 50 km wide coastal strip dominated by swampy areas, with the rest of the country consisting of dense tropical rainforest. Considering the small population, the number of infections is relatively high with an average annual incidence of >2/100,000.43 Construction of the Petit-Saut Dam in 1994 upstream the BU endemic area has been associated with a significant decline in case numbers, possibly by a better regulation of water flows. Recently, M. ulcerans DNA (IS2404 and the ketoreductase B domain gene) has been detected in specimens from three of 23 different freshwater bodies sampled in French Guiana, indicative for the presence of this species in the environment.43 The recent observation of genetic heterogeneity among 23 isolates from patients living along the coastline of French Guiana as revealed by Multilocus Variable Number Tandem Repeat Analysis44 is uncommon for M. ulcerans, which usually shows very limited genetic diversity within particular endemic areas.14,45,46 While two of the genotypes detected in French Guiana showed high similarity, one had distinctly different characteristics and shared more sequence similarity with the M. ulcerans ecovar liflandii. No geographical clustering of the genotypes was observed.44 While French Guiana is the only country in the Americas actively reporting BU cases to the WHO,13 sporadic infections have been reported from other American countries dating back several years.
From warm and humid areas of Peru, eight laboratory-confirmed BU cases have been recorded between 1996 and 2006. Five of these patients came from the Peruvian rainforest, while the other three patients lived near or visited Tumbes, a swampy area at the north coast of Peru. One of the patients presented with lesions on both knees and reported to often kneel on soil and organic mulch that contained wood shavings while carrying out gardening activities.47 In addition to a single case of BU reported from Brazil in 2007,48 a returning traveler from the UK in the same year was reported to most probably have contracted his M. ulcerans infection during a visit of the Pantanal Region of Southern Brazil.49 Cases of two laboratory-confirmed BU patients from Central Mexico with unusually disseminated infections have been reported in 2005.41
The larger number of reported BU cases from French Guiana as compared with the other South American countries might have various reasons, including increased awareness of the disease among the relatively small population and an efficient reporting system, the prevalence of more pathogenic variants of M. ulcerans, and/or a closer contact of the population to environments contaminated with the pathogen.
Asia (Japan and People’s Republic of China)
The causative agent of BU in Japan and People’s Republic of China is often referred to as “M. ulcerans subspecies shinshuense”.50 While the severity of the disease seems to be comparable to that caused by other M. ulcerans sublineages, pain was reported to be more common in Japanese than in African patients. Until 2014, a total of 53 BU cases have been reported from Japan with the first report dating back to 1980.50 After that no further cases had been reported until 2003.51 Between 2003 and 2011, there has been a steady increase in the number of reported cases, summing up to a total of 32 well-documented cases: 20 females and 12 males with an average age of 42 years. Most of these cases (25/32; 86%) were diagnosed during autumn and winter,52 which may speak for contraction of the slowly progressing infection in summer. All but one of the patients originated from different regions of the mountainous Honshu Island, a typical temperate region of Japan. None of the 19 patients that had been reported until 2010 could be linked to an aquatic environment.40 In contrast, a rare instance of familial occurrence of BU, in which three family members developed the disease, has been linked to a stagnant agricultural water channel. Detection of IS2404 from a crayfish within the water channel suggested that the pathogenic organism may reside in aquatic environmental reservoirs in Japan.53
The increased number of reported cases in Japan may rather be caused by increased awareness of the disease than by an actual rise in incidence. A case of BU in a patient with travel history in the People’s Republic of China infected with M. ulcerans ecovar shinshuense published in 2000 indicates the probable risk for M. ulcerans infection in other Asian countries.54
Classical lineage
Western Pacific (Australia, PNG, and Republic of Kiribati)
In Australia, two geographically and climatically very distinct BU endemic foci exist. An endemic area in the temperate southeastern state of Victoria, where the disease is known as “Bairnsdale ulcer”, has been extensively studied. A less well-known BU endemic area exists in tropical Far North Queensland, where the disease is often called “Daintree ulcer”.
In Victoria, BU outbreaks are typically observed along the highly populated coast and have been reported from Gippsland, Phillip Island,10 and towns located on the Mornington55 and Bellarine56,57 peninsulas. More than 430 cases have been reported for Victoria between 1939 and 2008,58 with the majority of cases notified after 1980. Disease foci are gradually moving along the coastal settlements, with the most severe BU outbreak in Point Lonsdale, where nearly 70 cases were recorded during 2004 and 2006.58 In a well-characterized cohort of 180 BU patients from the Bellarine Peninsula recorded between 1998 and 2011, the median patient age was 61 years and 49% of patients were male.57
In the BU endemic setting of Victoria, a new facet of the disease was revealed by the identification of possums as terrestrial animal reservoirs of M. ulcerans.59,60 Testing of environmental samples by polymerase chain reaction (PCR) revealed outstandingly high concentrations of M. ulcerans DNA in the feces of these animals. Subsequently, it was found that in areas endemic for human BU, a large proportion of possums had skin lesions caused by M. ulcerans. These observations may speak for a zoonotic risk of BU in Victoria.16 M. ulcerans DNA has also been detected in a small proportion of captured mosquitoes from Victoria.56 Furthermore, correlations have been found between the occurrence of BU and of other mosquito vector-borne diseases in this region, giving rise to speculations that mosquitoes might be vectors of M. ulcerans in southeastern Australia.61
The Daintree River catchment and adjacent swampy coastal lowlands in tropical Far North Queensland constitute another BU endemic focus in Australia. Settlement in this region is sparse and is composed of smaller communities and farms. While there are numerous anecdotal reports about patients having suffered from BU, a consistent recording was only available from 1964 onward. Between 1964 and 2008, 92 cases were recorded with a median age of 42 years. Most of the patients (53 males and 39 females) presented during the dry season and a number of patients reported spider or insect bites preceding development of their lesion.11
Historic reports indicate the presence of hundreds of BU cases in PNG, mainly in association with the Sepik and Kumusi River valleys.62 There is one published report on a case series of 13 BU patients detected between 1964 and 1965 in the Western (two cases) and Northern (eleven cases) districts of PNG.63 Another report described 46 cases of BU seen from 1979 to 1983 in PNG, mainly from villages on the Sepik River.64 Two cases of extensive limb ulcers with clinical features of M. ulcerans infection have been reported in the Republic of Kiribati.65
Southeast Asia (Indonesia, Malaysia, and Sri Lanka)
While a few cases have been historically reported to the WHO from Indonesia and Sri Lanka, no published reports are available. M. ulcerans infections have also been reported from Malaysia.66
Africa
The major burden of BU falls on populations living in West and Central Africa, where the disease typically occurs in rural, isolated foci associated with swampy lowlands and river valleys. Historically, outbreaks of BU have been associated with various ecological and environmental disturbances, including alterations of water systems such as irrigation or damming of streams or rivers connected with the creation of wetlands and impoundments67 or severe flooding of lakes and rivers exposing people to swampy terrain.68,69 Moreover, some infection foci were associated with agricultural activities leading to flooding or the creation of irrigation systems as well as land development accompanied by resettlement near water bodies.70 The first BU patients detected in West Africa came from Nigeria, where cases emerged in proximity to a small stream that was dammed to create an artificial lake.67 Step-by-step highly endemic BU foci – all located in and connected to river basins – were identified in several other West African countries including Benin,5 Côte d’Ivoire,6,7 Ghana,8 and Cameroon9 with more than 40,000 BU cases reported only from these four countries in the last decade.
Much of the basic knowledge about the distribution of BU cases among exposed populations in Africa can be extracted from an intensive study of 220 BU cases that occurred within a community of 2,500 Rwandan refugees between 1964 and 1969.71 The refugees settled in Kinyara, an area near the river Nile in Central Uganda, which turned out to be a focus for M. ulcerans infection, providing the opportunity of investigating epidemiological features of the disease in this population. Although BU was shown to affect individuals at every age, the highest incidence in the Rwandan refugee community was in children aged between 5 and 15 years. Despite the small extent of the settlement, a higher BU incidence was observed in sectors located close to the Nile and a small tributary, although actual contact with the river water appeared not necessary for infection. Seasonal fluctuation in BU incidence was observed with an estimated maximum spread between the two rainy seasons. The majority of patients presented with a single lesion, while the anatomical site of lesions varied with both sex and age. These valuable data were complemented by other studies conducted in different BU endemic areas in the last decades, including a recently published large study carried out between 2005 and 2011 at the BU Treatment Centre (Centre de Dépistage et de Traitement de l’Ulcère de Buruli [CDTUB]) in Benin.72 Prospective clinical data collected from 1,227 laboratory-confirmed BU patients from Benin revealed features similar to those found in many other African BU endemic areas. Typically, patients were children with a median age at diagnosis of 12 years and most lesions occurred on the limbs. The overall sex distribution was balanced. In all, 96% of the patients presented with a single lesion and 36% with an advanced lesion of more than 15 cm in diameter. Moreover, findings of this study support the view that the BU burden in Africa is underestimated, since in the past years, more patients from the neighboring country Nigeria were treated at the CDTUB in Benin than actually reported by Nigeria to the WHO.72
Risk factors and transmission
Since in recent years various environmental and animal reservoirs as well as different vectors were proposed to be involved in the transmission of M. ulcerans, consideration should be given to the possibility that multiple modes of infection may exist. In order to better understand how M. ulcerans is transmitted, a multitude of BU case series was analyzed. A number of case-control studies allowed for the direct identification of potential risk factors for contracting the disease (Table 2).6,73–87 A review article evaluating identified risk factors was published in 2010.88
Age and sex
While it has long been recognized that the majority of patients in the African BU endemic settings are children with a peak of BU incidence between 10 and 14 years of age,6,89 a bimodal age-related risk of developing BU is observed, when the population age distribution is taken into account. In one of the few case-control studies that did not match participants by age, an increased risk of BU in children aged below 15 years and adults aged above 49 years was found.87
A recent survey for BU in Cameroon has revealed that the risk is highest in children aged between 4 and 14 years and in the elderly aged above 50 years. Furthermore, a marked underrepresentation of children below 4 years of age became apparent.90 In line with this observation, sero-epidemiological studies in Ghana and Cameroon have indicated that children below 5 years of age are considerably less exposed to M. ulcerans than older children.91 Infection by M. ulcerans might thus occur outside the relatively small movement range of very young children. Exposure and the associated risk of developing BU disease appears to increase at an age when the children are having more intense environmental contacts, including direct exposure to water bodies peripheral to their homes. While young adults are less often affected by BU and may have developed a certain degree of immunity to M. ulcerans, the higher risk of BU in the elderly56,89 may be related to the age-dependent decrease in immunological competence. Sero-epidemiological studies also indicate that only a small proportion of exposed individuals develop clinical M. ulcerans disease.
The higher proportion of older people affected is very pronounced in southeastern Australia, where in a BU outbreak in Point Lonsdale, the risk of contracting BU was calculated to be about seven times higher for individuals ≥55 years of age than in those below 55 years of age.56 The high average age of BU patients from Victoria57 may at least be partly related to the high average age of the general population in the affected communities at the seaside, where many retired people have their homes.
A nearly equal sex distribution among patients was reported in most of the studies in African and Australian settings.6,8,9,57,72,89 However, differences in the occurrence of BU have been observed between groups, if stratified for age. In the refugee population in Kinyara, the incidence of BU among adults was considerably higher in women than in men.71 Several studies have reconfirmed this observation reporting that male patients were more prevalent in the younger age groups than females, but less prevalent in adults.8,72,92,93
Risk factors connected with behavior
Various behavioral factors that may lead to an increased or decreased probability of acquiring BU have been analyzed in populations living in African and Australian BU endemic settings (Table 2). In accordance with the prevailing assumption that M. ulcerans infection foci are associated with wetlands, most of the case-control studies conducted identified contact with or proximity to water bodies as a risk factor for contracting BU.6,74,76–78,80,83–87 Common factors associated with a lower risk for BU reported in several comparative studies were wound care and hygiene73,76,77,80,83,84 as well as wearing protective clothing.6,73,76,77,84
In southeastern Australia, exposure to mosquitoes was identified as an additional risk,73 implicating mosquitoes in the transmission of M. ulcerans in this region. Two studies conducted in Cameroon suggest an association between bed net use and protection against M. ulcerans infection.83,84
Only in a minority of the published case-control studies, clinical diagnosis of all suspected BU cases enrolled was confirmed by laboratory diagnosis. In view of the limited reliability of clinical diagnosis, laboratory confirmation should be a strict quality standard in future studies.
Host genetics
In a descriptive review of all BU cases that had occurred in the endemic region of Far North Queensland, ten asynchronous cases in genetically related family members suggested the possibility of a genetic predisposition.11 However, this has not been studied further. In one of the case-control studies conducted in Benin, a history of BU in the family was associated with an increased risk of BU.74 Whether family relationships of BU are due to genetic factors or simply due to similar exposure to the pathogen remains to be investigated. Only one human host genetic study has been published so far, reporting that susceptibility to BU may be associated with a polymorphism in a NRAMP gene, which had already been associated with tuberculosis and leprosy.94
Bacillus Calmette–Guérin vaccination
Even though Mycobacterium bovis Bacillus Calmette–Guérin (BCG) is the most widely administered vaccine in human history, its efficacy in preventing mycobacterial diseases remains controversial.95–97 Results of early clinical trials in Uganda indicated a protective effect of BCG vaccination against M. ulcerans infection.98,99 However, no evidence of a protective effect on the risk of developing BU was found in a number of case-control studies.75,77,82,87 BCG vaccination may at least confer some protection against severe forms of BU.100,101
HIV status
Information on the epidemiological and clinical consequences of BU–HIV coinfection is scarce and contradictory. While results of the case-control studies conducted in Benin suggested that HIV infection increases the risk of BU,79 no significant association was found in a comparative analysis in Ghana.77 Two recent case-control studies carried out in Benin and Ghana reported a significant effect of HIV infection on the severity of M. ulcerans infections and provided evidence suggestive of a higher HIV incidence in patients with BU compared with the general population.102,103 Furthermore, observations in case studies indicate a more severe disease in HIV patients.104–108
Transmission hypotheses
Incubation period – clues on exposure?
Due to the extremely slow growth rate of M. ulcerans, the incubation period of BU is very long. In the Kinyara refugee camp, the period between short stays of visitors and the development of BU was estimated to be 4–10 weeks. In a more recent study from Australia, the mean incubation period of BU patients who reported a single visit to the Victorian BU endemic area was 4.5 months. The shortest period recorded was 32 days and the longest was 254 days.109 The discrepancy between the two studies may possibly be related to differences in the mode of transmission and the inoculation dose of M. ulcerans which may be higher in the African setting.
The rapidly decreasing number of new infections after the move of the refugee community from Kinyara to a new locality in Uganda in late 1969, despite the continued presence of untreated patients with open, ulcerative wounds, speaks against an important role of direct person-to-person transmission and for the involvement of an environmental reservoir.71
Site of lesion – clues on vectors?
Since most of the BU patients present with a single lesion, it is commonly assumed that the site of the lesion is also the site of inoculation. Therefore, a number of studies have analyzed the distribution of lesions on the body in order to draw conclusions on the mechanism of infection. In the majority of the patients, lesions occur on the lower limbs, followed by the upper limbs, the trunk, and the neck/head.8,72,90,93,110 In the refugee population in Kinyara, the site of the lesion varied with both sex and age. Among children below 5 years of age, the lesions were distributed over all parts of the body, while with increasing age, lesions on the head and trunk were less common and largely confined to the limbs.71 These differences in the distribution of lesions among children and adults were reconfirmed in other studies.89,90,93 While some studies have reported that among females, arms were more71 and the trunk less90 frequently affected as compared with males, other data indicate a similar distribution of lesion sites in both sexes.93 Variations in the affected anatomical sites by age and sex may be due to differences in domestic or agricultural activities (eg, females fetching water and working in the fields with their bare hands; males farming, carrying out work using hoes and other tools) or different behaviors (males and children more commonly exposing their upper trunk than females).
While all studies agree that most lesions occur on body sites, which are not commonly protected by clothing, no definite mode of transmission can be established from these observations, since the described distribution of BU lesions may be related to insect bites, skin injuries, or both.
Hypotheses on the mode(s) of M. ulcerans transmission
In the BU focus in southeastern Australia, possums (small arboreal marsupials native to Australia) seem to represent an animal reservoir59,60 and mosquitoes are considered potential vectors61,111 of M. ulcerans.
In African BU endemic settings, no similar animal reservoir has been identified so far. However, shedding of M. ulcerans from ulcerative lesions of humans with active BU disease may play a role in the dissemination of M. ulcerans in the African BU endemic regions.14–16 Since recent studies indicated that M. ulcerans may persist for many months in underwater decaying organic matter,112 it may be considered that infection occurs at contaminated water contact sites. Sero-epidemiological studies in Ghana and Cameroon have shown that children are much earlier exposed to malaria parasites than to M. ulcerans, indicating that an involvement of insect vectors commonly found close to the households is highly unlikely.91 Results of other studies have been compiled in a conceptual model, where M. ulcerans, present in the aquatic environment such as in detritus, mud, or plant biofilms, is concentrated by water-filtering organisms70,113 and subsequently passed on to predatory aquatic vertebrates and invertebrates feeding on this prey. Infection from potential environmental reservoirs may take place via puncture wounds or lacerations after contact with concentrated M. ulcerans sources or via invertebrate vectors, such as aquatic insects.16,114,115
Disease burden for individuals and households affected
In Africa, BU typically affects rural, impoverished populations with limited access to medical care. In addition to geographical and financial constraints, beliefs and stigma regarding the origin of the disease, as well as the often slow progression of M. ulcerans infections combined with an indolent course result in delayed reporting of patients. According to the WHO classification, three stages of BU can be distinguished: 1) category I lesions with a cross-sectional diameter of less than 5 cm, 2) category II lesions with a size of 5–15 cm, and 3) category III lesions with diameter more than 15 cm as well as lesions at crucial sites (eye, breast, and genitalia), multiple lesions, and osteomyelitis.18 Until today only a minority of patients in Africa presents with category I disease.72 In the past, the only treatment option has been wide surgical excision of the affected tissue, followed by skin grafting, demanding wound management, and rehabilitative physiotherapy, if available.116 Major advances have been made in the management of BU after 2004 with the introduction of antibiotic therapy.117–119 However, while early stages of the disease can usually be treated with an 8-week course of streptomycin and rifampicin alone, a substantial proportion of patients presents in many African settings with massive necrotizing lesions, often necessitating adjunct surgery, skin grafting, and prolonged hospitalization.
Debilitating sequelae resulting from the massive destruction of tissue and joints are socially stigmatizing and may lead to a decreased quality of life. In hospitals of many BU endemic countries, medical treatment is free of charge. However, other expenditures such as costs for transportation, feeding, or accommodation of patients and their caregivers as well as indirect costs such as productivity loss often have devastating implications on household economies. Even if medical costs for hospital treatment and supplementary aid for everyday needs were provided to BU patients and their caretakers in a hospital in Central Cameroon, the median cost burden of hospitalization was reported to be 25% of household’s annual earnings due to high nonmedical costs and productivity loss.120 In another study carried out in the Ga West Municipality of Ghana, hospital-based treatment of BU patients with ulcerative lesions resulted in a mean loss of 265 productive workdays.121 For nonhospitalized BU patients, transportation and other costs can be overwhelming. A recent cost-of-illness study in the Ga South Municipality of Ghana including 63 BU outpatients has shown that the mean cost of BU treatment of US$570 constituted approximately 45% of the household annual income. It can be concluded that impoverishment is likely among BU-affected African households.122 Strategies to cope with the costs such as sale of assets, borrowing of money, use of savings, reduction of nonessentials and essentials, and the social isolation of the patient have been described.120,121 In one study, it was calculated that household costs were eight times higher for households involved in the care of patients compared with those for socially isolated patients.120 Therefore, new intervention strategies that are socially more compatible such as a decentralized system of care are of urgent need.
Conclusion
Intensified research activities after the establishment of the Global Buruli Ulcer Initiative by the WHO in 1998 have yielded: 1) advances in the treatment and management of the disease, 2) a better understanding of the pathogenesis of BU and the origin and evolution of M. ulcerans, 3) various hypotheses on potential reservoirs and modes of transmission, and 4) evidence for some more or less preventable risk factors for contracting the disease. Research activities toward the design of a BU vaccine have shown that in spite of the extracellular localization of M. ulcerans, the development of such a vaccine is a major challenge. Therefore, control of BU still relies mainly on active case search and adequate treatment of the patients coordinated by national BU control programs established in several endemic countries.
While in recent years a reduction of the worldwide incidence of BU was noted, infection foci continue to exist, particularly in remote, rural areas of West and Central Africa and represent a considerable disease burden for the affected populations. One of the main tasks for BU control will thus be to maintain public awareness of this rare, but highly debilitating disease and to sustain expertise to diagnose and treat it among local health staff.
Globally, chronic skin conditions with diverse infectious and noninfectious etiologies constitute a substantial public health concern. In contrast to BU, some other neglected infectious diseases with cutaneous manifestations such as lymphatic filariasis, onchocerciasis, yaws, and scabies can be simultaneously treated through once-annual administration of an integrated package of medicines.123 For BU, treatment regimens suitable for application at peripheral health centers should be further developed, and the development of a point-of-care rapid diagnostic test to avoid both over- and under-treatment is a key research priority. BU and other tropical skin diseases share similarities in terms of causing long-term disabilities, reinforcing poverty, and the geographical distribution. There is great need for a robust integrated diagnostic and therapeutic approach for the management of skin lesions at primary health care facilities of the resource poor African BU endemic countries. BU control and research activities should therefore be more efficiently integrated into combined programs with other skin diseases.
Acknowledgments
The authors thank Kobina Ampah for creating the map of the worldwide BU distribution with ArcGIS. Parental consent was obtained for the use of the images in Figure 1.
Disclosure
The authors report no conflicts of interest in this work.
References
Cook, A. The Mengo Hospital Notes. Kampala: Makere College Medical School Library; 1897. | |
MacCallum P, Tolhurst JC. A new mycobacterial infection in man. J Pathol Bacteriol. 1948;60(1):93–122. | |
Janssens PG, Quertinmont MJ, Sieniawski J, Gatti F. Necrotic tropical ulcers and mycobacterial causative agents. Trop Geogr Med. 1959;11:293–312. | |
Clancey JK, Dodge OG, Lunn HF, Oduori ML. Mycobacterial skin ulcers in Uganda. Lancet. 1961;2(7209):951–954. | |
Debacker M, Aguiar J, Steunou C, et al. Mycobacterium ulcerans disease (Buruli ulcer) in rural hospital, Southern Benin, 1997–2001. Emerg Infect Dis. 2004;10(8):1391–1398. | |
Marston BJ, Diallo MO, Horsburgh CR, et al. Emergence of Buruli ulcer disease in the Daloa region of Cote d’Ivoire. Am J Trop Med Hyg. 1995;52(3):219–224. | |
Kanga JM, Kacou ED. Aspects épidémiologiques de l’ulcère de Buruli en Côte d’Ivoire: résultats d’une enquête nationale [Epidemiological aspects of Buruli ulcer in Côte d’Ivoire: results of a national survey]. Bull Soc Pathol Exot. 2001; 94(1):46–51. French. | |
Amofah G, Bonsu F, Tetteh C, et al. Buruli ulcer in Ghana: Results of a national case search. Emerg Infect Dis. 2002;8(2):167–170. | |
Noeske J, Kuaban C, Rondini S, et al. Buruli ulcer disease in Cameroon rediscovered. Am J Trop Med Hyg. 2004;70(5):520–526. | |
Veitch MG, Johnson PD, Flood PE, Leslie DE, Street AC, Hayman JA. A large localized outbreak of Mycobacterium ulcerans infection on a temperate southern Australian island. Epidemiol Infect. 1997;119(3):313–318. | |
Steffen CM, Smith M, McBride WJH. Mycobacterium ulcerans infection in North Queensland: the “Daintree ulcer.” ANZ J Surg. 2010;80(10):732–736. | |
Wansbrough-Jones M, Phillips R. Buruli ulcer: Emerging from obscurity. Lancet. 2006;367(9525):1849–1858. | |
World Health Organization. Country data for Buruli ulcer. Available from: http://apps.who.int/neglected_diseases/ntddata/buruli/buruli.html. Accessed June 25, 2015. | |
Röltgen K, Qi W, Ruf M-T, et al. Single nucleotide polymorphism typing of Mycobacterium ulcerans reveals focal transmission of Buruli ulcer in a highly endemic region of Ghana. PLoS Negl Trop Dis. 2010;4(7):e751. | |
Williamson HR, Benbow ME, Campbell LP, et al. Detection of Mycobacterium ulcerans in the environment predicts prevalence of Buruli ulcer in Benin. PLoS Negl Trop Dis. 2012;6(1):e1506. | |
Röltgen K, Pluschke G. Mycobacterium ulcerans disease (Buruli ulcer): potential reservoirs and vectors. Curr Clin Microbiol Rep. 2015:1–9. | |
World Health Organization. Provisional Guidance on the Role of Specific Antibiotics in the Management of Mycobacterium Ulcerans Disease (Buruli Ulcer). Geneva: World Health Organization; 2004. | |
World Health Organization. Treatment of Mycobacterium Ulcerans Disease (Buruli Ulcer): Guidance for Health Workers. Geneva: World Health Organization; 2012. | |
Yip MJ, Porter JL, Fyfe JAM, et al. Evolution of Mycobacterium ulcerans and other mycolactone-producing mycobacteria from a common Mycobacterium marinum progenitor. J Bacteriol. 2007;189(5):2021–2029. | |
Qi W, Käser M, Röltgen K, Yeboah-Manu D, Pluschke G. Genomic diversity and evolution of Mycobacterium ulcerans revealed by next-generation sequencing. PLoS Pathog. 2009;5(9):e1000580. | |
Petrini B. Mycobacterium marinum: ubiquitous agent of waterborne granulomatous skin infections. Eur J Clin Microbiol Infect Dis. 2006; 25(10):609–613. | |
George KM, Chatterjee D, Gunawardana G, et al. Mycolactone: a polyketide toxin from Mycobacterium ulcerans required for virulence. Science. 1999;283(5403):854–857. | |
Hong H, Coutanceau E, Leclerc M, Caleechurn L, Leadlay PF, Demangel C. Mycolactone diffuses from Mycobacterium ulcerans-infected tissues and targets mononuclear cells in peripheral blood and lymphoid organs. PLoS Negl Trop Dis. 2008;2(10):e325. | |
Stinear TP, Seemann T, Pidot S, et al. Reductive evolution and niche adaptation inferred from the genome of Mycobacterium ulcerans, the causative agent of Buruli ulcer. Genome Res. 2007;17(2):192–200. | |
Stinear TP, Seemann T, Harrison PF, et al. Insights from the complete genome sequence of Mycobacterium marinum on the evolution of Mycobacterium tuberculosis. Genome Res. 2008;18(5):729–741. | |
Röltgen K, Stinear TP, Pluschke G. The genome, evolution and diversity of Mycobacterium ulcerans. Infect Genet Evol. 2012;12(3):522–529. | |
Doig KD, Holt KE, Fyfe JAM, et al. On the origin of Mycobacterium ulcerans, the causative agent of Buruli ulcer. BMC Genomics. 2012;13:258. | |
Eddyani M, De Jonckheere JF, Durnez L, Suykerbuyk P, Leirs H, Portaels F. Occurrence of free-living amoebae in communities of low and high endemicity for Buruli ulcer in southern Benin. Appl Environ Microbiol. 2008;74(21):6547–6553. | |
Gryseels S, Amissah D, Durnez L, et al. Amoebae as potential environmental hosts for Mycobacterium ulcerans and other mycobacteria, but doubtful actors in Buruli ulcer epidemiology. PLoS Negl Trop Dis. 2012;6(8):e1764. | |
Amissah NA, Gryseels S, Tobias NJ, et al. Investigating the role of free-living amoebae as a reservoir for Mycobacterium ulcerans. PLoS Negl Trop Dis. 2014;8(9):e3148. | |
Ranger BS, Mahrous EA, Mosi L, et al. Globally distributed mycobacterial fish pathogens produce a novel plasmid-encoded toxic macrolide, mycolactone F. Infect Immun. 2006;74(11):6037–6045. | |
Rhodes MW, Kator H, McNabb A, et al. Mycobacterium pseudoshottsii sp nov, a slowly growing chromogenic species isolated from Chesapeake Bay striped bass (Morone saxatilis). Int J Syst Evol Microbiol. 2005;55(Pt 3):1139–1147. | |
Trott KA, Stacy BA, Lifland BD, et al. Characterization of a Mycobacterium ulcerans-like infection in a colony of African tropical clawed frogs (Xenopus tropicalis). Comp Med. 2004;54(3):309–317. | |
Pidot SJ, Asiedu K, Käser M, Fyfe JAM, Stinear TP. Mycobacterium ulcerans and other mycolactone-producing mycobacteria should be considered a single species. PLoS Negl Trop Dis. 2010;4(7):e663. | |
Elsner L, Wayne J, O’Brien CR, et al. Localised Mycobacterium ulcerans infection in a cat in Australia. J Feline Med Surg. 2008;10(4):407–412. | |
Van Zyl A, Daniel J, Wayne J, et al. Mycobacterium ulcerans infections in two horses in south-eastern Australia. Aust Vet J. 2010;88(3):101–106. | |
O’Brien CR, McMillan E, Harris O, et al. Localised Mycobacterium ulcerans infection in four dogs. Aust Vet J. 2011;89(12):506–510. | |
O’Brien CR, Handasyde KA, Hibble J, et al. Clinical, microbiological and pathological findings of Mycobacterium ulcerans infection in three Australian Possum species. PLoS Negl Trop Dis. 2014;8(1):e2666. | |
Mitchell PJ, Jerrett IV, Slee KJ. Skin ulcers caused by Mycobacterium ulcerans in koalas near Bairnsdale, Australia. Pathology. 1984;16(3):256–260. | |
Nakanaga K, Hoshino Y, Yotsu RR, Makino M, Ishii N. Nineteen cases of Buruli ulcer diagnosed in Japan from 1980 to 2010. J Clin Microbiol. 2011;49(11):3829–3836. | |
Coloma JN-S, Navarrete-Franco G, Iribe P, López-Cepeda LD. Ulcerative cutaneous mycobacteriosis due to Mycobacterium ulcerans: report of two Mexican cases. Int J Lepr Other Mycobact Dis. 2005;73(1):5–12. | |
Boleira M, Lupi O, Lehman L, Asiedu KB, Kiszewski AE. Buruli ulcer. An Bras Dermatol. 2010;85(3):281–301. | |
Morris A, Gozlan R, Marion E, et al. First detection of Mycobacterium ulcerans DNA in environmental samples from South America. PLoS Negl Trop Dis. 2014;8(1):e2660. | |
Reynaud Y, Millet J, Couvin D, et al. Heterogeneity among Mycobacterium ulcerans from French Guiana revealed by multilocus variable number tandem repeat analysis (MLVA). PLoS One. 2015; 10(2):e0118597. | |
Vandelannoote K, Jordaens K, Bomans P, et al. Insertion sequence element single nucleotide polymorphism typing provides insights into the population structure and evolution of Mycobacterium ulcerans across Africa. Appl Environ Microbiol. 2014;80(3):1197–1209. | |
Bolz M, Bratschi MW, Kerber S, et al. Locally confined clonal complexes of Mycobacterium ulcerans in two Buruli ulcer endemic regions of Cameroon. PLoS Negl Trop Dis. 2015;9(6):e0003802. | |
Guerra H, Palomino JC, Falconí E, et al. Mycobacterium ulcerans disease, Peru. Emerg Infect Dis. 2008;14(3):373–377. | |
Dos Santos VM, Noronha FL, Vicentina EC, Lima CC. Mycobacterium ulcerans infection in Brazil. Med J Aust. 2007;187(1):63–64. | |
McGann H, Stragier P, Portaels F, et al. Buruli ulcer in United Kingdom tourist returning from Latin America. Emerg Infect Dis. 2009;15(11):1827–1829. | |
Tsukamura M, Kaneda K, Imaeda T, Mikoshiba H. [A taxonomic study on a mycobacterium which caused a skin ulcer in a Japanese girl and resembled Mycobacterium ulcerans]. Kekkaku. 1989;64(11):691–697. Japanese. | |
Kazumi Y, Ohtomo K, Takahashi M, et al. [Mycobacterium shinshuense isolated from cutaneous ulcer lesion of right lower extremity in a 37-year-old woman]. Kekkaku. 2004;79(7):437–441. Japanese. | |
Yotsu RR, Nakanaga K, Hoshino Y, Suzuki K, Ishii N. Buruli ulcer and current situation in Japan: a new emerging cutaneous Mycobacterium infection. J Dermatol. 2012;39(7):587–593. | |
Ohtsuka M, Kikuchi N, Yamamoto T, et al. Buruli ulcer caused by Mycobacterium ulcerans subsp shinshuense: a rare case of familial concurrent occurrence and detection of insertion sequence 2404 in Japan. JAMA Dermatol. 2014;150(1):64–67. | |
Faber WR, Arias-Bouda LM, Zeegelaar JE, et al. First reported case of Mycobacterium ulcerans infection in a patient from China. Trans R Soc Trop Med Hyg. 2000;94(3):277–279. | |
Johnson PD, Veitch MG, Leslie DE, Flood PE, Hayman JA. The emergence of Mycobacterium ulcerans infection near Melbourne. Med J Aust. 1996;164(2):76–78. | |
Johnson PDR, Azuolas J, Lavender CJ, et al. Mycobacterium ulcerans in mosquitoes captured during outbreak of Buruli ulcer, southeastern Australia. Emerg Infect Dis. 2007;13(11):1653–1660. | |
Boyd SC, Athan E, Friedman ND, et al. Epidemiology, clinical features and diagnosis of Mycobacterium ulcerans in an Australian population. Med J Aust. 2012;196(5):341–344. | |
Van Ravensway J, Benbow ME, Tsonis AA, et al. Climate and landscape factors associated with Buruli ulcer incidence in Victoria, Australia. PLoS One. 2012;7(12):e51074. | |
Fyfe JAM, Lavender CJ, Handasyde KA, et al. A major role for mammals in the ecology of Mycobacterium ulcerans. PLoS Negl Trop Dis. 2010;4(8):e791. | |
Carson C, Lavender CJ, Handasyde KA, et al. Potential wildlife sentinels for monitoring the endemic spread of human Buruli ulcer in South-East Australia. PLoS Negl Trop Dis. 2014;8(1):e2668. | |
Johnson PDR, Lavender CJ. Correlation between Buruli ulcer and vector-borne notifiable diseases, Victoria, Australia. Emerg Infect Dis. 2009;15(4):614–615. | |
Radford, AJ. Mycobacterium ulcerans infections in Papua New Guinea. Papua New Guinea Med J. 1974;(17):145–149. | |
Reid IS. Mycobacterium ulcerans infection: a report of 13 cases at the Port Moresby General Hospital, Papua. Med J Aust. 1967;1(9):427–431. | |
Igo JD, Murthy DP. Mycobacterium ulcerans infections in Papua New Guinea: correlation of clinical, histological, and microbiologic features. Am J Trop Med Hyg. 1988;38(2):391–392. | |
Christie M. Suspected Mycobacterium ulcerans disease in Kiribati. Med J Aust. 1987;146(11):600–604. | |
Pettit JHS, Marchette NJ, Rees RJW. Mycobacterium ulcerans infection. Br J Dermatol. 1966;78(4):187–197. | |
Oluwasanmi JO, Solankee TF, Olurin EO, Itayemi SO, Alabi GO, Lucas AO. Mycobacterium ulcerans (Buruli) skin ulceration in Nigeria. Am J Trop Med Hyg. 1976;25(1):122–128. | |
Barker DJ. Buruli disease in a district of Uganda. J Trop Med Hyg. 1971;74(12):260–264. | |
Hayman J. Postulated epidemiology of Mycobacterium ulcerans infection. Int J Epidemiol. 1991;20(4):1093–1098. | |
Merritt RW, Walker ED, Small PLC, et al. Ecology and transmission of Buruli ulcer disease: a systematic review. PLoS Negl Trop Dis. 2010;4(12):e911. | |
Epidemiology of Mycobacterium ulcerans infection (Buruli ulcer) at Kinyara, Uganda. Trans R Soc Trop Med Hyg. 1971;65(6):763–775. | |
Vincent QB, Ardant M-F, Adeye A, et al. Clinical epidemiology of laboratory-confirmed Buruli ulcer in Benin: a cohort study. Lancet Glob Health. 2014;2(7):e422–e430. | |
Quek TYJ, Athan E, Henry MJ, et al. Risk factors for Mycobacterium ulcerans infection, southeastern Australia. Emerg Infect Dis. 2007; 13(11):1661–1666. | |
Sopoh GE, Barogui YT, Johnson RC, et al. Family relationship, water contact and occurrence of Buruli ulcer in Benin. PLoS Negl Trop Dis. 2010;4(7):e746. | |
Phillips RO, Phanzu DM, Beissner M, et al. Effectiveness of routine BCG vaccination on Buruli ulcer disease: a case-control study in the democratic Republic of Congo, Ghana and Togo. PLoS Negl Trop Dis. 2015;9(1):e3457. | |
Kenu E, Nyarko KM, Seefeld L, et al. Risk factors for Buruli ulcer in Ghana – a case control study in the Suhum-Kraboa-Coaltar and Akuapem South Districts of the Eastern Region. PLoS Negl Trop Dis. 2014;8(11):e3279. | |
Raghunathan PL, Whitney EAS, Asamoa K, et al. Risk factors for Buruli ulcer disease (Mycobacterium ulcerans infection): Results from a case-control study in Ghana. Clin Infect Dis. 2005;40(10):1445–1453. | |
Aiga H, Amano T, Cairncross S, Domako JA, Nanas O-K, Coleman S. Assessing water-related risk factors for Buruli ulcer: A case-control study in Ghana. Am J Trop Med Hyg. 2004;71(4):387–392. | |
Johnson RC, Nackers F, Glynn JR, et al. Association of HIV infection and Mycobacterium ulcerans disease in Benin. AIDS. 2008;22(7):901–903. | |
Nackers F, Johnson RC, Glynn JR, Zinsou C, Tonglet R, Portaels F. Environmental and health-related risk factors for Mycobacterium ulcerans disease (Buruli ulcer) in Benin. Am J Trop Med Hyg. 2007; 77(5):834–836. | |
Nackers F, Tonglet R, Slachmuylder V, et al. Association between haemoglobin variants S and C and Mycobacterium ulcerans disease (Buruli ulcer): a case-control study in Benin. Trop Med Int Health. 2007;12(4):511–518. | |
Nackers F, Dramaix M, Johnson RC, et al. BCG vaccine effectiveness against Buruli ulcer: a case-control study in Benin. Am J Trop Med Hyg. 2006;75(4):768–774. | |
Landier J, Boisier P, Fotso Piam F, et al. Adequate wound care and use of bed nets as protective factors against Buruli ulcer: Results from a case control study in Cameroon. PLoS Negl Trop Dis. 2011;5(11):e1392. | |
Pouillot R, Matias G, Wondje CM, et al. Risk factors for Buruli ulcer: A case control study in Cameroon. PLoS Negl Trop Dis. 2007; 1(3):e101. | |
Ahoua L, Guetta AN, Ekaza E, Bouzid S, N’Guessan R, Dosso M. Risk factors for Buruli ulcer in Cote d’Ivoire: Results of a case-control study, Aug 2001. Afr J Biotechnol. 2009;8(4):536–546. | |
Barker DJ, Carswell JW. Mycobacterium ulcerans infection among tsetse control workers in Uganda. Int J Epidemiol. 1973;2(2):161–165. | |
Debacker M, Portaels F, Aguiar J, et al. Risk factors for Buruli ulcer, Benin. Emerg Infect Dis. 2006;12(9):1325–1331. | |
Jacobsen KH, Padgett JJ. Risk factors for Mycobacterium ulcerans infection. Int J Infect Dis. 2010;14(8):e677–e681. | |
Debacker M, Aguiar J, Steunou C, et al. Mycobacterium ulcerans disease: role of age and gender in incidence and morbidity. Trop Med Int Health. 2004;9(12):1297–1304. | |
Bratschi MW, Bolz M, Minyem JC, et al. Geographic distribution, age pattern and sites of lesions in a cohort of Buruli ulcer patients from the Mapé Basin of Cameroon. PLoS Negl Trop Dis. 2013;7(6):e2252. | |
Röltgen K, Bratschi MW, Ross A, et al. Late onset of the serological response against the 18 kDa small heat shock protein of Mycobacterium ulcerans in children. PLoS Negl Trop Dis. 2014;8(5):e2904. | |
Amofah GK, Sagoe-Moses C, Adjei-Acquah C, Frimpong EH. Epidemiology of Buruli ulcer in Amansie West district, Ghana. Trans R Soc Trop Med Hyg. 1993;87(6):644–645. | |
Hospers IC, Wiersma IC, Dijkstra PU, et al. Distribution of Buruli ulcer lesions over body surface area in a large case series in Ghana: Uncovering clues for mode of transmission. Trans R Soc Trop Med Hyg. 2005;99(3):196–201. | |
Stienstra Y, van der Werf TS, Oosterom E, et al. Susceptibility to Buruli ulcer is associated with the SLC11A1 (NRAMP1) D543N polymorphism. Genes Immun. 2006;7(3):185–189. | |
Setia MS, Steinmaus C, Ho CS, Rutherford GW. The role of BCG in prevention of leprosy: a meta-analysis. Lancet Infect Dis. 2006;6(3):162–170. | |
Luca S, Mihaescu T. History of BCG vaccine. Maedica (Buchar). 2013;8(1):53–58. | |
Roy A, Eisenhut M, Harris RJ, et al. Effect of BCG vaccination against Mycobacterium tuberculosis infection in children: systematic review and meta-analysis. BMJ. 2014;349:g4643. | |
The Uganda Buruli Group. BCG vaccination against Mycobacterium ulcerans infection (Buruli ulcer). First results of a trial in Uganda. Lancet. 1969;293(7586):111–115. | |
Smith PG, Revill WD, Lukwago E, Rykushin YP. The protective effect of BCG against Mycobacterium ulcerans disease: a controlled trial in an endemic area of Uganda. Trans R Soc Trop Med Hyg. 1976;70(5–6):449–457. | |
Portaels F, Aguiar J, Debacker M, et al. Prophylactic effect of Mycobacterium bovis BCG vaccination against osteomyelitis in children with Mycobacterium ulcerans disease (Buruli ulcer). Clin Diagn Lab Immunol. 2002;9(6):1389–1391. | |
Portaels F, Aguiar J, Debacker M, et al. Mycobacterium bovis BCG vaccination as prophylaxis against Mycobacterium ulcerans osteomyelitis in Buruli ulcer disease. Infect Immun. 2004;72(1):62–65. | |
Vincent QB, Ardant M-F, Marsollier L, Chauty A, Alcaïs A, Franco-Beninese Buruli Research Group (listed in appendix). HIV infection and Buruli ulcer in Africa. Lancet Infect Dis. 2014;14(9):796–797. | |
Christinet V, Comte E, Ciaffi L, et al. Impact of human immunodeficiency virus on the severity of Buruli ulcer disease: results of a retrospective study in Cameroon. Open Forum Infect Dis. 2014; 1(1):ofu021. | |
Johnson RC, Ifebe D, Hans-Moevi A, et al. Disseminated Mycobacterium ulcerans disease in an HIV-positive patient: a case study. AIDS. 2002;16(12):1704–1705. | |
Toll A, Gallardo F, Ferran M, et al. Aggressive multifocal Buruli ulcer with associated osteomyelitis in an HIV-positive patient. Clin Exp Dermatol. 2005;30(6):649–651. | |
Kibadi K, Colebunders R, Muyembe-Tamfum J-J, Meyers WM, Portaels F. Buruli ulcer lesions in HIV-positive patient. Emerg Infect Dis. 2010;16(4):738–739. | |
Komenan K, Elidjé EJ, Ildevert GP, et al. Multifocal Buruli ulcer associated with secondary infection in HIV positive patient. Case Rep Med. 2013;2013:348628. | |
Bayonne Manou LS, Portaels F, Eddyani M, Book AU, Vandelannoote K, de Jong BC. L’infection à Mycobacterium ulcerans (ulcère de Buruli) au Gabon de 2005 à 2011 [Mycobacterium ulcerans disease (Buruli ulcer) in Gabon: 2005–2011]. Med Sante Trop. 2013;23(4):450–457. French. | |
Trubiano JA, Lavender CJ, Fyfe JAM, Bittmann S, Johnson PDR. The incubation period of Buruli ulcer (Mycobacterium ulcerans infection). PLoS Negl Trop Dis. 2013;7(10):e2463. | |
Sopoh GE, Johnson RC, Chauty A, et al. Buruli ulcer surveillance, Benin, 2003–2005. Emerg Infect Dis. 2007;13(9):1374–1376. | |
Lavender CJ, Fyfe JAM, Azuolas J, et al. Risk of Buruli ulcer and detection of Mycobacterium ulcerans in mosquitoes in Southeastern Australia. PLoS Negl Trop Dis. 2011;5(9):e1305. | |
Bratschi MW, Ruf M-T, Andreoli A, et al. Mycobacterium ulcerans persistence at a village water source of Buruli ulcer patients. PLoS Negl Trop Dis. 2014;8(3):e2756. | |
Portaels F, Elsen P, Guimaraes-Peres A, Fonteyne PA, Meyers WM. Insects in the transmission of Mycobacterium ulcerans infection. Lancet. 1999;353(9157):986. | |
Portaels F, Meyers WM, Ablordey A, et al. First cultivation and characterization of Mycobacterium ulcerans from the environment. PLoS Negl Trop Dis. 2008;2(3):e178. | |
Williamson HR, Mosi L, Donnell R, Aqqad M, Merritt RW, Small PLC. Mycobacterium ulcerans fails to infect through skin abrasions in a guinea pig infection model: implications for transmission. PLoS Negl Trop Dis. 2014;8(4):e2770. | |
Sizaire V, Nackers F, Comte E, Portaels F. Mycobacterium ulcerans infection: control, diagnosis, and treatment. Lancet Infect Dis. 2006;6(5):288–296. | |
Etuaful S, Carbonnelle B, Grosset J, et al. Efficacy of the combination rifampin-streptomycin in preventing growth of Mycobacterium ulcerans in early lesions of Buruli ulcer in humans. Antimicrob Agents Chemother. 2005;49(8):3182–3186. | |
Chauty A, Ardant M-F, Adeye A, et al. Promising clinical efficacy of streptomycin-rifampin combination for treatment of Buruli ulcer (Mycobacterium ulcerans disease). Antimicrob Agents Chemother. 2007;51(11):4029–4035. | |
Sarfo FS, Phillips R, Asiedu K, et al. Clinical efficacy of combination of rifampin and streptomycin for treatment of Mycobacterium ulcerans disease. Antimicrob Agents Chemother. 2010;54(9):3678–3685. | |
Grietens KP, Boock AU, Peeters H, Hausmann-Muela S, Toomer E, Ribera JM. “It is me who endures but my family that suffers”: social isolation as a consequence of the household cost burden of Buruli ulcer free of charge hospital treatment. PLoS Negl Trop Dis. 2008;2(10):e321. | |
Adamba C, Owusu AY. Burden of Buruli ulcer: How affected households in a Ghanaian district cope. Afr Study Monogr. 2011; 32(1):1–23. | |
Amoakoh HB, Aikins M. Household cost of out-patient treatment of Buruli ulcer in Ghana: a case study of Obom in Ga South Municipality. BMC Health Serv Res. 2013;13:507. | |
Hotez PJ, Velasquez RM, Wolf JE Jr. Neglected tropical skin diseases: Their global elimination through integrated mass drug administration? JAMA Dermatol. 2014;150(5):481–482. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


