Back to Journals » Clinical Interventions in Aging » Volume 18
Eosinophil: A New Circulating Biomarker for Risk of Poor Outcome in Stroke Patients Undergoing Mechanical Thrombectomy
Authors Yu S , Huang ZC, Wang HS, Liu SW, You SJ, Hou J, Guo ZL, Xiao GD
Received 9 January 2023
Accepted for publication 22 March 2023
Published 28 March 2023 Volume 2023:18 Pages 523—531
DOI https://doi.org/10.2147/CIA.S404082
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Maddalena Illario
Shuai Yu,* Zhi-Chao Huang,* Huai-Shun Wang,* Shan-Wen Liu, Shou-Jiang You, Jie Hou, Zhi-Liang Guo, Guo-Dong Xiao
Department of Neurology and Suzhou Clinical Research Center of Neurological Disease, The Second Affiliated Hospital of Soochow University, Suzhou, 215004, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Guo-Dong Xiao; Zhi-Liang Guo, Department of Neurology and Suzhou Clinical Research Center of Neurological Disease, The Second Affiliated Hospital of Soochow University, No. 1055, Sanxiang Road, Suzhou, Jiangsu Province, 215004, People’s Republic of China, Email [email protected]; [email protected]
Objective: Acute ischemic stroke (AIS), caused by occlusion of large vessel, is a serious life-threatening disease. This study aimed to comprehensively investigate the association of 14 common and readily available circulating biomarkers with the 90-day modified Rankin Scale (mRS) score in patients undergoing mechanical thrombectomy (MT).
Methods: This study included patients with anterior circulation large vessel occlusive stroke treated with MT from 05/2017 to 12/2021. Baseline comparisons of poor outcome were performed among enrolled patients. Factors that may be associated with the mRS score were assessed using correlation analysis. Univariate and multivariate logistic regression analyses were used to evaluate the predictive value of circulating biomarkers and poor outcome.
Results: The mRS score has a strong correlation with neutrophil to lymphocyte ratio (NLR) and eosinophil levels (all rs> 0.4 in absolute value and all P< 0.001) in addition to a high correlation with National Institute of Health Stroke Scale (NIHSS) score (rs=0.40, P< 0.001). There was also a high correlation between NLR and eosinophil (rs=− 0.58, P< 0.001). In the multivariate regression analysis, only neutrophil (adjusted OR=1.301, 95% CI: 1.155− 1.465, P< 0.001), eosinophil (adjusted OR< 0.001, 95% CI: < 0.001− 0.016, P< 0.001), and NLR (adjusted OR=1.158, 95% CI: 1.082− 1.241, P< 0.001) were independently associated with poor outcome.
Conclusion: This study evaluated a series of circulating biomarkers and found that neutrophil, eosinophil, and NLR independently predicted poor outcome after MT in AIS patients. There was a significant negative correlation between eosinophil and NLR levels.
Keywords: acute ischemic stroke, mechanical thrombectomy, biomarker, eosinophil, poor outcome
Background
Acute ischemic stroke (AIS) is a serious life-threatening disease and a major cause of disability and death, particularly in patients with AIS caused by large vessel occlusion. Although early mechanical thrombectomy (MT) is significantly associated with lower rates of disability, there are still some patients failing to achieve good neurological improvement.1 A meta-analysis reported that poor outcome (defined as a 90-day modified Rankin Scale, ie, mRS score ≥3) can still be as high as 54% in patients treated with MT.1,2 Therefore, it is of great clinical value to effectively predict the adverse neurological outcome after MT in patients with AIS.
However, the neurological outcome of thrombectomy patients is difficult to explain and judge by a single factor. In recent years, as the pathophysiological mechanisms of stroke have become better understood, circulating biomarkers in patients have received increasing attention, especially when other predictors were not available easily. These circulating biomarkers are readily available and may provide objective and reliable information on the patient’s neurological outcome and even risk of death.3 However, few biomarker studies are focusing on AIS patients undergoing MT. Even though there is growing evidence that the neutrophil to lymphocyte ratio (NLR) is associated with outcome in stroke patients, these previous reports have rarely assessed the relationship between other circulating biomarkers and clinical outcomes in AIS patients with MT.4–6 Also, the predictive role of circulating biomarkers must be reviewed in the era of mechanical thrombectomy, as some previously reported serum biomarkers may no longer be predictive of outcomes associated with patients undergoing MT.7 We comprehensively evaluated 14 common and readily available circulating biomarkers, including lymphocyte, neutrophil, albumin, globulin, creatinine, glycated hemoglobin, eosinophil, platelet, prothrombin time (PT), activated partial thromboplastin time (APTT), D-dimer, fibrinogen, NLR and APTT/PT.
Methods
Study Population
This study was a retrospective study, retrospectively analyzing patients with anterior circulation large vessel occlusive stroke treated with mechanical thrombectomy at the neurology department of the author’s unit from 05/2017 to 12/2021.
All stroke patients who underwent MT received stroke management based on the recommendations of the latest guidelines. In this study, we excluded patients who 1) received only arterial thrombolysis, 2) had severe hepatic or renal insufficiency, heart failure, or other life-threatening complications, 3) had end-stage malignancy, 4) were confirmed by digital subtraction angiography not to have large vessel occlusion in the anterior circulation, and 5) were missing relevant imaging data, biomarkers, and 3-month prognosis. The 90-day prognostic follow-up was performed via telephone or in an outpatient setting.
This is a retrospective clinical study that has been approved by the Institutional Ethics Review Board of the Second Hospital of Soochow University (ethical review decision number: JD-HG-2022-11) and the informed consent was exempt due to the retrospective nature of this study. We followed the World Medical Association Declaration of Helsinki in conducting our research and kept patient data strictly confidential.
Data Collection
Data were obtained from medical records including age, female, hypertension, diabetes mellitus, dyslipidemia, atrial fibrillation, cardiovascular diseases, previous stroke, smoking, drinking, intravenous thrombolysis, preoperative blood glucose level, preoperative National Institute of Health Stroke Scale (NIHSS) score, preoperative Alberta Stroke Program Early CT Score (ASPECTS), vascular occlusion site, Stroke etiology, successful reperfusion, and poor collateral circulation. Circulating biomarkers were determined simultaneously within 24 h after MT. Lymphocyte, neutrophil and eosinophil were detected by flow cytometry + fluorescence staining. Platelet was detected by resistance method. The total protein was detected by biuret method. Albumin was detected by potassium bromide phenol green. Creatinine was tested by the creatinine oxidase method. PT and APTT were measured by coagulation method. The D-dimer was detected by immunoturbidimetry. Fibrinogen was detected by thrombin assay. Glycated hemoglobin was detected by ion exchange high performance liquid chromatography. NLR and APTT/PT were calculated by the formula. Patient follow-up was conducted objectively and independently, and the investigator had been unaware of any relevant clinical information about the patients.
Successful reperfusion is defined as modified thrombolysis in cerebral infarction (mTICI) of 2b to 3 grade.8 Poor collateral circulation is defined as grade 0 to 1 according to the American Society of Interventional and Therapeutic Neuro-radiology/Society of Interventional Radiology collateral circulation assessment criteria.8,9
Statistical Analyses
Continuous variables were expressed as mean (standard deviation, SD) or median [interquartile range, IQR], using Student’s t-test or Mann–Whitney U-test; categorical variables were expressed as several cases (composition ratio), ie, n (%), using the chi-square test or Fisher's exact test. First, patients were included for description and comparison of baseline information on poor outcome. Secondly, the association between patients’ mRS score and circulating biomarkers, blood glucose, NIHSS score and ASPECTS was assessed using Spearman correlation analysis. Finally, the predictive value of laboratory tests about poor outcome was assessed using univariate and multivariate logistic regression analyses. In the multivariate logistic regression analysis, potential confounders with P < 0.1 in the intergroup comparison of baseline data were adjusted. The regression results were further subjected to sensitivity analysis and ROC curves were plotted. Two-tailed p<0.05 was considered statistically significant. The data were analyzed with IBM SPSS Statistics 26.0 and R 4.1.0.
Results
The Baseline of Characteristics of Patients in Good Outcome Group and Poor Outcome Group
A total of 292 patients with anterior circulation large vessel occlusive stroke treated with MT were included in this study. The flow chart was shown in Figure 1. The median [IQR] of the overall patient mRS score was 3.00 [1.00, 4.00]. 189 of these patients failed to achieve good neurological improvement within 90 days. A comparison of the baseline characteristics of the included patients can be seen in Table 1. Patients with a poor outcome tended to be older, more likely to be female, and to have hypertension, atrial fibrillation, a history of stroke, and a lesser history of smoking and drinking. They also had higher preoperative glucose, NIHSS score, lower ASPECTS, and were more likely to have poor collateral circulation.
 |
Table 1 Baseline Characteristics in Good Outcome Group and Poor Outcome Group |
 |
Figure 1 Flow chart. |
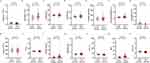
Comparison of Circulating Biomarkers Between Patients with Good Outcome and Poor Outcome
The levels of circulating biomarkers are shown in Figure 2. Compared to patients with a good outcome, patients with a poor outcome had lower lymphocyte counts, higher neutrophil counts, higher globulins, higher glycated hemoglobin, lower eosinophil counts, higher D-dimers, and shorter APTT. Also, the NLR was greater and the APTT/PT was smaller.
Association Between mRS Score and Circulating Biomarkers, Blood Glucose, NIHSS Score and ASPECTS
A correlation analysis (Figure 3) showed a positive correlation between mRS score and PT (rs=0.13, P=0.029), glycated hemoglobin (rs=0.19, P=0.001), blood glucose (rs=0.21, P<0.001), globulin (rs=0.20, P=0.001), NIHSS score (rs=0.40, P<0.001), D-dimer (rs=0.32, P<0.001), age (rs=0.34, P<0.001), neutrophil (rs=0.29, P<0.001), and NLR (rs=0.44, P<0.001); and a negative correlation with ASPECTS (rs=−0.28, P<0.001), lymphocyte (rs=−0.34, P<0.001), eosinophil (rs=−0.46, P<0.001), APTT (rs=−0.12, P=0.042), APTT/PT (rs=−0.20, P<0.001), and platelet (rs=−0.12, P=0.035).
From Figure 3, we can find that the patients’ mRS score have strong correlations with NLR and eosinophil levels (Spearman correlation coefficients were all greater than 0.4 in absolute value, and the p-values were all less than 0.001) in addition to a high correlation with NIHSS score. Furthermore, there was also a high correlation between NLR and eosinophil (rs=−0.58, P<0.001).
Circulating Biomarkers and Poor Outcome
The results of the univariate and multivariate logistic regression can be viewed in Table 2. In the univariate analysis, we found that neutrophil, globulin, eosinophil, D-dimer, and NLR were significantly associated with poor outcome (all P<0.05). After adjusting for potential confounders (age, female, hypertension, diabetes mellitus, atrial fibrillation, previous stroke, smoking, drinking, blood glucose, NIHSS score, ASPECTS, vascular occlusion site, successful reperfusion and poor collateral circulation) and performing a multivariate regression analysis of these variables, we identified that only neutrophil (adjusted OR=1.301, 95% CI: 1.155−1.465, P<0.001), eosinophil (adjusted OR<0.001, 95% CI: <0.001−0.016, P<0.001), and NLR (adjusted OR=1.158, 95% CI: 1.082−1.241, P<0.001) were independently associated with poor outcome.
 |
Table 2 Binary Logistic Regression Between Circulating Biomarkers and Poor Outcome |
To further evaluate the sensitivity, specificity, and predictive values of these variables for poor outcome, the receiver operator characteristic (ROC) curve was plotted (Figure 4). The AUC for neutrophil, eosinophil and NLR were 0.662 (95% CI: 0.598–0.726, P<0.001), 0.731 (95% CI: 0.671–0.791, P<0.001) and 0.738 (95% CI: 0.679–0.798, P<0.001) respectively. Their corresponding optimal cutoff values were 7.35*109/L (neutrophil, sensitivity 66.7% and specificity 56.3%), 0.005*109/L (eosinophil, sensitivity 55.0% and specificity 82.5%) and 7.455 (NLR, sensitivity 66.7% and specificity 70.9%), respectively.
 |
Figure 4 Receiver operator characteristic curve analysis. |
Discussion
In the present study, our main findings were that 1) neutrophil, eosinophil, and NLR were independent predictors of poor outcome in patients with acute anterior circulation large vessel occlusive stroke after MT, and 2) eosinophils were highly negatively correlated with NLR. Consistent with previous studies, several generic indicators, such as age, hypertension, diabetes, atrial fibrillation, baseline NIHSS score, ASPECTS, and poor collateral circulation, were associated with poor outcome after MT in AIS patients.10–12 Regarding circulating biomarkers, also similar to other studies, neutrophil and NLR levels were independently associated with clinical outcome in patients undergoing MT.13 In addition, this study found that higher eosinophil level can predict a good outcome for patients. Eosinophils may have a neuroprotective role in the stroke process and regulate the inflammatory response of stroke patients in the same way as neutrophils and lymphocytes.
A growing number of studies indicate the importance of the inflammatory response in the pathogenesis of stroke.14–16 Inflammation in ischemic stroke involves the release of cytokines, chemokines, and injury-related molecular patterns that exacerbate the destruction of brain tissue during the acute and repair phases of ischemic stroke.17 Proinflammatory signals from immune mediators rapidly activate resident cells and influence various inflammatory cells (neutrophil, monocyte/macrophage, different subtypes of T cell, and other inflammatory cells) to infiltrate the ischemic zone, exacerbating brain injury.16 Neutrophil has been reported to promote disruption of the blood–brain barrier exacerbating brain edema and brain injury, and neutrophil counts are dynamic parameters associated with hemorrhagic complications and long-term outcome.13,18 Compared to neutrophils, increased levels of their derivative index NLR can independently predict good clinical outcome in patients after MT.19
Usually, think before, eosinophil plays a major role in host defense against worms, plays an intermediary role in allergic disease transmission, in systemic eosinophils and eosinophil infiltration characteristics of target organs plays a role of pathology in clinical disease, is a destructive effect of inflammatory medium of granular tissue injury and disease.20,21 However, this view has shifted in recent years to suggest that the eosinophil is an important regulator of local immunity and damaged tissue repair in both health and disease, has receptors for a range of inflammatory mediators, and is capable of producing large amounts of pro-inflammatory and homeostatic mediators.20,22 The presence of eosinophil has been reported to be associated with prolonged survival rather than the death of the parasite in animal models.20 In addition, instead of increasing the severity of allergic and hypersensitivity reactions, eosinophils seem to play a role in regulating local inflammation, rather than increasing it.20 Based on the “LIAR hypothesis” proposed by Lee et al, the eosinophil is now recognized as an important component of a complex regulatory network that regulates the local and systemic immune and inflammatory responses together with other elements of the immune system, including polymorphonuclear leukocytes (PMNLs), lymphocytes and macrophages.20,22 Eosinophil could be able to secrete more than 35 cytokines, growth factors, and chemokines, including IL-4 and IL-13, which induce the activation of M2-phenotype microglia.23–26 M2-phenotype microglia promotes the regression of inflammation and has neuroprotective properties.25,26 Eosinophil plays a complex role in the progression of ischemic injury in stroke patients, and its role with pro-inflammatory and anti-inflammatory properties and neuroprotective effects plays a delicate balance in the progression of stroke.
Previous studies though have shown that inflammatory markers in the blood can predict early and long-term functional outcomes in patients with AIS treated with MT. However, these studies were mainly limited to a single neutrophil and its derived indicator NLR and may not have focused on other circulating biomarkers. In this study, a comprehensive evaluation of 14 commonly available circulating biomarkers found that eosinophil was as highly valuable as NLR in predicting functional outcome after MT of patients, even after adjustment for confounding factors. The addition of eosinophil levels to conventional predictive models may help to improve the ability to predict poor outcome. In addition, eosinophil levels combined with NLR appear to provide an integrated and comprehensive assessment of the systemic inflammatory status of stroke patients.
This study still has several limitations. First, this was a single-center retrospective study with small sample size. In addition, studies of these biomarkers, whether in NLR or eosinophil, are not yet reliable enough to guide clinical practice. Therefore, validation in additional large clinical trials would still be needed. Second, it is not clear whether eosinophil is a static variable. In the future, we expect to further investigate whether eosinophil is dynamic after MT in AIS patients as in NLR and whether its possible indicators of dynamic change could provide greater predictive value for poor outcome.
Conclusion
In summary, our study evaluated a series of circulating biomarkers and found that neutrophil, eosinophil, and NLR independently predicted poor outcome after MT in AIS patients. There was a significant negative correlation between eosinophil and NLR levels. Higher eosinophil level may be a new inflammatory marker to predict a good outcome after MT in AIS patients, playing a delicate balance in the progression of stroke.
Abbreviations
AIS, acute ischemic stroke; MT, mechanical thrombectomy; NIHSS, the National Institute of Health Stroke Scale; NLR, neutrophil to lymphocyte ratio; mTICI, modified thrombolysis in cerebral infarction; PT, prothrombin time; APTT, activated partial thromboplastin time; ASITN/SIR, American society of interventional and therapeutic; neuroradiology/society of interventional radiology; ASPECTS, Alberta Stroke Program Early Computed Tomography Score; ICA, internal carotid artery; MCA, middle cerebral artery; SD, standard deviation; IQR, interquartile range; mRS, modified rankin scale.
Statements and Declarations
This study has been approved by the Institutional Ethics Review Board of the Second Hospital of Soochow University (ethical review decision number: JD-HG-2022-11) and the informed consent was exempt. We followed the Declaration of Helsinki in conducting our research and kept patient data strictly confidential.
Acknowledgments
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The work has been supported in part by the grant from the Suzhou Science and Technology Bureau Medical-Industrial Collaborative Innovation Research Project (SLJ2021014) and the Suzhou Medical and Health Science and Technology Innovation (SKY2022160).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–1731. doi:10.1016/s0140-6736(16)00163-x
2. Zhang X, Zhang S, Chen Q, Ding W, Campbell BCV, Lou M. Ipsilateral prominent thalamostriate vein on susceptibility-weighted imaging predicts poor outcome after intravenous thrombolysis in acute ischemic stroke. AJNR Am J Neuroradiol. 2017;38:875–881. doi:10.3174/ajnr.A5135
3. Soldozy S, Yagmurlu K, Norat P, et al. Biomarkers predictive of long-term outcome after ischemic stroke: a meta-analysis. World Neurosurg. 2022;163:e1–e42. doi:10.1016/j.wneu.2021.10.157
4. Celikbilek A, Ismailogullari S, Zararsiz G. Neutrophil to lymphocyte ratio predicts poor prognosis in ischemic cerebrovascular disease. J Clin Lab Anal. 2014;28:27–31. doi:10.1002/jcla.21639
5. Brooks SD, Spears C, Cummings C, et al. Admission neutrophil-lymphocyte ratio predicts 90 day outcome after endovascular stroke therapy. J Neurointerv Surg. 2014;6:578–583. doi:10.1136/neurintsurg-2013-010780
6. Komurcu HF, Gozke E, Dogan AP, Kalyoncu Aslan I, Salt I. Changes in neutrophil, lymphocyte, platelet ratios and their relationship with NIHSS after rtPA and/or thrombectomy in ischemic stroke. J Stroke Cerebrovasc Dis. 2020;29:105004. doi:10.1016/j.jstrokecerebrovasdis.2020.105004
7. Iwamoto T, Kitano T, Oyama N, Yagita Y. Predicting hemorrhagic transformation after large vessel occlusion stroke in the era of mechanical thrombectomy. PLoS One. 2021;16:e0256170. doi:10.1371/journal.pone.0256170
8. Zaidat OO, Yoo AJ, Khatri P, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44:2650–2663. doi:10.1161/STROKEAHA.113.001972
9. Wang D, Shu H, Meng Y, Zhang H, Wang H, He S. Factors promoting futile recanalization after stent retriever thrombectomy for stroke affecting the anterior circulation: a retrospective analysis. World Neurosurg. 2020;133:e576–e582. doi:10.1016/j.wneu.2019.09.098
10. van de Graaf RA, Samuels N, Chalos V, et al. Predictors of poor outcome despite successful endovascular treatment for ischemic stroke: results from the MR CLEAN Registry. J Neurointerv Surg. 2022;14:660–665. doi:10.1136/neurintsurg-2021-017726
11. Wang H, Zhang M, Hao Y, et al. Early prediction of poor outcome despite successful recanalization after endovascular treatment for anterior large vessel occlusion stroke. World Neurosurg. 2018;115:e312–e321. doi:10.1016/j.wneu.2018.04.042
12. Rabinstein AA, Albers GW, Brinjikji W, Koch S. Factors that may contribute to poor outcome despite good reperfusion after acute endovascular stroke therapy. Int J Stroke. 2019;14:23–31. doi:10.1177/1747493018799979
13. Semerano A, Laredo C, Zhao Y, et al. Leukocytes, collateral circulation, and reperfusion in ischemic stroke patients treated with mechanical thrombectomy. Stroke. 2019;50:3456–3464. doi:10.1161/STROKEAHA.119.026743
14. Jiang M, Yin P, Bai X, Yang L, Zhang J, Proinflammatory XS. Anti-inflammatory genes in stroke pathogenesis. Curr Pharm Des. 2020;26:4220–4233. doi:10.2174/1381612826666200701212859
15. Kawabori M, Yenari MA. Inflammatory responses in brain ischemia. Curr Med Chem. 2015;22:1258–1277. doi:10.2174/0929867322666150209154036
16. Jayaraj RL, Azimullah S, Beiram R, Jalal FY, Rosenberg GA. Neuroinflammation: friend and foe for ischemic stroke. J Neuroinflammation. 2019;16:142. doi:10.1186/s12974-019-1516-2
17. Zhang Y, Xing Z, Zhou K, Jiang S. The predictive role of Systemic Inflammation Response Index (SIRI) in the prognosis of stroke patients. Clin Interv Aging. 2021;16:1997–2007. doi:10.2147/CIA.S339221
18. Jickling GC, Liu D, Ander BP, Stamova B, Zhan X, Sharp FR. Targeting neutrophils in ischemic stroke: translational insights from experimental studies. J Cereb Blood Flow Metab. 2015;35:888–901. doi:10.1038/jcbfm.2015.45
19. Aly M, Abdalla RN, Batra A, et al. Follow-up neutrophil-lymphocyte ratio after stroke thrombectomy is an independent biomarker of clinical outcome. J Neurointerv Surg. 2021;13:609–613. doi:10.1136/neurintsurg-2020-016342
20. Chusid MJ. Eosinophils: friends or foes? J Allergy Clin Immunol Pract. 2018;6:1439–1444. doi:10.1016/j.jaip.2018.04.031
21. Coden ME, Berdnikovs S. Eosinophils in wound healing and epithelial remodeling: is coagulation a missing link? J Leukoc Biol. 2020;108:93–103. doi:10.1002/JLB.3MR0120-390R
22. Lee JJ, Jacobsen EA, McGarry MP, Schleimer RP, Lee NA. Eosinophils in health and disease: the LIAR hypothesis. Clin Exp Allergy. 2010;40:563–575. doi:10.1111/j.1365-2222.2010.03484.x
23. Davoine F, Lacy P. Eosinophil cytokines, chemokines, and growth factors: emerging roles in immunity. Front Immunol. 2014;5. doi:10.3389/fimmu.2014.00570
24. Piehler D, Stenzel W, Grahnert A, et al. Eosinophils contribute to IL-4 production and shape the T-helper cytokine profile and inflammatory response in pulmonary cryptococcosis. Am J Pathol. 2011;179:733–744. doi:10.1016/j.ajpath.2011.04.025
25. Orihuela R, McPherson CA, Harry GJ. Microglial M1/M2 polarization and metabolic states. Br J Pharmacol. 2016;173:649–665. doi:10.1111/bph.13139
26. Juceviciute N, Mikuzis P, Balnyte R. Absolute blood eosinophil count could be a potential biomarker for predicting haemorrhagic transformation after intravenous thrombolysis for acute ischaemic stroke. BMC Neurol. 2019;19:127. doi:10.1186/s12883-019-1359-6
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.