Back to Journals » Infection and Drug Resistance » Volume 14
Epidemiological, Clinical and Laboratory Characteristics of Patients with Brucella Infection in Anhui Province, China
Authors Shi C , Wang L, Lv D, Wang G , Mengist HM, Jin T , Wang B, Huang Y, Li Y , Xu Y
Received 13 May 2021
Accepted for publication 8 July 2021
Published 16 July 2021 Volume 2021:14 Pages 2741—2752
DOI https://doi.org/10.2147/IDR.S319595
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Cuixiao Shi,1 Lianzi Wang,1 Dongmei Lv,1 Gang Wang,1 Hylemariam Mihiretie Mengist,2 Tengchuan Jin,2 Bo Wang,1 Ying Huang,1 Yajuan Li,1 Yuanhong Xu1
1Department of Clinical Laboratory, the First Affiliated Hospital of Anhui Medical University, Hefei, 230022, People’s Republic of China; 2Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, 230027, People’s Republic of China
Correspondence: Yuanhong Xu; Yajuan Li Email [email protected]; [email protected]
Background: Brucellosis is currently one of the most widespread zoonotic diseases caused by Brucella genus, and the Brucella melitensis is the major pathogen. The number of people infected with Brucella has gradually increased in Anhui Province.
Purpose: To retrospectively evaluate the epidemiological, clinical, and laboratory data of brucellosis patients in Anhui Province.
Patients and Methods: A total of 109 brucellosis patients were admitted to the First Affiliated Hospital of Anhui Medical University from January 2012 to March 2021. Data from all patients were retrieved from the hospital’s electronic medical system. The final results were grouped and compared according to the presence or absence of bacteremic brucellosis and three phases of brucellosis.
Results: The most common symptoms among all 109 brucellosis patients were fever (89.0%), followed by chills (52.3%), arthralgia (48.6%), and weight loss (30.3%), and laboratory results presented with anemia (65.1%), elevate of C-reactive protein (CRP) (91.7%), erythrocyte sedimentation rate (ESR) (86.2%), aspartate aminotransferase (AST) (40.4%), and lactate dehydrogenase (LDH) (43.1%). The percentage of fever (96.1%), arthralgia (58.8%), anorexia (35.3%), leukopenia (31.4%), and the AST (51.0%) were higher in bacteremic than nonbacteremic group. Additionally, the median level of LDH (332.0 mg/L, IQR, 209.0– 553.0) was higher in bacteremic than nonbacteremic group. Nevertheless, the albumin (36.0 mg/L, IQR, 33.9– 38.2) was lower in the bacteremic group. The percentage of fever (94.9%) and the median LDH level (316.0 U/L (IQR,218.0– 517.5)) in the acute phase of brucellosis were higher than the percentage of fever (72.0%) and the median LDH level (209.0 U/L (IQR,162.0– 276.0)) in the subacute phase of brucellosis.
Conclusion: Brucellosis has become an important public health issue in Anhui Province. Brucellosis is a disease with diverse clinical manifestations. Our data showed that unexplained fever, arthralgia, and elevated AST and LDH should be considered as a diagnosis of bacteremia brucellosis for early treatment intervention.
Keywords: Brucella melitensis, brucellosis, bacteremia, epidemiological, clinical and laboratory features
Introduction
Brucella, is a gram-negative coccobacillus causing infection mainly in the livestock and animals, and humans acquire the infection through contact with infected animals or eating diseased animals and their products.1 Brucella belongs to the Brucellae genus; there are six species, including B. melitensis, B. abortus, B. suis, B. canis, B. ovis, B. neotomaes.2 Each species has its optimal host, of which only the first four species can infect humans.3 Among them, B. melitensis is the most virulent and dominant species associated with human brucellosis in China.3,4 Brucellosis is a disease caused by Brucella with symptoms including long-term fever, hyperhidrosis, arthralgia, fatigue, and pain.5 Brucellosis a very common but long-forgotten zoonosis that was repopulated in China since 1995, and the affected areas were mainly localized in northern China such as Inner Mongolia, Shanxi, Heilongjiang, Hebei, Jilin, and Shaanxi.6 The increment in brucellosis in southern China is mainly driven by occupational exposure to the infected animal or connection with persons who had been exposed to the animals or animal products in the region of previous bacterial contamination.7 In the past decade, the increasing incidence of brucellosis gradually move toward Anhui Province thus increasing disease prevalence.
Although brucellosis is an old zoonotic disease with low mortality in humans, it can cause severe weakness, disability and non-reversible effects.8 Brucellosis is a febrile disease with overlapping symptoms with other diseases that often being confused2 and is a serious threat to human physical and mental health due to the lack of proper treatment and reliable diagnostic methods.9 The disease has chronic and long-lasting properties where the granuloma formation can compromise any organs and cause a variety of clinical symptoms.10 The infrequent presentations and non-specific symptoms of brucellosis pose a challenge to the diagnosis of brucellosis.11 Laboratory confirmation of brucellosis is achieved mainly through serological examination and blood culture.6 Currently, blood culture is the gold standard in the diagnosis of brucellosis.12 Positive blood culture provided confirmatory diagnosis when serology is sometimes showing false-negative results for a variety of possible confounding factors, and the presence of bacteremia does seem to be associated with the increase of recurrence brucellosis.13 Brucella can cause severe bacteremic brucellosis, which is not present in all infected patients because it is a facultative organism with intracellular replication capacity and avoid being detection from the immune system.14 From previous studies, several clinical manifestations, and laboratory results were found to be associated with bacteremic brucellosis, including fever, chills, leukopenia, thrombocytopenia, increased liver enzymes, and elevated C-reactive protein levels.15,16
Although Brucella bacteremia is not uncommon in patients with Brucellosis, the reports regarding brucella bacteremia are scarce in Anhui Province. Furthermore, results obtained from the epidemiology, laboratory tests, and the clinical presentation of brucellosis patients are not fully investigated. Therefore, the present study was designed to explore the epidemiology of patients with Brucella infection and assess the clinical presentations, laboratory findings, and the risk factors associated with the disease progression in Anhui Province which may hold great promise to reduce the misdiagnosis of brucellosis.
Patients and Methods
Recruitment Criteria and Brucellosis Diagnosis
This retrospective study was conducted using data of brucellosis patients collected between January 2012 and March 2021 at the First Affiliated Hospital of Anhui Medical University. It is one of the largest tertiary medical institutions with a capacity of 2800 beds positioned in Hefei, Anhui Province, China. Brucella infection was initially suspected in the presence of clinical presentation and epidemiological investigation and confirmed by laboratory examination. Brucellosis was diagnosed based on the national guideline of “Diagnosis for Brucellosis (WS 269–2019)”17 issued by National Health Commission of the people’s Republic of China in 2019: (1) Epidemiological history: patient has a history of close contact with livestock and animal products suspected of brucellosis infection, or has eaten raw cow, sheep milk or meat products, etc. (2) Clinical manifestation: symptoms such as fever (including low-grade fever) lasting for days or weeks, hyperhidrosis, fatigue, arthralgia and myalgia, etc. (3) Positive rose bengal plate agglutination test (RBT) reported by Anhui Prevention and Treatment Center for Occupational Disease. (4) Isolation of Brucella species in blood culture or positive serum agglutination test (SAT) detected by Anhui Provincial Center for Disease Control and Prevention. The titer of the SAT was ≥1:100 or the duration of the course more than 1 year and still having clinical symptoms was ≥1:50. The confirmed cases must comply with (1), (2), and any one of (4) or (1), (2), (3), and any one of (4) at the same time. Overall, fifty-one patients with positive blood culture results confirmed with Brucella melitensis were grouped into the bacteremic brucellosis group, while fifty-eight patients with negative blood culture results were grouped into non-bacteremic brucellosis group. Diagnosis of patients with non-bacteremic brucellosis is based on clinical and epidemiological characteristics and the titer of the SAT.
Blood specimens were collected from the bloodstream by venipuncture and subsequently inoculated in aerobic and anaerobic bottles before using antibiotics. The Bac-Tac™ Blood culture system (BacT/ALERT 3D (240), BioMérieux) was used for determining the blood culture result and followed by Matrix-Assisted Laser Desorption Ionization Time of Flight Mass Spectrometry (MALDI-TOF MS, BioMérieux, France) for bacteria identification. The laboratory result of confirmed bacteremic brucellosis was defined by the blood culture positive for Brucella.
Based on the duration of the manifestation and symptom before admission to the hospital, the patients were further divided into three groups: acute brucellosis (less than 2 months), subacute brucellosis (2–6 months), and chronic brucellosis (more than 6 months).18
Data Collection
Data obtained from 109 brucellosis patients were retrieved from the hospital’s electronic medical system. Clinical data collected for each patient included the following details: (1) Demographic and epidemiological characteristics, such as age, sex, occupation, place of residence, animal contact history, and travel history to outbreak area; (2) Clinical and laboratory data including the date of hospitalization, symptom onset, symptoms, complications, physical examination and laboratory test results; (3) Data regarding the treatment history for brucellosis.
Statistical Analysis
All data were analyzed using the software SPSS version 25.0. The continuous variables assumed normal distribution were expressed as mean ± standard deviation (SD). The statistical methods compared with two groups were used the Student’s t-test while those without a normal distribution were described as medians (interquartile range) and examined by the Mann–Whitney U-tests. Comparison of three groups was used the One-way ANOVA or the Kruskal–Wallis H-tests followed by the post-doc Bonferroni correction. For categorical variables, data were presented as frequency or percentage and were compared by the chi-square or by the Fisher’s exact test. Variables with P < 0.05 in the univariate analysis were further analyzed by the binary logistic regression model, and the results were presented as odds ratio (OR) and 95% confidence intervals (95% CIs). Statistical significance was defined as P < 0.05.
Results
Demographic and Epidemiological Characteristics
From January 2012 to March 2021, 109 brucellosis patients were admitted to the First Affiliated Hospital of Anhui Medical University and were later enrolled in this research. Of the 109 patients, 72 (66.1%) were males, with ages ranging from 1 to 81 years old (median age: 49 years). Seventeen of these cases were from Hefei and its adjacent districts, the others from nearby cities such as Luan (24 cases), Fuyang (16 cases), Anqing (13 cases), Bozhou (12 cases), Huainan (9 cases), Suzhou (6 cases), Chuzhou (4 cases), Huaibei (3 cases), Chizhou (1 case), Wuhu (1 case), and Xinyang, Henan province (3 cases).
More than half of the participants, 58 cases (53.2%) were farmers and herdsmen (occupation information: 78.0% were recorded and 22% were not recorded). In terms of epidemiological history, the most common risk factors of infection were exposure to sheep or cattle, which is accounts for 59 cases (54.2%). The highest number of admissions occurred in spring and summer, with an overall 70 cases (64.2%). There were 79 cases (72.5%) in the acute phase, 24 cases (22.0%) in the subacute phase, and 6 cases (5.5%) in the chronic phase. The brucellosis distribution between bacteremic and nonbacteremic patients was significantly different in acute, subacute, and chronic phases (P = 0.016). Distribution of patients with bacteremia and nonbacteremia in acute, subacute, and chronic phases was 43 (84.3%), 7 (13.7%), 1 (2.0%) and 35 (60.3%), 18 (31.0%), 5 (8.6%) cases, respectively. All detailed demographics and epidemiological characteristics are shown in Table 1.
 |
Table 1 Demographic and Epidemiological Analysis of Brucellosis Patients (N = 109) |
Clinical Characteristics and Complications
The most common symptoms of 109 patients were fever (89.0%), followed by chills (52.3%), arthralgia (48.6%), weight loss (30.3%), fatigue (28.4%), hyperhidrosis (25.7%), anorexia (25.7%), myalgia (24.8%), lymphadenectasis (24.8%), and cough (23.9%). The most common complication in the patients was pneumonia (14.7%) and splenomegaly (13.8%), while other least common complications including epididymo-orchitis (7.3%), arthrophlogosis (4.6%), and meningitis (2.8%) were also observed. More patients with bacteremic presented with fever (p=0.027), arthralgia (p=0.046), and anorexia (p=0.031) compared with nonbacteremic patients. In clear contrast to more nonbacteremic patients presented with myalgia (P= 0.039) than bacteremic patients (Table 2). The differences in other clinical parameters between the bacteremia and nonbacteremia were not statistically significant. Table 2 shows the symptoms, and complications of these patients.
 |
Table 2 Clinical Symptoms and Complications of Patients with Brucellosis |
Laboratory Results
Common hematological changes include anemia 71 cases (65.1%), and leukopenia 27 cases (24.8%). There were 94 (86.2%) patients with an elevated erythrocyte sedimentation rate (ESR) and 100 (91.7%) patients with increased C-reactive protein (CRP). The other laboratory findings were high ALT (36.7%), high AST (40.4%), high GGT (44.0%), high LDH (43.1%), and high PCT (25.7%). The median levels of hemoglobin (Hb), white blood cell (WBC) count, albumin (ALB), activated partial thromboplastin time (APTT) and prothrombin time (PT) was 121.0 g/L (IQR, 108.0–128.0), 5.4 ×109/L (IQR, 3.5–7.3), 36.8 g/L (IQR, 34.5–39.9), 41.3 s (IQR, 37.1–47.1) and 13.9 s (IQR, 13–14.5), respectively (Note: the PCT value was account for 82.6% of patients and 17.4% of patients were lack of records; the value for APTT and PT were account for 87.2% of patients and 12.8% of patients were missing).
In the comparison of the laboratory results between the bacteremic group and the nonbacteremic group, we found that the percentage of leukopenia (31.4%) and the elevated AST (51.0%) were higher in the bacteremic patients than the percentage of leukopenia (19.0%) and the elevated AST (31.0%) in the nonbacteremic patients. Furthermore, the median level of LDH (332.0 mg/L, IQR = 209.0–553.0) was also higher in the bacteremic group. However, the ALB (36.0 mg/L, IQR = 33.9–38.2) was lower in the bacteremic group. Although the median level of PLT, TBIL, DBIL, and TT was within the normal range, there is a statistically significant difference between bacteremia and nonbacteremic patients. All the above parameters were higher in the bacteremia group except PLT (P values are 0.012, 0.034, 0.049, and 0.020, respectively). A comparative dataset on laboratory findings at admission between the two groups is shown in Table 3.
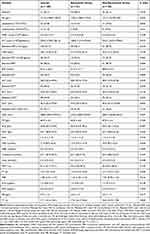 |
Table 3 Laboratory Result of Brucellosis Patients |
Phases of Patients with Brucellosis
In the comparison of different phases in patients, there was no statistical significance in the demographic, clinical, and laboratory results between the acute, subacute and chronic phases of brucellosis. Fever, chills, arthralgia, and loss of weight were the main symptoms at every phase. However, fever occurs more frequently in the acute phase (94.9%) than in the subacute phase (72.0%) while lose weight occurs more frequently in the subacute phase (52.0%) than in the acute phase (33.3%), both were statistical significance with the adjusted p < 0.05. In addition, the median LDH level was 316.0 U/L (IQR,218.0–517.5) in the acute phase and it was higher than the median level of 209.0 U/L (IQR,162.0–276.0) in the subacute phase. Similarly, the median APTT level was 43.5 s (IQR,38.1–48.9) in the subacute phase and it was higher than the APTT of 33.1 s (IQR, 29.5–41.2) in the chronic phase. All of the details and comprehensive results are shown in Table 4.
 |
Table 4 Comparative Analysis of Demographic, Clinical, and Laboratory Data in Acute, Subacute, and Chronic Phases of Brucellosis Patients |
Compared with nonbacteremic brucellosis, bacteremic brucellosis was diagnosed more often during the acute phase (Table 1). The differences of bacteremia and nonbacteremia between acute and subacute phases were not statistically significant. However, the CRP (P = 0.010) and LDH (P = 0.010) were found to be statistically significant in the acute phase of bacteremia compare to the subacute phase of bacteremia. The median levels of CRP and LDH in the acute phase of bacteremia were 38.7 mg/L (IQR, 21.0–66.4) and 355.0 U/L (IQR, 215.0–517.0), respectively. It was higher than the median levels of CRP and LDH of 8.2 mg/L (IQR,7.1–32.0) and 209.0 U/L (IQR, 180.0–216.0), respectively, in the subacute phase of bacteremia. Detailed results are shown in Table 5.
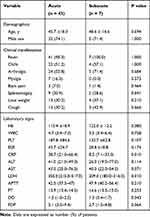 |
Table 5 Comparative Analysis of Demographic, Clinical, and Laboratory Data in Acute and Subacute Phase of Bacteremic Patients |
Risk Factors for Brucella Bacteremia
In the multivariate logistic regression analysis, the only factor was identified as the major risk factor associated with bacteremic brucellosis was fever (OR = 8.391, 95% CI = 1.210–58.170). Table 6 shows the results of multivariate analysis.
 |
Table 6 The Multivariate Logistic Regression Analysis for Bacteremic Patients |
Treatment and Outcomes
The antibiotic treatment regimens contain three options: levofloxacin in combination with minocycline, rifampicin in combination with minocycline or levofloxacin in combination with rifampicin. After the antibiotic treatment, the temperature of some patients returned to normal very quickly within one day. Most of the patients were discharged within 3 to 5 days after the improvement of body temperature and other symptoms, and the antibiotics were prescribed to be taken for six weeks after discharge.
Discussion
Most of the successful programs for the control of brucellosis take place in developed countries, while developing countries continue to struggle with the burden of the disease.10 Therefore, brucellosis is still an imperative public health problem in developing countries including China. After the resurgence of brucellosis in China in 1995, the geographical distribution of the affected areas gradually expanded.19 The prevalence of brucellosis has gradually shifted from rural pasturing areas (Inner Mongolia, Xinjiang, Tibet, Qinghai, and Ningxia) to semi-agricultural, agricultural areas and moved toward small towns (Shanxi, Liaoning, Hebei, Shandong, and Jilin) and the southern provinces of China.19,20 In China, brucellosis is now distributed to all provinces. This may be due to the vigorous development of the animal husbandry industry and subsequently increased the chance of human infection from an infected animal or animal products.21 Before this study, the information on epidemiology, clinical and laboratory results of the brucellosis, and their treatment history is very limited. The number of reported brucellosis cases in Anhui Province of southeast China has been increasing yearly in the last decade. It is mainly distributed in Luan, Hefei, Fuyang, Anqing, and Bozhou. This study enrolled 109 brucellosis patients and within these patients, 72 were males (66.1%) and 37 were females (33.9%). Our data showed that the farmers and herdsmen were at a high risk of getting the infection and it was not too surprising due to their occupation were suitable for disease transmission. We also found that the disease tends to cause epidemics in spring and summer and this may be due to active production and overpopulation of domestic animals in spring. Our results were similar to many other pieces of literature reported from other provinces or cities in China.5,22,23
The conclusion drawn from previous studies, the positive rates of brucellosis from blood cultures had a wide-ranged from 16% to 90%.24 This was consistent with the current study of 46.8% positive rate and mostly were discovered in the acute phase of brucellosis. Another study was completed in Hulunbuir, Inner Mongolia and they showed that the positive rate of blood culture was 14.9%,1 which is lower than our finding. The positive rate of blood cultures is mainly affected by two major factors. The first major factor is associated with the disease progression in the individual patient. Many variables can influence disease progressions such as age, duration of symptoms, systemic vs focal disease, first infection verse recurrence, and any previous or current antibiotic treatment. The second major factor is attributed to the method used in blood culture. Methods affecting results including the total volume of the blood specimen, number of cultures obtained, frequency of monitoring, the sensitivity of the blood culture system, incubation period, recovery of microorganism by the periodic performance of blind subcultures, and performance of terminal blind subcultures.25 Concluding from our regression analysis, we found that patients with a history of fever were more likely to be positive in blood culture. Human brucellosis is traditionally described as a multisystemic disease with a protean clinical manifestations.5 A study indicated that fever is a constant symptom that can be spiking accompanied by chills, if bacteremia is present, or may be relapsing, mild, or protracted.26 Although serological diagnosis of brucellosis is not perfect but it is indeed indispensable. Clinically, the RBT was used for preliminary screening of brucellosis in the laboratory and the SAT result was the most common serodiagnostic assay used for diagnosis of B. abortus, B. melitensis, and B. suis infections.25
The most common clinical manifestations of brucellosis in Anhui Province were fever. In contrast to a previous study, fatigue was the most common symptom in Xinjiang Uygur Autonomous Region.11 In our study, there were 97 patients (89.0%) with brucellosis who had a fever, and the rate of fever was 96.1% in the bacteremic group and it has been significantly higher than 82.8% of fever in the no-bacteremic group. In contrast to a previous study15 which reported a higher rate of arthritis in the nonbacteremic patients and another study27 reported that the arthritis rate was no difference between the two groups. Our study showed that arthritis was more common in the bacteremic group. This contradictory result could be attributed to geographical differences, differences in the study sample size, and study design. Further to this, there was no significant difference in the ratio of hepatomegaly and splenomegaly between bacteremic and nonbacteremic patients, which is contradicting another study that reported a higher prevalence of hepatomegaly and splenomegaly in the bacteremic patients.27
Our liver, the largest organ of the reticuloendothelial system, plays an important part in the defense against Brucella infection.28 However, the liver function has been significantly affected because of the infection of bacteria to the hepatocytes and intracellular replication of the bacteria in the liver.29 In this study, we observed a portion of patients showed liver function impairment, evidenced as increased ALT (37.7%), AST (40.4%), GGT (44.0%), and LDH (43.1%). However, most liver enzymes were not differentially expressed between bacteremic and nonbacteremic groups. We found that there were no statistically significant differences in the level of liver enzyme of the above parameters. The only exception was the level of LDH, which was higher in the bacteremic group (332.0 U/L (209.0–553.0)) than in the nonbacteremic group (258.5 U/L (186.8–442.5)). Although previous studies have also found CRP and ESR levels were moderately increased in a portion of patients with brucellosis, our study discovered dramatic changes of elevated CRP in 100 patients (91.7%) and ESR in 94 patients (86.2%) out of 109 patients with brucellosis in Anhui Province. Our study reported a larger and significantly higher portion of patients than previous studies reported elevated CRP and ESR ratio of 44.2% and 64.7% in Xinjiang,30 70.7% and 55.7% in Hulunbuir,1 and 45.4% and 73.2% in Turkey.31 Therefore, we hypothesized that monitoring CRP and ESR levels can assist in the diagnosis of brucellosis. Nevertheless, these two markers may not be sufficient to distinguish bacteremic and nonbacteremic patients. Interestingly, our data suggested that leukopenia occurred more frequently in patients with bacteremia, indicating a level of immune cells defense mechanism inversely correlated to circulating bacteria in the blood.
In this study, we observed that our patients were mainly diagnosed with the acute phase of brucellosis. With 78 (71.6%) out of the 109 patients were in the acute phase in Anhui and similar findings have been described in Shandong.18 Among the acute phase patients, 43 patients were confirmed with bacteremia and 35 patients were confirmed with nonbacteremic. Typically, the clinical manifestations of acute brucellosis patients presented with systematic manifestation, such as intermittent fever, lack of appetite, and weight loss.11 On the contrary, subacute and chronic brucellosis patients showed a larger variation of clinical presentation; and in general, those symptoms are less severe than the acute cases.32 Fever has been the most common symptom in the acute phase than in the subacute phase (adjusted p < 0.05) in our study, it was consistent with a previous study completed in Xinjiang.11 However, contrary to this study, lose weight was significantly different and more frequent in the subacute phase than in the acute phase in our study (adjusted p < 0.05). Our clinical data revealed no significant differences between acute and subacute bacteremia patients. Nevertheless, the median level of some markers was elevated, we found that the median levels of CRP (38.7 mg/L, IQR = 21.0–66.4) and LDH (355.0 U/L, IQR = 215.0–517.0) in the acute bacteremia phase were higher than the median levels of CRP (8.2 mg/L, IQR = 7.1–32.0) and LDH (209.0 U/L, IQR = 180.0–216.0) in the subacute bacteremia phase.
Fever was found to be the major risk factor for bacteremic brucellosis in our study. It was also known that minocycline has been recognized as the first-choice antibiotic for the treatment of brucellosis as early as 1987.33 Whereas, a combination of antibiotics therapy for brucellosis can lead to faster recovery, shortening of the symptomatic interval, and reduction the overall morbidity.34 The First Affiliated Hospital of Anhui Medical University had adopted the scheme of combination antibiotics treatment for 6 weeks for patients diagnosed with brucellosis.
Early diagnosis and treatment of brucellosis is paramount for reducing the overall morbidity of brucellosis. The current diagnosis of brucellosis was based on three criteria: (1) Clinical manifestations, such as fever, chills, hyperhidrosis, fatigue, arthralgia, myalgia, and weight loss. (2) Travel history to the infected areas or exposure to raw animal products. (3) Laboratory test results. For those patients with fever from unknown origin, detection of Brucella antibody or isolation of pathogen must be carried out as soon as possible in the clinical practice for the diagnosis of brucellosis.
There are several limitations to our study. Firstly, this was a retrospective observational analysis based on a small sample size so that the interpretation of our findings might not represent in the bigger population. Secondly, we were able to retrieve ~80% of relevant information regarding patient’s demographics and laboratory tests. Markers with ~20% missing value including the APTT and their occupation. Interpretation of these data might be underestimated. Finally, there was also no long-term follow-up for patients after discharge. However, having these limitations in our study, our study still provides important perspectives for brucellosis in Anhui. We believed our study provides a solid foundation for the prevention and control of brucellosis in the future.
Conclusion
The most common risk factors of Brucella infection in Anhui Province are associated with the occupation of patients. Farmers and herdsmen whose exposure to infected cattle and sheep or consumption of contaminated animal products were most often diagnosed with brucellosis. Fever was the most common symptom, followed by chills, arthralgia, weight loss, and fatigue. A larger majority of patients experienced anemia, elevated CRP and ESR, and abnormal liver function in the laboratory result. There was no statistical significance in the demographic, clinical, and laboratory results between the acute, subacute and chronic phases of brucellosis. Our data suggested that patients with obvious fever and arthralgia accompanied by elevated AST, LDH, and CRP should be considered as a diagnosis of bacteremia brucellosis and received urgently antibiotics treatment before the blood test result. This study summarized 10 years of patient data in Anhui Province and could be helpful to improved strategies for prevention, diagnosis, and treatment of brucellosis in other cities and provinces.
Ethical Approval
This study was reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Anhui Medical University. The written informed consent was obtained from patients in accordance with the Declaration of Helsinki.
Acknowledgments
We would like to thank the First Affiliated Hospital of Anhui Medical University staffs for their support in sample and clinical data collections. This work was financially supported by Anhui Natural Science Foundation (grant number: 9021138201) and Scientific Research Project of Universities in Anhui Province (grant number: KJ2020A0170).
Disclosure
The authors declare that there are no conflicts of interest in this work.
References
1. Liang C, Wei W, Liang X, De E, Zheng B. Spinal brucellosis in Hulunbuir, China, 2011–2016. Infect Drug Resist. 2019;12:1565–1571. doi:10.2147/IDR.S202440
2. Deng Y, Liu X, Duan K, Peng Q. Research progress on brucellosis. Curr Med Chem. 2019;26(30):5598–5608. doi:10.2174/0929867325666180510125009
3. Ye HY, Xing FF, Yang J, et al. High index of suspicion for brucellosis in a highly cosmopolitan city in southern China. Bmc Infect Dis. 2020;20(1):22. doi:10.1186/s12879-019-4748-y
4. Zhao Z, Li J, Ma L, et al. Molecular characteristics of Brucella melitensis isolates from humans in Qinghai Province, China. Infect Dis Poverty. 2021;10(1). doi:10.1186/s40249-021-00829-0.
5. Jiang W, Chen J, Li Q, et al. Epidemiological characteristics, clinical manifestations and laboratory findings in 850 patients with brucellosis in Heilongjiang Province, China. Bmc Infect Dis. 2019;19(1):439. doi:10.1186/s12879-019-4081-5
6. Lai S, Zhou H, Xiong W, et al. Changing epidemiology of human brucellosis, China, 1955–2014. Emerg Infect Dis. 2017;23(2):184–194. doi:10.3201/eid2302.151710
7. Chen S, Zhang H, Liu X, et al. Increasing threat of brucellosis to low-risk persons in urban settings, China. Emerg Infect Dis. 2014;20(1):126–130. doi:10.3201/eid2001.130324
8. Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. The new global map of human brucellosis. Lancet Infect Dis. 2006;6(2):91–99. doi:10.1016/S1473-3099(06)70382-6
9. Wang H, Xu WM, Zhu KJ, et al. Molecular investigation of infection sources and transmission chains of brucellosis in Zhejiang, China. Emerg Microbes Infect. 2020;9(1):889–899.
10. Franco MP, Mulder M, Gilman RH, Smits HL. Human brucellosis. Lancet Infect Dis. 2007;7(12):775–786. doi:10.1016/S1473-3099(07)70286-4
11. Shi Y, Gao H, Pappas G, et al. Clinical features of 2041 human brucellosis cases in China. PLoS One. 2018;13(11):e205500. doi:10.1371/journal.pone.0205500
12. Araj GF. Update on laboratory diagnosis of human brucellosis. Int J Antimicrob Agents. 2010;36(Suppl 1):S12–S17. doi:10.1016/j.ijantimicag.2010.06.014
13. Pappas G, Papadimitriou P. Challenges in Brucella bacteraemia. Int J Antimicrob Agents. 2007;30(Suppl 1):S29–S31. doi:10.1016/j.ijantimicag.2007.06.011
14. Memish Z, Mah MW, Mahmoud SA, Shaalan MA, Khan MY. Brucella bacteraemia: clinical and laboratory observations in 160 patients. J Infect. 2000;40(1):59–63. doi:10.1053/jinf.1999.0586
15. Kadanali A, Ozden K, Altoparlak U, Erturk A, Parlak M, Kadanali A. Bacteremic and nonbacteremic brucellosis: clinical and laboratory observations. Infection. 2009;37(1):67–69. doi:10.1007/s15010-008-7353-3
16. Qie C, Cui J, Liu Y, Li Y, Wu H, Mi Y. Epidemiological and clinical characteristics of bacteremic brucellosis. J Int Med Res. 2020;48(7):1410563826. doi:10.1177/0300060520936829
17. National Health Commission of the People’s Republic of China. Diagnosis for Brucellosis (WS 269—2019). Beijing: National Health Commission of the People’s Republic of China; 2019.
18. Xu N, Dong X, Yao Y, et al. Improved early detection of focal brucellosis complications with anti-Brucella IgG. J Clin Microbiol. 2020;58(10):10. doi:10.1128/JCM.00903-20
19. Deqiu S, Donglou X, Jiming Y. Epidemiology and control of brucellosis in China. Vet Microbiol. 2002;90(1–4):165–182. doi:10.1016/S0378-1135(02)00252-3
20. Kong W. Brucellosis infection increasing in Southern China. Eur J Intern Med. 2018;51:e16–e18. doi:10.1016/j.ejim.2018.03.004
21. Jiang H, O’Callaghan D, Ding J-B. Brucellosis in China: history, progress and challenge. Infect Dis Poverty. 2020;9(1):55. doi:10.1186/s40249-020-00673-8
22. Zheng R, Xie S, Lu X, et al. A systematic review and meta-analysis of epidemiology and clinical manifestations of human brucellosis in China. Biomed Res Int. 2018;2018:5712920. doi:10.1155/2018/5712920
23. Yuan HT, Wang CL, Liu LN, et al. Epidemiologically characteristics of human brucellosis and antimicrobial susceptibility pattern of Brucella melitensis in hinggan league of the inner mongolia autonomous region, China. Infect Dis Poverty. 2020;9(1):79. doi:10.1186/s40249-020-00697-0
24. Dokuzoğuz B, Ergönül O, Baykam N, et al. Characteristics of B. melitensis versus B. abortus bacteraemias. J Infect. 2005;50(1):41–45. doi:10.1016/j.jinf.2004.02.005
25. Yagupsky P, Morata P, Colmenero JD. Laboratory diagnosis of human brucellosis. Clin Microbiol Rev. 2019;33(1). doi:10.1128/CMR.00073-19
26. Pappas G, Akritidis N, Bosilkovski M, Tsianos E. Brucellosis. N Engl J Med. 2005;352(22):2325–2336. doi:10.1056/NEJMra050570
27. Apa H, Devrim I, Memur S, et al. Factors affecting Brucella spp. blood cultures positivity in children. Vector Borne Zoonotic Dis. 2013;13(3):176–180. doi:10.1089/vbz.2012.0997
28. Ariza J, Pigrau C, Cañas C, et al. Current understanding and management of chronic hepatosplenic suppurative brucellosis. Clin Infect Dis. 2001;32(7):1024–1033. doi:10.1086/319608
29. Ozturk-Engin D, Erdem H, Gencer S, et al. Liver involvement in patients with brucellosis: results of the Marmara study. Eur J Clin Microbiol Infect Dis. 2014;33(7):1253–1262. doi:10.1007/s10096-014-2064-4
30. Jia B, Zhang F, Lu Y, et al. The clinical features of 590 patients with brucellosis in Xinjiang, China with the emphasis on the treatment of complications. PLoS Negl Trop Dis. 2017;11(5):e5577. doi:10.1371/journal.pntd.0005577
31. Yoldas T, Tezer H, Ozkaya-Parlakay A, Sayli TR. Clinical and laboratory findings of 97 pediatric brucellosis patients in central Turkey. J Microbiol Immunol Infect. 2015;48(4):446–449. doi:10.1016/j.jmii.2014.04.016
32. Patra S, Tellapragada C, Mukhopadhyay C. Human brucellosis: experience from a tertiary care hospital in southern India. Trop Doct. 2018;48(4):368–372. doi:10.1177/0049475518788467
33. Grasso E, Conversa P, Mondino P, Spirolazzi MP, Dalmasso G, Raineri G. [295 cases of brucellosis treated with minocycline]. Minerva Med. 1987;78(19):1443–1447. [Norwegian]
34. Ranjbar M, Keramat F, Mamani M, et al. Comparison between doxycycline-rifampin-amikacin and doxycycline-rifampin regimens in the treatment of brucellosis. Int J Infect Dis. 2007;11(2):152–156. doi:10.1016/j.ijid.2005.11.007
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
