Back to Journals » Journal of Blood Medicine » Volume 11
Epidemiological Characteristics and Etiology of Budd-Chiari Syndrome in Upper Egypt
Authors Abdel Hameed MR , Elbeih EAMS, Abd El-Aziz HM, Afifi OAH, Khalaf LMR, Ali Abu Rahma MZ , Sabry A
Received 13 October 2020
Accepted for publication 14 December 2020
Published 30 December 2020 Volume 2020:11 Pages 515—524
DOI https://doi.org/10.2147/JBM.S278678
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Muhamad R Abdel Hameed,1 Esam Abdel-Moneim Sadek Elbeih,1 Heba Mahmoud Abd El-Aziz,2 Ola Abdel-Haleem Afifi,3 Lamiaa Mohammed Refaat Khalaf,4 Mohammed Zakaria Ali Abu Rahma,5 Abeer Sabry6
1Department of Internal Medicine & Hematology Unit, Assiut University Hospitals and Bone Marrow Transplantation Unit, South Egypt Cancer Institute, Assiut University, Assiut, Egypt; 2Department of Internal Medicine, Assiut University Hospitals, Assiut University, Assiut, Egypt; 3Department of Clinical Pathology, Assiut University Hospitals, Assiut University, Assiut, Egypt; 4Department of Radiology, South Egypt Cancer Institute, Assiut University, Assiut, Egypt; 5Department of Tropical Medicine and Gastroenterology, Assiut University, Assiut, Egypt; 6Department of Internal Medicine, Helwan University, Helwan, Egypt
Correspondence: Muhamad R Abdel Hameed
Department of Internal Medicine & Hematology Unit, Assiut University Hospitals and Bone Marrow Transplantation Unit, South Egypt Cancer Institute, Assiut University, Assiut, Egypt
Tel (+2)01097510010
Email [email protected]
Background and Objectives: Budd-Chiari syndrome (BCS) is a rare disorder caused by obstruction to hepatic venous outflow. It affects all races, usually during the third or fourth decade of life. Higher prevalence had being evident in developing countries. The aim of the present study was to clarify sociodemographic features, clinical, radiological presentations, and etiology of BCS among Upper Egyptian patients.
Patients and Methods: This retrospective cohort study enrolled 50 Upper Egyptian Patients with confirmed primary BCS. Liver, coagulation, and thrombophilia workup profiles were performed as anticardiolipin antibodies, lupus anticoagulant, protein C, protein S, and antithrombin III assays. Factor V Leiden and JAK2 mutations were assessed. Full radiological assessment was done.
Results: Fifty patients were included. There were 28 males (56%) and 22 females (44%) with mean age (32.5 ± 11.1 years). The etiological factor was not identified in 22% of cases (n=11). Isolated factor C deficiency was found in 26% (n=13) with male predominance 39.3% and protein S deficiency in 10% (n=5). Factor V Leiden mutation was the etiology in 5 patients (10%). Membranous web and antiphospholipid syndrome each were the etiology in 8% (n=4). Behςet’s disease was diagnosed in 4% (n=2). Cases of liver cirrhosis(LC) were 41/50(82%)they were :33/50(66%) LC child class C, 8 /50(16%) LC child class B, and 0/50 (0%) LC child class A. Abdominal pain was the most common symptom (96%), and ascites was the most common sign (82%). Obstruction of hepatic veins was present in 80%.
Conclusion: BCS in Upper Egyptian patients was mainly occurred in males in the third and fourth decade of life, mostly with liver cirrhosis. The most common etiology is isolated protein C deficiency followed by Factor V Leiden mutation and isolated protein S deficiency. Hepatic veins obstruction was the most common pattern of vascular involvement.
Keywords: Budd-Chiari syndrome, Upper Egypt, factor V Leiden, thrombophilia, etiology, protein C
Introduction
Budd–Chiari syndrome (BCS) was defined according to EASL guidelines as an obstruction of the hepatic venous outflow tract in absence of right-sided heart failure or constrictive pericarditis.1 Lesions causing BCS could be located in the small or large hepatic veins (HV) or in the suprahepatic part of inferior vena cava (IVC).
BCS is a rare disease, with the prevalence of 2 per 100,000 inhabitants and an annual incidence of approximately 0.2 cases per million.2 It has higher prevalence in developing countries such as China, India, Nepal, and South Africa.3
Budd-Chiari syndrome has four clinical variants; acute, subacute, chronic, and fulminant. The acute form is characterized by abdominal pain, ascites, and hepatomegaly, without evidence of portal hypertension. This form can be complicated by fulminant hepatitis. The chronic form is difficult to distinguish from cirrhosis regardless of the etiology. The subacute form is characterized by features of acute BCS with portal hypertension. It reflects an extension of a previous occurrence of thrombosis in the HV.4
BCS can also be classified according to etiology either as primary or secondary. Primary BCS is often associated with the presence of thrombotic risk factors. While, secondary BCS could be caused by obstruction in hepatic veins outflow by surgical injury, extrinsic compression, or tumor infiltration.5 At least one hereditary or acquired pro-coagulative disorder is present in 74% of cases. As many as 30% of BCS patients carry a Factor V Leiden mutation (FVLM) and some showed the inherited deficiency of Protein C, S, and antithrombin III.2
India, Pakistan, Middle East, and Europe share many characteristics in terms of the level of obstruction and causal factors. However, in China, involvement of the IVC may be more common.6 The clinical differences among different areas can be explained by different causes and area-related prevalence, eg, oral contraceptives in Europe or extreme poverty in certain Asian countries and South Africa.5
In Egypt, there are few studies on the character of BCS and its etiology with lack of studies from Upper Egypt region. Thus, we aimed to study the sociodemographic features, clinical, radiological presentations of Budd-Chiari syndrome among Upper Egyptian patients and to identify the possible etiology in this group of patients.
Patients and Methods
The present retrospective cohort study enrolled 50 patients with confirmed diagnosis of primary BCS referred to Assiut University Hospital Internal, Medicine Department and Al-Rajhi Liver University Hospital as tertiary centers (Assiut, Egypt) from different Upper Egyptian cities, eg, Minia, Assiut, Sohag, Qena, Luxor and Aswan. An informed written consent for data collection was obtained from all subjects who participated in the study during follow up visits. A parent provides informed consent for any participants under the age of 18 years. The protocol of the study was approved by the ethical committee of Assiut University. Full history and clinical examination were recorded for all patients.
- Laboratory investigations included complete blood picture, liver profile, and coagulation screen which were assessed using Cell-Dyn Ruby (Abbot), Cobas-integra 800 plus and Sysmex CA-1500 (Siemens) respectively.
- To determine the etiology of BCS, investigations of thrombophilia were performed including:
Protein C global test was first performed, as a screening test for identifying the defects of protein C and S anticoagulant pathways, then followed by protein C and S assays. Antithrombin III and lupus anticoagulant assays were done. All coagulation tests were performed using auto-analyzer Sysmex CA-1500 and reagents supplied by Siemens (Marburg, Germany). In addition, anti-cardiolipin antibodies, antinuclear antibodies, and Factor V Leiden mutation were investigated. Janus kinas 2 (JAK2) mutational statuses were assessed by PCR exploring the possible presence of a myeloproliferative disorder.
Protein C global test is an activated partial thromboplastin (aPTT) time-based assay in which snake venom was used for activation of endogenous protein C and of the intrinsic coagulation cascade. The activated protein C in conjunction with endogenous protein S inactivated the procoagulatory cofactors VIII and Va. This delayed clot formation. The time taken for a clot to form was determined. In addition, before use in the test the sample was mixed 1+4 with coagulation factor V deficient plasma. This minimized the influence of other factors and a reduced normalized ratio was then almost exclusively due to the presence of Factor V Leiden.
Protein C activity assay: Protein C in the patient sample was activated by a specific snake venom activator. The resulting activated protein C (protein Ca) was assayed in a kinetic test by measuring the increase in absorbance at 405 nm.
Protein S Activity Assay
Protein Ca proteolytically cleaves FVa which is generated during the activation of the coagulation cascade by Russel viper venom (RVV). In this reaction protein S acts as a cofactor which accelerates the reaction. As a result, the coagulation time increases proportionally to the activity of protein S in the sample. The addition of deficient plasma ensures that the test mixture has a sufficient supply of fibrinogen, Factor V, and the other necessary coagulation factors.
Antithrombin III (ATIII) Activity Assay
The antithrombin III in the sample is converted by heparin into an immediate inhibitor and inactivates the thrombin present. The residual thrombin content is determined in a kinetic test measuring the increase in absorbance at 405 nm according to the following reaction:
ATIII sample+Thrombin excess (ATIII-Thrombin) + Thrombin residual
The absorbance change is inversely correlated to the antithrombin III activity in the sample.
Lupus Anticoagulant (LA) Assay
Russell’s viper venom present in LA1 Screening Reagent initiates plasma clotting by directly activating factor X. LA antibodies prolong the LA1 Screening Reagent clotting time. LA2 Confirmation Reagent is similar to LA1Screening Reagent but with high phospholipid concentration. The extra phospholipid counteracts the LA antibody and largely corrects the clot time. DRVV test “bypass” factor VII of the extrinsic pathway and the contact and antihemophilic factor of the intrinsic pathway. Therefore, LA1Screening Reagent is more specific for LA than aPTT as they are not affected by contact factor abnormalities or by factor VIII deficiencies or antibodies.
Anti-Cardiolipin IgM, IgG Assay
The determination was based on an indirect enzyme linked immune reaction. The Alegria test strip was used with the diagnostic instrument Alegria - a fully automated random access analyzer (Orgentec Diagnostika, Germany).
Antinuclear Antibodies Assay
Antinuclear antibodies test is an indirect fluorescent antibody test that involves incubating dilutions of patient sera with a monolayer of fixed, permeabilized cells. Antibodies adherent to the cell monolayer were visualized with an anti-human immunoglobulin reagent that had been conjugated to a fluorescent tag. The reagents were provided by Diagnostic automation Inc. (CA, USA). The presence or absence of nuclear staining and the pattern of nuclear staining were identified by fluorescence microscopy.
DNA extraction for PCR: Genomic DNA was isolated from ethylene diaminetetraacetic acid (EDTA)-whole blood using QIAamp DNA Mini Kit (Qiagen, Germany) according to manufacturer’s instructions.
Detection of Factor V Leiden (FVL)
Genotyping by real-time PCR was performed using hydrolysis probes. Each genotyping primer/probe mix contained two labelled probes homologous to the two genotypes under investigation. The probes included one specific for the wild-type G1691allele (FAM-labeled) and the other specific for the mutant A1961 allele (VIC-labeled). The mixture was placed in a 96-well PCR plate using 7500 Fast Real-Time PCR system (Applied Biosystems, CA, USA). During qPCR amplification of the target DNA with 40 cycles of alternating temperature (95°C for 15 s and 60°C for 1 min), the probes competed for binding across the variant region. The probe that was 100% homologous to the DNA binding site preferentially bonded and gave a fluorescent signal as PCR proceeded. Results were analyzed by the instrument software to indicate the presence of one of the following in each DNA sample: wild-type alleles only (normal), mutant alleles only (homozygous FVL), or both alleles (heterozygous FVL).
JAK2 V617F Mutation Analysis
Real-time quantitative polymerase chain reaction (RT-PCR)-based allelic discrimination assay was used to detect the JAK2 V617F mutation employing TaqMan real-time technology and 7500 Fast Real-Time PCR system (Applied Biosystems, CA, USA). Extracted DNA (80 ng) from the patients was used to amplify the mutated and unmutated exon 14 of JAK2 in an allele-specific PCR. Genomic DNA amplification was done in a 40-cycle PCR at an annealing temperature at 61°C. All reactions were carried out in a final volume of 25 µL containing 1×PCR Master Mix (Applied Biosystems, USA),900 nM of both forward and reverse primers and 100 nM of each probe.7
The sequence of the forward and reverse primers was as follows:
(F5ʹAAGCTTTCTCACAAGCAAGCATTTGGTTT) and (R5ʹAGAAAGGCATTAGA AAGCCTGTAGTT) respectively. PCR products were separated by electrophoresis on a 3% agarose gel, stained with ethidium bromide, and viewed under UV light. A 203 base-pair fragment indicates the presence of the 1849G>T mutation. A ratio of cycle threshold (Ct) between the Ct (JAK2 V617F) and Ct (JAK2 wild type) was calculated for each sample.
(JAK2 V617F ≥ 0.042) indicates that JAK2 V617F mutation is detected, while (JAK2 V617F < 0.042) indicates thatJAK2 V617F mutation is not detected.
Radiological assessment using abdominal ultrasound and Duplex ultrasonography (US) was performed to assess the status of liver disease and patency of the hepatic veins (HVs), the portal vein, and the IVC. Abdominal multislice computed tomography (MSCT) and/or magnetic resonance imaging (MRI) and/or IVC venography were performed to confirm all diagnoses and to assess vascular anatomy. Patients with secondary BCS caused by intraluminal invasion by a parasite or a malignant tumor, or extraluminal compression by an abscess, cyst, or solid tumor were excluded.
Statistical Analysis
All data were analyzed using SPSS version 16. Results were presented as means and standard deviations (SD). Data were expressed as number and frequencies. The chi-squared and t-test tests were used to test for differences among variables. P value less than 0.05 was considered significant; a P value less than 0.01 was highly significant (HS); and a P value less than 0.001 was very highly significant (VHS).
Results
Fifty Upper Egyptian patients were enrolled in the study. There were 28 males (56%) and 22 females (44%). Their age was between (13–57 years) with mean (32.5 ± 11.1). A total of46 (92%) patients showing chronic BCS while 4(8%) acute BCS presented with the classic triad of abdominal pain, hepatomegaly and ascites and were noncirrhotic as shown in Table 1.
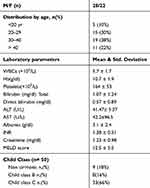 |
Table 1 Baseline Sociodemographic and Laboratory Features of the Included Patients (n=50) |
Table 2 summarizes the disease etiologies. More than one etiological factor were identified in 32% (n=16). The etiology was not identified in 22% of cases (n=11). The first commonest etiology was isolated factor C deficiency in 26% (n=13) followed by protein S deficiency in 10% (n=5) and Factor V Leiden mutation in 10% (n= 5) and membranous web in 8% (n=4). Antiphospholipid syndrome diagnosed by positive lupus anticoagulant and anticardiolipin antibodies was present in 8% (n=4) also. History of hormonal therapy in the form of oral contraceptives in 6% of the females (n=3).
 |
Table 2 Etiology of Budd-ChiariSyndrome in the Studied Patients |
Behςet’s disease was diagnosed in 4% (n=2) based on the diagnostic criteria of the International Study Group of Behçet’s Disease.8
No patients had JAK2 mutation.
As regard the relation between the etiology and gender, there was a highly statistical significant difference with male predominance in both cases with isolated protein C deficiency (P = 0.001) and others with unidentified etiology (P = 0.002) (Table 3).
 |
Table 3 Relation Between Etiology and Gender |
Most of the included patients, 82% had liver cirrhosis on admission (n=41) while 9 patients have no liver cirrhosis. Child-Pugh classification among patients who had liver cirrhosis (n=41), 33 patients were Child class C (66%) while Child class B were in 8 patients (16%) and no patients had Child class A. Mean MELD score was 12.5 ± 5.5. None of the included cases received anticoagulants (Table 1).
Abdominal pain was the most common symptoms in BCS patients (96%), abdominal distension in 41 patients (82%) as shown in Table 4.
 |
Table 4 Clinical Presentations of Budd-Chiari Syndrome in the Studied Patients (N= 50) |
The most common clinical signs were moderate to tense ascites (82%), edema of lower limbs (68%), and hepatomegaly (50%) Table 4.
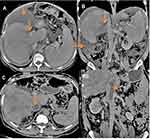
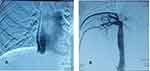
As shown in Table 5: most of the patients had obstruction at the level of hepatic veins with high statistical significance (P = 0.000) =40 (80%). Patients with protein c deficiency and those with unidentified etiology had obstruction at the level of hepatic vein with high statistical significance p values 0.001and 0.002, respectively. Five patients (10%) had obstruction at both HV and IVC and 5 patients (10%) had obstruction at the level of IVC. Isolated protein C deficiency was the most common cause associated with H.V obstruction (Figure 1). All cases with factor V Leiden mutation had obstruction at the level of hepatic veins (p value 0.050) and all cases of membranous web were associated with IVC obstruction (Figure 2).
 |
Table 5 Distribution of the Site of Venous Obstruction as Detected by Radiological Assessment According to the Etiology of Budd-Chiari Syndrome |
Discussion
Budd-Chiari syndrome is a rare disorder of the liver with heterogenous nature, characterized by obstruction of the hepatic venous outflow.9
Our current epidemiological study describes the socio-demographic features, etiology, and risk factors for BCS of 50 Upper Egyptian patients with confirmed diagnoses of primary BCS.
Previous studies found that BCS was usually occurring during the third or fourth decade of life, with slightly more common predominance in females.10 Another study in Egypt describing disease pattern in Delta region and Cairo found more females affection than males with M/F ratio 43/57 and mean patient age about 28 years,11 while our study showed that BCS is mainly occurring at middle age with the mean age about 32 years with male predominance: M/F 28/22. AN Algerian study found that M/F was 45/70 and the mean age was 34 years.4 However, A Chinese study by Lin found that patients with BCS had mean age of (52.5 ± 11.7) with M/F ratio was 11/4.12
Most of the patients in the current study were diagnosed during the chronic phase (92%) of the disease; the rest of the patients were diagnosed during acute (8%) stage. These results support other studies.13–16
In this study, Liver cirrhosis was noticed in 82% and most of them were Child class C (66%) while 16% were Child class B and no cases with Child class A. Their presentation by symptoms and signs of portal hypertension and hepatic decompensation, explaining common symptoms of abdominal pain, distension, and upper GIT bleeding 96%, 82%, and 36%, respectively. While ascites, lower limb edema, and hepatomegaly were the most common presenting signs in 82%, 68%, and 50%, respectively. Most of these patients were referred to our centers to be treated from the complications of liver cirrhosis after failure of catching definite diagnosis of cirrhosis.
Patients with BCS may show nonspecifically decreased levels of protein C, protein S, and antithrombin, attributable to impaired hepatic synthesis of these anticoagulants.17
In the current study isolated protein C deficiency was the most common identified etiology in 26% of patients while isolated protein S in 10% and no case with isolated antithrombin III. Similar to Afredj et al anticoagulant protein deficiency was observed in 28% of patients (n = 18), dominated by protein C deficiency (n = 13). Only 5 patients demonstrated a protein S deficiency, one had a mutation in the prothrombin gene and no patient demonstrated an antithrombin deficiency.4
Sakr et al 2011 identified deficiencies in antithrombin III, protein C, and protein S in 43%, 43%, and 1.1%, of BCS patients, respectively.14 Similarly, Mohanty et al studied BCS patients in India and found deficiencies in antithrombin III, protein C, and protein S in 3.8%, 13.2%, and 5.7% of patients, respectively.18 Uskudar and his colleagues in Turkey reported deficiencies in Antithrombin ш, Protein C, and Protein S in 3%, 9%, and 7% of BCS patients, respectively.19
In this study assessment for prothrombin gene mutation (PGM) was not done. Dutta et al study reported that there were no patients with BCS had PGM.13 G20210A PT gene mutation is a relatively uncommon prothrombotic risk factor with a weak prothrombotic risk.20
In Europe, factor V Leiden is a second prothrombotic factor and is found in 12–31% of BCS patients5,21 and antiphospholipid syndrome appears to be the third most common prothrombotic factor.22 Factor V Leiden induces resistance of activated factor V to degradation by activated protein C.23
In this study factor V Leiden mutation was present in 10%. Both antiphospholipid syndrome and membranous web were detected in 8% of the patients. Behcet’s disease was found in 4% similar to other studies which showed that 4–13% of BCS patients have Behcet’s disease.14,19
In the current study, we found FVLM in 10% of patients and all were heterozygous. Meanwhile, Sakr studied Egyptian BCS patients in Delta found that FVLM was the most common cause of disease (53.1% of patients); 37.5% of patients were FVLM heterozygotes, and 15.6% FVLM homozygotes.14 Another Egyptian study by Sakr and his colleagues observed that factor V Leiden mutation was present in 23% of patients.11
Similarly, a study in India by Mohanty et al found that FVLM was the most common etiology of BCS (26% of patients).18 However, another Egyptian study showed that FVLM was the second most common mutation found in 48.6% of patients (42.9% heterozygous and 5.7% homozygous).24 Nevertheless, observational studies did not find any Chinese BCS patients with either of the two FVLM mutations.25,26
In this study hormonal therapy in the form of oral contraceptives was considered an etiological factor only in 6% of all BCS patients. This could explain the higher ratio of BCS found in males in our study compared to other Egyptian studies because of lower exposure to oral contraceptives among Upper Egyptian females than in Delta region females.
Exposure to oral contraceptives does not play a role as an etiology of BCS in the East. In both India and China, the use of oral contraceptives is less than 2%, compared with 30% in Europe and above 40% in France.27,28 The current study showed that JAK2-V617F mutation was not detected in any case; thus, myeloproliferative disorder was not an identified etiology. Similar to Chinese study where the prevalence of the JAK2-V617F mutation appears to be particularly low.5
In the present study Most patients without identified etiologies had advanced liver disease and were child class C (n=10) and more than one etiological factor were identified in 32% while other studies showing nearly 80% of BCS patients have at least one thrombotic risk factor, and 30–50% of them have two or more thrombotic risk factors.29,30 Uskudar and his colleagues in Turkey identified at least two etiological factors in 18.6% and three in 1.3%.13 Egyptian patients by Sakr and his colleagues identified at least one etiological factor in 48.9% of patients, two in 30.9%, three in 8.5%, and four in 3.2%.14
The patterns of vascular involvement in BCS patients show variation between countries. IVC obstruction is prevalent in Asia, Nepal, South Africa, India, and China, whereas pure HVs obstruction predominates in Western countries.31
This study showed that HVs obstruction was the most common pattern of venous obstruction (80%), combined HVs and IVC obstruction was similar to isolated IVC obstruction and were observed in 10%, while in another Egyptian study by Sakr et al, HVs obstruction, IVC obstruction, and combined HVs and IVC obstruction were found in 43%, 3%, and 21% of patients, respectively.11
Darwish Murad reported isolated obstruction of HV, IVC, and both veins in 62%, 7%, and 31% of patients, respectively. Behcet’s disease was the main etiology associated with IVC obstruction in this study.32
Regarding the relation between the pattern of venous obstruction and the etiology, FVLM appears to be common in patients with IVC obstruction33 and the use of oral contraceptives, or pregnancy, is specifically associated with hepatic vein involvement.34
The present study showed that isolated protein C, protein S deficiency, and FVLM were associated with HVs obstruction. Meanwhile, membranous web was associated with IVC obstruction. Sakr et al reported protein S (48.8%), protein C (41.9%), antiphospholipid syndrome (32%), and FVLM (20.9%) were the most common causes of isolated hepatic veins thrombosis.11
Conclusion
BCS is a multifactorial disease in Upper Egyptian patients that occurs mainly in middle-aged males, mostly with chronic form. Isolated factor C deficiency was the most common etiology, while factor S deficiency and Factor V Leiden mutation were the second most common etiologies. Hepatic venous outflow was the main level of venous obstruction in our cohort. Identification of certain cause of BCS should not exclude searching for other etiological factors. Comparing our data with those of previous studies indicate that BCS of different geographic regions showing demographic difference.
Ethics Approval and Consent to Participate
This study was approved by the Regional Ethics Committee, Faculty of Medicine, Assuit University and was conducted in accordance with the provisions of the Declaration of Helsinki. Informed written consent was obtained from all participants before enrolment. A parent or legal guardian provided informed consent for any participants under the age of 18 years.
Author Contributions
E.A.S. conceived and designed the research. M.R.A., M.Z.A., A.M.M. and H.M.A. recruited patients, carried out the clinical investigations, collected clinical data and followed up the cases. O.A.A. and L.M.R. contributed in the interpretation of data for the work. M.R.A., M.Z.A., A.M.M., O.A.A. and L.MR prepared the original draft of the manuscript. All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. EASL. Clinical practice guidelines. Vascular diseases of the liver. J Hepatol. 2016;64(1):179–202. doi:10.1016/j.jhep.2015.07.040
2. Valla DC. Primary Budd-Chiari syndrome. J Hepatol. 2009;50(1):195–203. doi:10.1016/j.jhep.2008.10.007
3. Wang ZG, Jones RS. Budd-Chiari syndrome. Curr Probl Surg. 1996;33(2):83–211. doi:10.1016/S0011-3840(96)80001-3
4. Afredj N, Guessab N, Nani A, et al. Aetiological factors of Budd-Chiari syndrome in Algeria. World J Hepatol. 2015;7(6):903–909. doi:10.4254/wjh.v7.i6.903
5. Qi X, Han G, Guo X, et al. Review article: the aetiology of primary Budd–Chiari syndrome—differences between the West and China. Aliment Pharmacol Ther. 2016;44(11–12):1152–1167. doi:10.1111/apt.13815
6. Valla D. Budd–Chiari syndrome/hepatic venous outflow tract obstruction. Hepatol Int. 2017;12(S1):168–180. doi:10.1007/s12072-017-9810-5
7. Baxter EJ, Scott M, Campbell PJ, et al. Cancer genome project: acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365(9464):1054–1061. doi:10.1016/S0140-6736(05)71142-9
8. International Study Group for Behçet’s Disease. Criteria for diagnosis of Behçet’s disease. Lancet. 1990;335(8697):1078–1080.
9. Martens P, Nevens F. Budd–Chiari syndrome. United European Gastroenterol J. 2015;3(6):489–500. doi:10.1177/2050640615582293
10. Valla DC. Hepatic vein thrombosis (Budd-Chiari syndrome). Semin Liver Dis. 2002;22(1):5–14. doi:10.1055/s-2002-23202
11. Sakr MA, Abdelhakam SM, Dabbous HM, et al. Pattern of vascular involvement in egyptian patients with Budd-Chiari syndrome: relation to etiology and impact on clinical presentation epidemiological aspects of Budd-Chiari in Egyptian patients: a single-center study. Ann Hepatol. 2018;17(4):638–644. doi:10.5604/01.3001.0012.0933
12. Lin M, Zhang F, Wang Y, et al. Liver cirrhosis caused by chronic Budd–Chiari syndrome. Medicine. 2017;96(34):34. doi:10.1097/MD.0000000000007425
13. Dutta AK, Chacko A, George B, Joseph JA, Nair SC, Mathews V. Risk factors of thrombosis in abdominal veins. World J Gastroenterol. 2008;14(28):4518–4522. doi:10.3748/wjg.14.4518
14. Sakr M, Barakat E, Abdelhakam S, et al. Epidemiological aspects of Budd–Chiari in Egyptian patients: a single-center study. World J Gastroenterol. 2011;17(42):4704–4710. doi:10.3748/wjg.v17.i42.4704
15. Dilawari JB, Bambery P, Chawla Y, et al. Hepatic outflow obstruction (Budd–Chiari syndrome). Experience with 177 patients and a review of the literature. Medicine (Baltimore). 1994;73(1):21–36. doi:10.1097/00005792-199401000-00003
16. Bayraktar UD, Seren S, Bayraktar Y. Hepatic venous outflow obstruction: three similar syndromes. World J Gastroenterol. 2007;13(13):1912–1927. doi:10.3748/wjg.v13.i13.1912
17. Dayal S, Pati HP, Pande GK, Sharma MP, Saraya AK. Multilineage hemopoietic stem cell defects in Budd Chiari syndrome. J Hepatol. 1997;26(2):293–297. doi:10.1016/S0168-8278(97)80044-X
18. Mohanty D, Shetty S, Ghosh K, Pawar A, Abraham P. Hereditary thrombophilia as a cause of Budd-Chiari syndrome: a study from Western India. Hepatology. 2001;34(4):666–670. doi:10.1053/jhep.2001.27948
19. Uskudar O, Akdogan M, Sasmaz N, Yilmaz S, Tola M, Sahin B. Etiology and portal vein thrombosis in Budd-Chiari syndrome. World J Gastroenterol. 2008;14(18):2858–2862. doi:10.3748/wjg.14.2858
20. Darwish MS, Plessier A, Hernandez-Guerra M, et al. Etiology, management, and outcome of the Budd-Chiari syndrome. Ann Intern Med. 2009;151(3):167–175. doi:10.7326/0003-4819-151-3-200908040-00004
21. Seijo S, Plessier A, Hoekstra J, et al. Good long-term outcome of Budd–Chiari syndrome with a step-wise management. Hepatology. 2013;57(5):1962–1968. doi:10.1002/hep.26306
22. Espinosa G, Font J, Garcia-Pagan JC, et al. Budd–Chiari syndrome secondary to antiphospholipid syndrome: clinical and immunologic characteristics of 43 patients. Medicine (Baltimore). 2001;80(6):345–354. doi:10.1097/00005792-200111000-00001
23. Dahlbäck B, Hildebrand B. Inherited resistance to activated protein C is corrected by anticoagulant cofactor activity found to be a property of factor V. Proc Natl Acad Sci USA. 1994;91(4):1396–1400. doi:10.1073/pnas.91.4.1396
24. El Sebay HM, Safan MA, Daoud AA, Tayel SI, Nouh MA, El Shafie S. Association of factor V Leiden, Janus kinase 2, prothrombin, and MTHFR mutations with primary Budd–Chiari syndrome in Egyptian patients. J Gastroenterol Hepatol. 2016;31(1):235–240. doi:10.1111/jgh.13066
25. Qi X, Wu F, Ren W, et al. Thrombotic risk factors in Chinese Budd-Chiari syndrome patients. An observational study with a systematic review of the literature. Thromb Haemost. 2013;109(05):878–884. doi:10.1160/TH12-10-0784
26. Wang H, Sun G, Zhang P, et al. JAK2 V617F mutation and 46/1 haplotype in Chinese Budd-Chiari syndrome patients. J Gastroenterol Hepatol. 2014;29(1):208–214. doi:10.1111/jgh.12379
27. Li J, Temmerman M, Chen Q, Xu J, Hu L, Zhang WH. A review of contraceptive practices among married and unmarried women in China from 1982 to 2010. Eur J Contracept Reprod Health Care. 2013;18(3):148–158. doi:10.3109/13625187.2013.776673
28. Skouby SO. Contraceptive use and behavior in the 21st century: a comprehensive study across five European countries. Eur J Contracept Reprod Health Care. 2010;15(Suppl 2):S42–S53. doi:10.3109/13625187.2010.533002
29. DeLeve LD, Valla DC, Garcia-Tsao G. Vascular disorders of the liver. Hepatology. 2009;49:1729–1764.
30. Senzolo M, Riggio O, Primignani M. Vascular disorders of the liver: recommendations from the Italian association for the study of the liver (AISF) ad hoc committee. Dig Liver Dis. 2011;43(7):503–514. doi:10.1016/j.dld.2010.11.006
31. Poddar P, Gurizala S, Rao S. Endovascular stenting of IVC using Brockenborough’s needle in Budd–Chiari syndrome—a case report. Indian Heart J. 2014;66(3):363–365. doi:10.1016/j.ihj.2014.03.014
32. Darwish MS, Valla DC, de Groen PC, et al. Determinants of survival and the effect of portosystemic shunting in patients with Budd-Chiari syndrome. Hepatology. 2004;39(2):500–508. doi:10.1002/hep.20064
33. Deltenre P, Denninger MH, Hillaire S, et al. Factor V Leiden related Budd-Chiari syndrome. Gut. 2001;48(2):264–268. doi:10.1136/gut.48.2.264
34. Valla DC. Hepatic venous outflow tract obstruction etiopathogenesis: Asia versus the West. J Gastroenterol Hepatol. 2004;19(s7):S204–S211. doi:10.1111/j.1440-1746.2004.03642.x
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.