Back to Journals » Infection and Drug Resistance » Volume 16
Epidemiological Features and Impact of High Glucose Level on Virulence Gene Expression and Serum Resistance of Klebsiella pneumoniae Causing Liver Abscess in Diabetic Patients
Authors Tang L , Wang H, Cao K , Li Y , Li T, Huang Y, Xu Y
Received 25 October 2022
Accepted for publication 18 February 2023
Published 28 February 2023 Volume 2023:16 Pages 1221—1230
DOI https://doi.org/10.2147/IDR.S391349
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Ling Tang,* Hui Wang,* Kangli Cao,* Yajuan Li, Tingting Li, Ying Huang, Yuanhong Xu
Department of Clinical Laboratory, First Affiliated Hospital of Anhui Medical University, Hefei, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yuanhong Xu, Email [email protected]
Purpose: Klebsiella pneumoniae (K. pneumoniae) is a Gram-negative bacterium that is predominantly associated with liver abscesses in global diabetic patients. High levels of glucose in the surrounding of K. pneumonia increase its pathogenicity including capsular polysaccharide (CPS) and fimbriae. Other important virulent factors include outer membrane protein A (ompA) and regulator mucoid phenotype A (rmpA). The objective of this investigation was to elucidate the effects of high glucose on rmpA and ompA gene expression and serum resistance of K. pneumoniae causing liver abscess.
Patients and Methods: The clinical history of 57 patients suffering from K. pneumoniae-caused liver abscesses (KLA) was acquired and their clinical and laboratory manifestations in the presence or absence of diabetes were analyzed. The antimicrobial susceptibility, serotypes, and virulence genes were tested. Clinical isolates of 3 serotype-K1 hypervirulent K. pneumoniae (hvKP) were used to detect the effect of exogenous high glucose on rmpA, ompA, and clbB genes expression, and bacterial serum resistance.
Results: KLA patients with diabetes showed higher C-reactive protein (CRP) compared to non-diabetic KLA patients. Furthermore, the diabetic group showed increased incidences of sepsis and invasive infections, and their length of hospital stay was also prolonged. Pre-incubation of K. pneumoniae in high glucose (0.5%) concentration up-regulated rmpA, ompA, and clbB genes expression. However, cAMP supplementation, which was inhibited by environmental glucose, reversed the increase of rmpA and ompA in a cAMP-dependent manner. Moreover, hvKP strains incubated in high glucose also exhibited enhanced protection from serum killing.
Conclusion: High glucose levels reflected by poor glycemic control has increased gene expression of rmpA and ompA in hvKP by the cAMP signaling pathway and enhanced its resistance to serum killing, thus providing a new and reasonable explanation for the high incidences of sepsis and invasive infections in KLA patients with diabetes.
Keywords: Klebsiella pneumoniae, liver abscess, diabetes, rmpA, ompA, serum resistance
Introduction
Klebsiella pneumoniae after colonization (in its virulent state) crosses the intestinal-mucosal barrier and targets the liver through the portal vein system, subsequently causing K. pneumoniae-caused liver abscesses (KLA). K. pneumoniae is bacteria that predominantly contributes to pyogenic liver abscesses worldwide.1 KLA is generically cryptogenic and frequently becomes complicated because of invasive infection to other organs due to K. pneumoniae virulence factors, which include, capsular serotype, rmpA, and aerobactin.2 Because of invasive systemic infections, the KLA mortality rate equates to 10% within 30 days of hospitalization.3 Unfortunately, the dramatically increased multi-drug resistance (MDR) of hyper-virulent K. pneumoniae (hvKP), especially resistance to carbapenems and third-generation cephalosporins, presents a great challenge for clinical treatment.
KLA is accompanied by many chronic diseases. Studies have shown that diabetes mellitus is a concomitant condition in approximately 29.3–44.3% of liver abscess cases.4,5 Hyperglycemia weakens the immune barrier and increases the susceptibility to hvKP infection by inhibiting phagocytosis6 and neutrophil extracellular traps (NETs) generation,7 reducing macrophages’ function,8 and impairing the bacteriolysis of antibodies and complement.9 Poor glycemic control, also affects the pathogenicity of K. pneumoniae. Lee et al found that high glucose levels stimulated the biosynthesis of polysaccharide and gene expression of hvKP’s cps, thereby increasing phagocytosis resistance and development of invasive syndrome.10 Exogenous glucose can also activate the production of K. pneumoniae’s type 3 fimbriae. These functions are under the control of global modulator cyclic AMP (cAMP) receptor protein (CRP) and cAMP-CRP signaling pathway.11 However, it is unknown if the expressions of other known pathogenic factors, for instance, the rmpA, ompA, and colibactin are also affected.
The K. pneumoniae pathogenic factors help its survival and colonization outside the gastrointestinal tract. RmpA is present at the large virulence plasmid and promotes capsular production.12 Outer membrane protein A (OmpA) is a unique protein, abundantly located in the outer membrane of Gram-negative bacteria, and it is highly conserved throughout its evolution.13 Since ompA expression is under the control of two stress-stimulated ribonucleolytic mechanisms, many environmental triggers can modulate ompA expression, for example, acid challenge,14 antimicrobial peptide,15 cAMP,16 and polyamines.17 Moreover, in Gram-negative bacteria, ompA performs many functions such as toxicity, invasion, serum resistance, adhesion, and biofilm formation.18
Enhanced polysaccharide biosynthesis stimulated by glucose further increased the K. pneumoniae resistance to phagocytosis of neutrophils and destruction of blood leukocytes. However, it has not been studied whether K. pneumoniae cultured with high-glucose increases its resistance to serum killing. Serum comprises >30 complement system proteins, this system is associated with the host’s innate immune response.19 The structural integrity of the cellular envelope is crucial for serum survival. Capsule and OmpA have been implicated in serum resistance.18,20
In this investigation, the clinical manifestations associated with KLA patients in the presence or absence of diabetes and the association between glycemic control and the invasive syndrome were determined. Then, the present study aimed to explore the impact of high exogenous glucose levels on rmpA, ompA, and colibactin clbB gene expression and serum resistance of K. pneumoniae in in-vitro assays. It is expected that this will provide a new and reasonable explanation for the high incidences of sepsis and invasive infections in KLA patients with diabetes.
Materials and Methods
Study Population
This retrospective cohort investigation analyzed the KLA patients who were also diabetic and were enrolled at The First Affiliated Hospital of Anhui Medical University, from January 2020 to November 2022 in a tertiary medical center with a 3000-bed capacity. And was duly authorized by the human ethics council of First Affiliated Hospital of Anhui Medical University. The KLA patients were confirmed after the culture reports from the Department of Microbiology were reviewed and the diagnosis was based on the typical clinical symptoms, liver abscess cavity imaging examinations, and culture identification via K. pneumoniae blood isolate or puncture fluid samples. A total of 24 K. pneumoniae isolates were collected in non-diabetic group, including 14 strains from puncture fluid and 10 strains from blood. In diabetic group, 33 K. pneumoniae isolates were collected, including 23 strains from puncture fluid and 10 strains from blood. Diabetic patients were elaborated as those who were previously diagnosed with either type 2 or type 1 diabetes and/or those who were either taking insulin and/or oral hypoglycemic drugs. Participants’ laboratory reports, sex, underlying diseases, age, clinical manifestations, and management were gathered. Sepsis-3.0 criteria were used for defining sepsis.21 And the infection was deemed metastasized when the infection spread to a distant site by the same pathogen as the pyogenic liver abscess (K. pneumoniae).
Microbiologic Data
For recognizing all the isolates, Matrix-Assisted Laser Desorption/Ionization-Time of Flight mass spectrometry (MALDI-TOF MS, Vitek MS, bioMérieux, France) was utilized. In accordance with the 2019 Clinical and Laboratory Standards Institute’s recommendations, 28 antibiotics were tested for their susceptibility via VITEK 2 (Card number: AST-GN13) system or Kirby-Bauer Disk Diffusion (Oxoid, UK) (CLSI) method. K. pneumoniae (ATCC700603) and Escherichia coli (ATCC25922) isolates were kept as standards to ensure quality control. Agar dilution assessment with the help of ceftazidime and cefotaxime combined with clavulanate was performed to confirm ESBL. Resistance to carbapenem (meropenem, imipenem, and ertapenem) was confirmed via disk diffusion protocol.
Detection of Capsular Serotypes and Virulent Genes
Columbia blood agar plates were prepared for inoculating the strains which were then incubated for 18 hours at 37°C in 5% CO2 chamber. K. pneumoniae isolates DNA was collected via a DNA extraction kit (Sangon Biotech, Shanghai, China) by following the instructions provided by the manufacturer. K1, K2, K5, K20, K54, and K57 capsular serotypes and virulence-associated genes, including wcaG, magA, rmpA, alls, iucb, aerobactin, iroN, kfu, entB, irp1, clbA, clbB, and clbN, were determined by PCR. 25μL reactions were prepared with 2× Spark Taq PCR Master Mix (Solely Bio, Shandong, China) (12.5μL), forward and reverse primer (10 pmol/μL primer stock) (both 1μL), genomic DNA (1μL), and water (9.5μL). PCR was run; for 4 min initial activation at 95°C, then 30s cycles 35 times at 94 °C, 60 °C for 30s, and 72 °C for 60s, and lastly, a 10 min final extension at 72 °C. 1% agarose gel electrophoresis was performed for separating PCR products. Table S1 enlists all the primers employed.
Quantitative RT-PCR
3 K. pneumoniae (serotype K1) strains isolated from the participants and classic K. pneumoniae ATCC700603 were considered the representing strains in the subsequent experiments. The three clinical isolates were named KP133, KP164 and KP188. Among these isolates, KP133 was isolated from the non-diabetic group and the remaining two were from the diabetic group. K. pneumoniae were cultured in glucose-free LB broth, 0.5% glucose LB broth, or 0.5% glucose containing 1mM cAMP LB broth, respectively. After incubated at 37°C for 6h, total RNA was acquired from exponential growth phase bacterial cells via the Bacterial RNA Kit (YEASEN, China) according to the developer’s protocol. RNA was reverse-transcribed with the RT Master Mix (Takara, Japan) using random primers. qRT-PCR was carried out in a Light-Cycler 480 (Roche, Basel, Switzerland) instrument using SYBR Premix Ex TaqII (Takara, Japan). The cycling parameters included; 30s at 95°C, 5s of 40 cycles at 95°C, and 60°C for 20s. Relative gene expression was measured by the comparative threshold cycle 2−ΔΔCTmethod. 23S rRNA was selected as an endogenous reference. All samples were analyzed thrice. The primer’s sequences are listed in Table S1.
Serum Killing Assays
The bacterial susceptibility to human serum was assayed using a modified method by Yeh et al.22 KP133, KP164 and KP188 strains were isolated from the participants and were considered the representing strains in the subsequent experiments. The serotypes and virulence-associated genes of the three clinical hvKP strains are presented in Table S2. Briefly, human serum from 8 healthy donors was incubated with LB broth cultured hvKP strains in the presence and absence of 0.5% glucose. Bacteria were diluted to 4×106 colony-forming units (c.f.u.)/mL in physiological saline. The bacterial suspensions (25μL) and the acquired nonimmune human serum (75μL) were dispensed in 96 well-plate, mixed, and incubated at 37 °C. Viability was determined immediately after 1h, 2h, and 3 h incubation. After mixing, samples were taken and serial dilutions were plated on MH agar, then incubated at 37°C for 18h for colony counts.
Statistical Analysis
SPSS (25.0 version) was utilized for assessing the statistics. Normally distributed continuous variables were demonstrated as the mean ± standard deviation and for their comparison, Student’s t-test was applied. Categorical variable data were disclosed as n (%) and Fisher’s exact test was performed for their comparative analysis. Statistical analysis was performed using GraphPad Prism 6.01. All histograms related quantitative data were expressed as mean ± standard deviation (SD). Comparisons between two groups were made using an unpaired t-test. P<0.05 was considered significant.
Results
Comparison of Clinical Characteristics of KLA with Diabetes and without Diabetes
The 57 K. pneumoniae liver abscess diagnosed patient’s demographic manifestations, underlying disorder, and complications associated with diabetes (n=33, 57.9%) and without diabetes (n=24, 42.1%) are summarized in Table 1. More than half of the patients were male (42/57, 73.7%) and the average age of the patients at diagnosis was 58.5±14.7 years (range 30 to 99). Fever and abdominal pain were the predominant manifestations in these two groups. Diabetes followed by hypertension (29.8%, 17/57) and biliary tract diseases (28.1%, 16/57) was the most common underlying disease. Poor glycemic control patients had the trend of higher sepsis rate (51.5% vs 20.8%, P=0.028) and invasive infections (45.5% vs 12.5%, P=0.01), and therefore longer hospital stays (23.3±7.4 vs 17.3±8.6, P=0.01) than those with controlled glycaemia. Laboratory investigations recorded on admission are also shown in Table 1. The levels of CRP were positively correlated with the degree of infection, and >100 mg/L CRP indicated a serious infection. In this research, the proportion of CRP>100 mg/L in the diabetic group was significantly higher than that in the non-diabetic group.
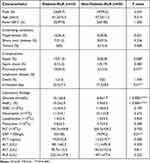 |
Table 1 Baseline Characteristics, Clinical Presentation, and Outcome of KLA Patients with Diabetes and without Diabetes |
Microbiological Characteristics and Antimicrobial Susceptibility
In addition to its natural ampicillin resistance, the isolated K. pneumoniae was highly sensitive to other antibiotics. A few strains were resistant to levofloxacin, ciprofloxacin, ampicillin/sulbactam, and cotrimoxazole, but there was no difference between the two groups (Table 2). Unfortunately, compared to the high antibiotic susceptibility of K. pneumoniae in the diabetic group, the non-diabetic group had two multi-drug resistant strains, including an extended-spectrum β-lactamase-producing K. pneumoniae (ESBL-KP) and a carbapenem-resistant K. pneumoniae (CR-KP) isolate.
 |
Table 2 Antimicrobial Susceptibility of K. pneumoniae Isolates from KLA Patients with Diabetes and without Diabetes |
Four serotypes were detected in K. pneumonia patients, these were K1 (57.9%, 33/57), K2 (19.3%, 11/57), K5 (1.8%, 1/57), and K57 (7%, 4/57), while K20 and K54 were not detected in any tested isolates (Table 3). Eight isolates (14.0%, 8/57) were not classified successfully (referred to as; K-non-typable isolates). The capsular serotypes prevalence among diabetic and non-diabetic isolates were similar. The expression of virulence genes including wcaG, magA, rmpA, kfu, iro, entB, irp1, aero, and alls showed no marked change between the diabetic and non-diabetic K. pneumoniae isolates. However, all diabetic isolates harbored rmpA and irp1, while 87.5% of non-diabetic isolates expressed these important virulence-associated genes (P=0.068). Colibactin encoded by the pks gene cluster (clbA, clbB, and clbN), damages DNA and enhances virulence.23 In this research, the colibactin system markers clbA, clbB, and clbN were simultaneously identified in 42.1% (24/57) isolates, which were considered as pks+ K. pneumoniae.24 The positive rates of pks+ K. pneumoniae among diabetic isolates (51.5%, 17/33) were higher than those among non-diabetic isolates (29.2%, 7/24). However, no notable difference (p=0.11) in pks expression between the two cohorts was noticed.
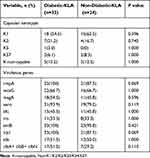 |
Table 3 Capsular Serotypes and Virulence Factors of K. pneumoniae Isolated from Patients with Diabetes and without Diabetes |
Effect of Exogenous Glucose on the rmpA, ompA, and clbB Transcription of K. pneumoniae
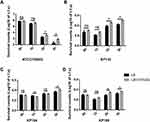
The impact of exogenous high glucose level on the transcriptions of K. pneumoniae virulent genes was analyzed. With the help of classic K. pneumoniae ATCC700603 and 3 serotype-K1 hvKP clinical isolates, the effect of exogenous high glucose level on rmpA, ompA, and clbB genes expression were assessed. Virulence genes expression of the three isolates was shown in Table S2. The clinical isolates were cultured in LB broth (in the presence or absence of 0.5% glucose). The mRNA levels of rmpA, ompA, and clbB were quantified by qRT-PCR. As Figure 1B–D depicts, adding exogenous glucose (0.5%) in LB broth increased the mRNA of rmpA, ompA, and clbB genes expression. The intracellular cAMP levels were reduced in LB broth with glucose25,26 and exogenous 1mM cAMP was added to the glucose-augmented broth to observe the mRNA levels of these genes. The exogenous cAMP addition suppressed the glucose effect on the transcription of rmpA and ompA, but did not affect clbB (Figure 1B–D). K. pneumoniae ATCC700603 did not express virulence factors rmpA and clbB. Our results showed high glucose levels also increased the transcription of ompA in K. pneumoniae ATCC700603, and this up-regulation was controlled by cAMP (Figure 1A). These results suggested that high glucose concentration up-regulated the transcription of rmpA and ompA in K. pneumoniae by the cAMP signaling pathway.
Environmental Glucose Stimuli Increased K. pneumoniae Resistance to Serum Killing
High glucose levels could enhance cps genes expression of hvKP, which increases resistance to phagocytosis.6 However, the literature suggesting whether K. pneumoniae cultured with high-glucose increases its resistance to serum killing is still missing. To evaluate the serum’s sensitivity to the K. pneumoniae pathogenic effect, grown in high glucose, serum killing assays were performed. Bacterial survival in normal human serum was recorded at different time points. As shown in Figure 2A, the survival rate of K. pneumoniae ATCC700603 strain decreased over time. However, after co-cultured with serum for 2 and 3 hours, the survival rate of the strain cultured in LB with high glucose level (0.5%) was increased compared with that in LB broth. Compared with classic K. pneumoniae ATCC700603, clinical isolates (KP133, KP164 and KP188) expressed virulence factors and showed a hypermucoviscous phenotype. The rate of hvKP strains survival was also markedly enhanced when grown in LB with 0.5% glucose than that in LB broth lacked glucose (Figure 2B–D).
Discussion
Diabetes is a risk factor for infectious diseases, such as KLA, tuberculosis, melioidosis, and COVID-19.27–30 Previous studies have shown that diabetes impairs the innate and adaptive immune system, impairing macrophages, T cells, neutrophils, and NK cells.31 This immune dysfunction increases the risk of infection in diabetic patients. Lin et al found that diabetic control was crucial for the clinical KLA characteristics, especially when dealing with metastatic complications associated with KLA.30 Consistent with the above study, we observed that substandard control of glucose in patients leads to a higher rate of invasive infections than in those with controlled glycaemia. The major abnormalities of KLA in the laboratory were increased CRP and liver dysfunction.32,33 It was also demonstrated in this investigation that the levels of CRP in diabetic-KLA patients were higher, which suggested a more severe infection. Sepsis is a lethal organ dysfunction caused by a dysregulated host response to an infection. Septic-KLA patients had an increased risk of chronic metastatic complications and prolonged hospital stays.33 The results from this research indicated that the incidence of sepsis was higher and the hospital stays were longer in KLA with diabetes.
In this study, the virulence genes and capsular serotypes were detected by PCR. 94.7% (55/57) K. pneumoniae expressed rmpA and at least one siderophore. Moreover, hvKP strains isolated from KLA patients were sensitive to a range of antibiotics. So, empirical antibiotic therapy for liver abscesses (including three generation of cephalosporin, carbapenems and piperacillin-tazobactam) was generally effective. However, compared to the high antibiotic susceptibility of K. pneumoniae in the diabetic group, the non-diabetic group had two multi-drug resistant strains, including an ESBL-KP and a CR-KP isolate. The CR-KP isolate belonged to K2 serotype and expressed siderophores virulence genes (entB and irp1). Both the two multi-drug resistant K. pneumoniae isolates recovered from patients with hepatic diseases and repeated hospitalizations. Therefore, it is also necessary to pay attention to the occurrence of KLA after liver surgery, especially caused by multi-drug resistant K. pneumoniae.
Diabetes is an important risk factor for KLA.34 Hyperglycaemia dysregulates the neutrophil phagocytosis of K. pneumoniae capsule serotypes K1 and K2.6 While, high glucose levels also affect K. pneumoniae pathogenicity, including enhancement of CPS and type 3 fimbriae.10,11 The glucose levels used in our experiments is supposed to be similar to the human bloodstream concentrations. Literature suggests that healthy control would have 0.1% glucose blood concentration, while diabetic one would have 0.2–0.5% glucose blood concentrations.10 Our study showed that addition of exogenous glucose (0.5%) in LB broth enhanced mRNA of ompA and rmpA gene expression. RmpA is an important virulent factor in hvKP isolate, which could stimulate capsular production, resulting in the formation of hypermucoviscous phenotype.12 In our study, 87.5% (21/24) of K. pneumoniae isolated from KLA without diabetes harbored the rmpA gene, while 100% of K. pneumoniae in KLA with diabetes were rmpA-positive. Furthermore, high glucose increased the mRNA of rmpA by regulating the cAMP-CRP signal pathway. As rmpA activates K. pneumoniae’s CPS biosynthesis, the results indicated that this is done not only by the direct cps genes regulation but also probably by increasing rmpA gene expression.
OmpA is an abundant predominant outer membrane protein of Enterobacteriaceae13 and is under the influence of many environmental stimuli. Gibert et al revealed that glucose augmented culture medium reduced the transcription of the ompA gene in Escherichia coli.16 Increased glucose inhibits intracellular second messenger cAMP production and inactivates the cAMP-CRP signaling pathway.25,26 Liu et al demonstrated that ompA increased in protein levels in the crp deletion K. pneumoniae by proteomics analysis. However, there was no significant difference in the level of transcription in the high-glucose environment.35 Therefore, the regulation of ompA by high glucose is still controversial. This investigation revealed that high glucose enhanced the mRNA of ompA and the addition of exogenous cAMP suppressed its enhancement.
Resistance to serum killing is associated with K. pneumoniae hyper-virulence. The CPS, O-antigen of LPS, and outer membrane proteins are important pathogenic factors that protect K. pneumoniae from being killed by the serum.19 K. pneumoniae was able to prevent complement activation by modifying sugar structures.36 A thick outside polysaccharide layer creates a physical barrier in the bacterium that inhibits the penetration of the membrane attack complex (MAC), thereby preventing bacterial lysis.37 Apart from the capsular polysaccharides and LPS structures, K. pneumoniae also utilizes outer membrane proteins to escape from the complement system detection.38 OmpA directly restricts the complement cascade to mediate serum resistance.39 Our results indicate that pre-incubation of K. pneumoniae strains in media containing high glucose enhanced rmpA and ompA gene expression, which might lead to increased bacterial resistance to serum killing.
Conclusion
In summary, KLA patients with diabetes showed a higher incidences of sepsis and invasive infections, and their length of hospital stay was also prolonged than those without diabetes. But there was no significant differences in antibiotic susceptibility and virulence genes between the two groups. Furthermore, high glucose levels increased the transcription of rmpA and ompA in hvKP by the cAMP signaling pathway and enhanced the resistance to serum killing, thus providing a new and reasonable explanation for the high incidence of sepsis and invasive infections in KLA patients with diabetes.
Data Sharing Statement
All primers used in this article and virulence genes expression of the three clinical isolates are available in Supplementary Materials. Readers can contact the corresponding author if they want access to additional materials.
Ethics Approval and Consent to Participate
This study complies with the guidelines for human studies and is in accordance with the Declaration of Helsinki. All clinical data of the patients were collected in accordance with the Local Research Ethics Committee of the First Affiliated Hospital of Anhui Medical University (Quick-PJ 2023-01-16). The need for written informed consent was waived because the samples were routinely collected and patients’ anonymous information was provided by the microbiology hospital laboratory. This study completely followed the principles outlined in the Declaration of Helsinki.
Funding
This work was supported by Anhui Provincial Key Research and Development Plan Project (201904a07020049).
Disclosure
The authors have no conflicts of interest to declare for this work.
References
1. Lederman ER, Crum NF. Pyogenic liver abscess with a focus on Klebsiella pneumoniae as a primary pathogen: an emerging disease with unique clinical characteristics. Am J Gastroenterol. 2005;100(2):322–331. doi:10.1111/j.1572-0241.2005.40310.x
2. Chen J, Zhang M, Chen J, et al. Cryptogenic and non-cryptogenic liver abscess: a retrospective analysis of 178 cases revealed distinct characteristics. J Int Med Res. 2018;46(9):3824–3836. doi:10.1177/0300060518781256
3. Chen YC, Lin CH, Chang SN, Shi ZY. Epidemiology and clinical outcome of pyogenic liver abscess: an analysis from the National Health Insurance Research Database of Taiwan, 2000–2011. J Microbiol Immunol Infect. 2016;49(5):646–653. doi:10.1016/j.jmii.2014.08.028
4. Lee KT, Wong SR, Sheen PC. Pyogenic liver abscess: an audit of 10 years’ experience and analysis of risk factors. Dig Surg. 2001;18(6):459–465; discussion 465–456. doi:10.1159/000050194
5. Tian LT, Yao K, Zhang XY, et al. Liver abscesses in adult patients with and without diabetes mellitus: an analysis of the clinical characteristics, features of the causative pathogens, outcomes and predictors of fatality: a report based on a large population, retrospective study in China. Clin Microbiol Infect. 2012;18(9):E314–E330. doi:10.1111/j.1469-0691.2012.03912.x
6. Lin JC, Siu LK, Fung CP, et al. Impaired phagocytosis of capsular serotypes K1 or K2 Klebsiella pneumoniae in type 2 diabetes mellitus patients with poor glycemic control. J Clin Endocrinol Metab. 2006;91(8):3084–3087. doi:10.1210/jc.2005-2749
7. Joshi MB, Lad A, Bharath Prasad AS, Balakrishnan A, Ramachandra L, Satyamoorthy K. High glucose modulates IL-6 mediated immune homeostasis through impeding neutrophil extracellular trap formation. FEBS Lett. 2013;587(14):2241–2246. doi:10.1016/j.febslet.2013.05.053
8. Pavlou S, Lindsay J, Ingram R, Xu H, Chen M. Sustained high glucose exposure sensitizes macrophage responses to cytokine stimuli but reduces their phagocytic activity. BMC Immunol. 2018;19(1):24. doi:10.1186/s12865-018-0261-0
9. Mauriello CT, Hair PS, Rohn RD, Rister NS, Krishna NK, Cunnion KM. Hyperglycemia inhibits complement-mediated immunological control of S. aureus in a rat model of peritonitis. J Diabetes Res. 2014;2014:762051. doi:10.1155/2014/762051
10. Lee CH, Chen IL, Chuah SK, et al. Impact of glycemic control on capsular polysaccharide biosynthesis and opsonophagocytosis of Klebsiella pneumoniae: implications for invasive syndrome in patients with diabetes mellitus. Virulence. 2016;7(7):770–778. doi:10.1080/21505594.2016.1186315
11. Lin CT, Lin TH, Wu CC, Wan L, Huang CF, Peng HL. CRP-Cyclic AMP regulates the expression of type 3 fimbriae via cyclic di-GMP in Klebsiella pneumoniae. PLoS One. 2016;11(9):e0162884. doi:10.1371/journal.pone.0162884
12. Cheng HY, Chen YS, Wu CY, Chang HY, Lai YC, Peng HL. RmpA regulation of capsular polysaccharide biosynthesis in Klebsiella pneumoniae CG43. J Bacteriol. 2010;192(12):3144–3158. doi:10.1128/JB.00031-10
13. Confer AW, Ayalew S. The OmpA family of proteins: roles in bacterial pathogenesis and immunity. Vet Microbiol. 2013;163(3–4):207–222. doi:10.1016/j.vetmic.2012.08.019
14. Sainz T, Pérez J, Villaseca J, et al. Survival to different acid challenges and outer membrane protein profiles of pathogenic Escherichia coli strains isolated from pozol, a Mexican typical maize fermented food. Int J Food Microbiol. 2005;105(3):357–367. doi:10.1016/j.ijfoodmicro.2005.04.017
15. Carlsson A, Nyström T, de Cock H, Bennich H. Attacin--an insect immune protein--binds LPS and triggers the specific inhibition of bacterial outer-membrane protein synthesis. Microbiology. 1998;144(Pt 8)):2179–2188. doi:10.1099/00221287-144-8-2179
16. Gibert I, Barbé J. Cyclic AMP stimulates transcription of the structural gene of the outer-membrane protein OmpA of Escherichia coli. FEMS Microbiol Lett. 1990;56(3):307–311. doi:10.1111/j.1574-6968.1988.tb03197.x
17. Yohannes E, Thurber AE, Wilks JC, Tate DP, Slonczewski JL. Polyamine stress at high pH in Escherichia coli K-12. BMC Microbiol. 2005;5:59. doi:10.1186/1471-2180-5-59
18. Smith SG, Mahon V, Lambert MA, Fagan RP. A molecular Swiss army knife: ompA structure, function and expression. FEMS Microbiol Lett. 2007;273(1):1–11. doi:10.1111/j.1574-6968.2007.00778.x
19. Doorduijn DJ, Rooijakkers SH, van Schaik W, Bardoel BW. Complement resistance mechanisms of Klebsiella pneumoniae. Immunobiology. 2016;221(10):1102–1109. doi:10.1016/j.imbio.2016.06.014
20. Lin CT, Chen YC, Jinn TR, Wu CC, Hong YM, Wu WH. Role of the cAMP-dependent carbon catabolite repression in capsular polysaccharide biosynthesis in Klebsiella pneumoniae. PLoS One. 2013;8(2):e54430. doi:10.1371/journal.pone.0054430
21. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
22. Yeh FC, Yeh KM, Siu LK, et al. Increasing opsonizing and killing effect of serum from patients with recurrent K1 Klebsiella pneumoniae liver abscess. J Microbiol Immunol Infect. 2012;45(2):141–146. doi:10.1016/j.jmii.2011.12.006
23. Faïs T, Delmas J, Barnich N, Bonnet R, Dalmasso G. Colibactin: more Than a New Bacterial Toxin. Toxins. 2018;10(4):151. doi:10.3390/toxins10040151
24. Lai YC, Lin AC, Chiang MK, et al. Genotoxic Klebsiella pneumoniae in Taiwan. PLoS One. 2014;9(5):e96292. doi:10.1371/journal.pone.0096292
25. McDonough KA, Rodriguez A. The myriad roles of cyclic AMP in microbial pathogens: from signal to sword. Nat Rev Microbiol. 2011;10(1):27–38. doi:10.1038/nrmicro2688
26. Peterkofsky A, Gazdar C. Glucose and the metabolism of adenosine 3’:5’-cyclic monophosphate in Escherichia coli. Proc Natl Acad Sci U S A. 1971;68(11):2794–2798. doi:10.1073/pnas.68.11.2794
27. Kumar Nathella P, Babu S. Influence of diabetes mellitus on immunity to human tuberculosis. Immunology. 2017;152(1):13–24. doi:10.1111/imm.12762
28. Kronsteiner B, Chaichana P, Sumonwiriya M, et al. Diabetes alters immune response patterns to acute melioidosis in humans. Eur J Immunol. 2019;49(7):1092–1106. doi:10.1002/eji.201848037
29. Shen Y, Zhang L, Fan X, Zhou J. Effectiveness of remote continuous glucose monitoring on adverse outcomes among patients with diabetes complicated with COVID-19. J Diabetes Investig. 2021;12(10):1923–1924. doi:10.1111/jdi.13537
30. Lin YT, Wang FD, Wu PF, Fung CP. Klebsiella pneumoniae liver abscess in diabetic patients: association of glycemic control with the clinical characteristics. BMC Infect Dis. 2013;13:56. doi:10.1186/1471-2334-13-56
31. Berbudi A, Rahmadika N, Tjahjadi AI, Ruslami R. Type 2 Diabetes and its Impact on the Immune System. Curr Diabetes Rev. 2020;16(5):442–449. doi:10.2174/1573399815666191024085838
32. Zhang S, Zhang X, Wu Q, et al. Clinical, microbiological, and molecular epidemiological characteristics of Klebsiella pneumoniae-induced pyogenic liver abscess in southeastern China. Antimicrob Resist Infect Control. 2019;8:166. doi:10.1186/s13756-019-0615-2
33. Li S, Yu S, Peng M, et al. Clinical features and development of Sepsis in Klebsiella pneumoniae infected liver abscess patients: a retrospective analysis of 135 cases. BMC Infect Dis. 2021;21(1):597. doi:10.1186/s12879-021-06325-y
34. Thomsen RW, Jepsen P, Sørensen HT. Diabetes mellitus and pyogenic liver abscess: risk and prognosis. Clin Infect Dis. 2007;44(9):1194–1201. doi:10.1086/513201
35. Liu L, Li F, Xu L, et al. Cyclic AMP-CRP modulates the cell morphology of Klebsiella pneumoniae in high-glucose environment. Front Microbiol. 2019;10:2984. doi:10.3389/fmicb.2019.02984
36. Sahly H, Keisari Y, Ofek I. Manno(rhamno)biose-containing capsular polysaccharides of Klebsiella pneumoniae enhance opsono-stimulation of human polymorphonuclear leukocytes. J Innate Immun. 2009;1(2):136–144. doi:10.1159/000154812
37. de Astorza B, Cortés G, Crespí C, Saus C, Rojo JM, Albertí S. C3 promotes clearance of Klebsiella pneumoniae by A549 epithelial cells. Infect Immun. 2004;72(3):1767–1774. doi:10.1128/IAI.72.3.1767-1774.2004
38. Merino S, Camprubí S, Albertí S, Benedí VJ, Tomás JM. Mechanisms of Klebsiella pneumoniae resistance to complement-mediated killing. Infect Immun. 1992;60(6):2529–2535. doi:10.1128/iai.60.6.2529-2535.1992
39. Prasadarao NV, Blom AM, Villoutreix BO, Linsangan LC. A novel interaction of outer membrane protein A with C4b binding protein mediates serum resistance of Escherichia coli K1. J Immunol. 2002;169(11):6352–6360. doi:10.4049/jimmunol.169.11.6352
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.