Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 16
Eosinopenia Predicting Long-term Mortality in Hospitalized Acute Exacerbation of COPD Patients with Community-acquired Pneumonia—A Retrospective Analysis
Authors Mao Y , Qian Y, Sun X, Li N, Huang H
Received 9 November 2021
Accepted for publication 20 December 2021
Published 30 December 2021 Volume 2021:16 Pages 3551—3559
DOI https://doi.org/10.2147/COPD.S347948
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Yanxiong Mao,1,* Yuanyuan Qian,2,* Xiaoyan Sun,3 Na Li,1 Huaqiong Huang1
1Key Laboratory of Respiratory Disease of Zhejiang Province, Department of Respiratory and Critical Care Medicine, Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, Zhejiang, People’s Republic of China; 2Department of Respiratory and Critical Care Medicine, Haining People’s Hospital, Haining Branch, The First Affiliated Hospital, Zhejiang University, Haining, Zhejiang, People’s Republic of China; 3Department of Gynecology, Women’s Hospital School of Medicine Zhejiang University, Hangzhou, Zhejiang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Huaqiong Huang
Key Laboratory of Respiratory Disease of Zhejiang Province, Department of Respiratory and Critical Care Medicine, Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, Zhejiang, People’s Republic of China
Tel/Fax +86-571-87783552
Email [email protected]
Background: Acute exacerbation of chronic obstructive pulmonary disease (AECOPD) could be triggered by community-acquired pneumonia (CAP). Peripheral blood eosinopenia are strongly associated with increased mortality. In hospitalized AECOPD patients with CAP, eosinopenia may be used to identify patients with high risk of death on admission.
Methods: We conducted a retrospective cohort study in 82 hospitalized AECOPD patients with CAP. Patients who had received systemic corticosteroids preadmission were excluded. The patients were identified by individual case file review. According to blood eosinophil count, patients were grouped as eosinopenia (< 50/μL) and non-eosinopenia (≥ 50/μL). Association of eosinopenia with infection and 18-month survival were analyzed using appropriate statistical methods.
Results: Baseline demographic, comorbidity, CURB65 and Pneumonia Severity Index scores were similar between two groups. The eosinopenia group had significantly higher pro-brain natriuretic peptide, D-dimer, neutrophil percentage, and lower lymphocyte count and lymphocyte percentage. The eosinopenia group had significantly higher C-reactive protein (median 77.30 vs 16.55, p=0.008) and procalcitonin (median 0.32 vs 0.12, p=0.001). Survival at 18 months after hospital discharge was significantly lower in the eosinopenia group vs non-eosinopenia group (log rank, p=0.002).
Conclusion: Eosinopenia (< 50/μL) was a strong predictor of 18-month mortality and associated with more severe infection in hospitalized AECOPD patients with CAP. Eosinophil count at admission can be used as a prognosis marker of mortality in those population.
Keywords: eosinopenia, chronic obstructive pulmonary disease, community-acquired pneumonia, mortality
Background
Chronic obstructive pulmonary disease (COPD) is a common respiratory disease worldwide.1 Acute exacerbations of chronic obstructive pulmonary disease (AECOPD) are important causes of hospital admission and mortality.2 Community-acquired pneumonia (CAP) occurs commonly in patients with COPD. It is estimated that there is an approximately 18-fold greater incidence of CAP in patients with COPD than in those without COPD.3 COPD is also frequent among CAP patients. COPD as a comorbidity is reported in 35% to 50% of hospitalized patients with CAP.4 The CAP and AECOPD come together when COPD patients acquire AECOPD triggered by CAP. The clinical presentations of these episodes meet the accepted criteria for the diagnosis of AECOPD and CAP in which a pulmonary infiltrate is found by chest radiograph.
Eosinopenia, defined as a reduced eosinophil count in peripheral blood, could be caused either by acute infection or acute stress.5 Eosinopenia is reported to be a useful predictor of bloodstream infection or sepsis,6,7 and an early marker of increased mortality in intensive care unit (ICU) patients.5,8 Eosinopenia in AECOPD is also concluded to have some clinical implications in published studies. In AECOPD patients, an inverse relationship between bacterial infection and eosinophil count has been reported.9 The AECOPD patients with bacterial infection have a significant decrease in the blood eosinophil count compared to the stable state. Eosinopenia is also an independent predictor of mortality and length of stay in AECOPD patients.10–12
The number of published articles on AECOPD with CAP is relatively small, compared to articles on AECOPD or CAP. The potential utility of using eosinopenia to guide optimal management of AECOPD with CAP has not been explored. The aim of the present study was to evaluate association between eosinopenia and clinical outcomes in hospitalized AECOPD patients with CAP.
Methods
The present study was a retrospective cohort study of hospitalized AECOPD patients with CAP at an academic teaching tertiary care hospital (Second Affiliated Hospital of Zhejiang University School of Medicine, China). Ethical approval was sought and granted by the Ethics Committee of Second Affiliated Hospital of Zhejiang University School of Medicine. As the non-interventional retrospective study was determined to be no greater than minimal risk, the Ethics Committee issued a waiver of informed consent. Patient data privacy and confidentiality were maintained as this study was conducted in compliance with the ethical standards of the Declaration of Helsinki.
Patient Selection
All patients admitted to the study hospital between January 2019 and June 2019 with discharge diagnosis of both AECOPD and CAP were retrieved from the electronic medical record system (EMRS). Patients who had received systemic corticosteroids pre-admission were identified from medical record and excluded from study.
Confirmation of Diagnosis
The definition of AECOPD was based on baseline clinical data obtained by chart review as described before.13 This strategy is widely used in the application of prognostic prediction rules and reflects the methods used in the original Pneumonia Severity Index (PSI) score studies.14
Records were inspected by a pulmonologist (YYQ), who was kept blinded to eosinophil designation, to confirm CAP. In Chinese tertiary care hospitals, it is common practice to order a chest computed tomography (CT) for patients suspected of CAP. So the diagnosis of CAP in this study were all based on chest CT. Patients were defined as having CAP as described by the Chinese CAP guideline in adults,15 when the following four criteria were met: (1) onset in community; (2) at least one of the following, (a) new onset of cough or expectoration, or aggravation of existing symptoms of respiratory tract diseases, with or without purulent sputum, chest pain, dyspnea, or hemoptysis, (b) fever >37.5°C, (c) signs of pulmonary consolidation and/or moist rales, (d) peripheral white blood cell (WBC) count >10×109 /L or <4×109/L, with or without a left shift; (3) chest CT scan at the time of hospitalization showing new patchy infiltrates, lobar or segmental consolidation, ground-glass opacities, or interstitial changes, with or without pleural effusion, defined by a radiologist’s reading; and (4) no alternative diagnosis at the time of hospital discharge that justified the presence of criteria 2 and 3.
Data Collection
Demographic data, lab test results on admission, disease comorbidities, and pharmacotherapy were collected from EMRS. The eosinophil count from the first whole blood cell count obtained in the hospital was used to designate eosinophil group. In accordance with previous studies, we defined eosinopenia as <50/μL and non-eosinopenia as ≥50/μL.12 Mortality status was assessed by phone call during early June 2021.
Data Analysis
The results were analyzed using IBM SPSS Statistics v. 20. Continuous data was presented as the mean with stand deviation (SD) or median with interquartile range (IQR), depending on the distribution of data. Variables were compared using the unpaired Student’s t-test, Welch's t-test or the Wilcoxon rank sum test with continuity correction, depending on data normality and homogeneity of variance. Categorical data were presented as absolute value and percentage, and analyzed using chi-squared test or Fisher’s exact test according to test assumptions. Statistical significance was set at p<0.05.
Results
A total number of 82 admissions with discharge diagnosis of both AECOPD and CAP were identified from EMRS. After review case by case, 59 admissions of AECOPD with CAP were confirmed and eligible for further analysis (Figure 1). The eosinopenia group had 41 admissions, and the non-eosinopenia group had 18 admissions.
 |
Figure 1 Flow chart of study population. Abbreviations: CT, computed tomography; CAP, community-acquired pneumonia. |
Demographic, Comorbidity and Clinical Expression
Baseline demographic and comorbidity data were similar between two groups (Table 1). The proportion of patients with atrial fibrillation or history of tuberculosis were higher in the eosinopenia group, but without statistical significance. Both the CURB65 and PSI scores were not different between two groups, which indicated that the severity of CAP between two groups was similar (Table 2). Compared to the non-eosinopenia group, the eosinopenia group had a higher proportion of patients admitted via emergency department (ED) (97.6% vs 77.8%, p=0.012). The eosinopenia group had significantly higher pro-brain natriuretic peptide (pro-BNP), D-dimer, neutrophil percentage than the non-eosinopenia group. Conversely, the eosinopenia group had significantly lower lymphocyte count and lymphocyte percentage. There was no significant difference in baseline arterial pressure of oxygen (PaO2) and arterial pressure of carbon dioxide (PaCO2) between two groups.
 |
Table 1 Comparison of Baseline Demographic and Comorbidity Data Between Eosinopenia and Non-eosinopenia Groups |
 |
Table 2 Comparison of Clinical Expression Between Eosinopenia and Non-eosinopenia Groups |
Infection Severity
Regarding biomarkers of infection, the eosinopenia group consistently presented with more severe infection status than the non-eosinopenia group (Figure 2). The eosinopenia group had significantly higher C-reactive protein (CRP) (median 77.30 vs 16.55, p=0.008) and procalcitonin (median 0.32 vs 0.12, p=0.001).
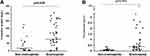 |
Figure 2 Infection biomarkers between eosinopenia and non-eosinopenia groups. (A) CRP levels; (B) procalcitonin levels. Abbreviation: CRP, C-reactive protein. |
Treatment
The length of hospital stay was similar between two groups (Table 3). But the eosinopenia group had higher hospital cost with statistical significance (median 20,598.00 vs 14,295.50, p=0.043). There was no significant difference in duration of antibiotic use and use of non-invasive ventilation between the two groups, but there was a clear trend to a higher proportion of invasive ventilation in the eosinopenia group (34.1% vs 11.1%, p=0.067). The eosinopenia group had a higher proportion of systemic corticosteroids use (73.2% vs 50.0%, p=0.083), but without statistical significance. Moreover the eosinopenia group had a significantly higher total (median 160.00 vs 40.00, p=0.019) and daily (median 40.00 vs 18.40, p=0.028) dose of systemic corticosteroids use. But there was no difference in total and daily dose of inhaled corticosteroids between the two groups.
 |
Table 3 Comparison of Treatment Between Eosinopenia and Non-eosinopenia Groups |
Long-term Mortality
Kaplan–Meier analyses identified a significant difference between two groups in all-cause mortality after hospital discharge. Survival at 18 months after hospital discharge was significantly lower in the eosinopenia group vs non-eosinopenia group (log rank, p=0.002) (Figure 3).
 |
Figure 3 Eighteen-month survival between eosinopenia and non-eosinopenia groups. Kaplan–Meier analysis of survival to 18 months after hospital discharge. |
Discussion
The potential utility of eosinopenia has not been evaluated in hospitalized AECOPD patients with CAP. The current study showed that eosinopenia (<50/μL) was a strong predictor of 18-month mortality. In addition, the eosinopenia group had significantly elevated CRP and procalcitonin, which indicated that eosinopenia was associated with more severe infection. Therefore, eosinopenia may be helpful for physicians to identify patients with high risk of mortality and optimize management strategies accordingly. Prospective studies are warranted to evaluate if adopting this strategy could improve prognosis of hospitalized AECOPD patients with CAP.
COPD is one of the most important cause of death worldwide, which was worsened by exacerbations.2 The CAP occurs commonly in patients with COPD as impairment in lung defense, especially in those older than 65 years.16 COPD is reported as a comorbidity in at least one third of hospitalized patients with CAP.4 There is a controversy about the impact of COPD on mortality of patients hospitalized for CAP.17 Several studies supported that COPD patients with CAP had higher mortality.13,18–20 However, some studies and meta-analysis showed that mortality was not increased in patients with COPD hospitalized with CAP.21–23 Regardless of co-existence of CAP, AECOPD caused high mortality worldwide, which warranted attention from physicians. So a simple, accessible and cheap marker to identify those patients with high risk of mortality was helpful in practice, especially in developing countries. Eosinopenia gained our attention, which was further examined in the current study.
Normally, eosinophils account for only a small part of white blood cells in blood. Its roles have been extensively investigated in asthma and COPD. High blood eosinophil count was a reliable predictor of response to inhaled corticosteroid in COPD.24–27 Furthermore, oral corticosteroid during AECOPD had better effect in patients with high blood eosinophil count.28 However studies about low blood eosinophil count or eosinopenia were relatively limited. Eosinopenia could be caused by either acute infection or acute stress, so it was not a biomarker of infection status. In essence, the eosinopenia of acute infection has been assumed to be a secondary expression of adrenal corticosteroid stimulation produced by the stress of the infection.29,30 Therefore, it was suggested that eosinopenia was a response to the acute inflammatory process rather than to a specific type of pathogen.
The principal finding of the current study was that eosinopenia (<50/μL) was a strong predictor of 18-month mortality. This finding was in agreement with previous studies. Numerous studies supported that eosinopenia was strongly associated with increased mortality in various clinical settings and diseases. In a large retrospective cohort study of 2311 patients with bacteremia, eosinopenia (<50/μL) was associated with a 4.77-fold increase in risk of dying compared with a normal eosinophil count.31 Yip et al found that eosinopenia (<10/μL) on ICU discharge was associated with increased post-ICU mortality.32 Similarly eosinopenia (<40/μL) at ICU admission was reported to be an independent predictor of 28-day mortality.8 Eosinopenia was reported to be able to predict mortality not only in adults, but also in children.5
A strong association between eosinopenia and mortality in AECOPD had also been described by some studies. A study by Holland et al reported that eosinopenia (<40/μL) was associated with higher in-hospital mortality.10 Moreover eosinopenia was identified as one of five strongest predictors of inpatient mortality in hospitalized AECOPD patients as part of the dyspnea, eosinopenia, consolidation, acidemia, and atrial fibrillation (DECAF) score.11 MacDonald et al further demonstrated that eosinopenia (<50/μL) was strongly associated with higher 12-month mortality in hospitalized AECOPD patients.12 In our dataset, eosinopenia (<50/μL) was associated with increased mortality at 18 months. So our findings corroborated with MacDonald’s, but our study population was different. In our study, only hospitalized AECOPD patients with confirmed CAP were analyzed; in MacDonald’s study, hospitalized AECOPD patients with all causes were included. Based on detection of chest X-ray consolidation, it was roughly estimated that there were 43 cases of pneumonia out of 242 cases in MacDonald’s study. So there were more hospitalized AECOPD patients with CAP in our study than MacDonald’s (59 vs 43). Moreover in our study, the diagnosis of CAP was based on chest CT, which was more reliable than X-ray. But still the study population was relatively small, further studies with a larger population were warranted.
Although the prognostic value of eosinopenia in COPD has been established by various studies including the current one, the mechanism of eosinopenia in COPD remains unclear. This area of research is still in its infancy, thus no conclusions could be drawn due to insufficient evidence. But in sepsis, some hypotheses about the mechanism of eosinopenia have been proposed, which may provide some possible directions for future studies in COPD.6 The first hypothesis is that eosinopenia may be due to low type 2 inflammation.6 Type 2 inflammation is usually associated with eosinophilia, and low type 2 inflammation may cause immune imbalance and worse clinical outcomes.29,33 But COPD is most commonly associated with type 1 and type 3 inflammation, so future studies are needed to determine whether this assumption holds in COPD.34 Another hypothesis is that eosinopenia may be a consequence of increased tissue eosinophil recruitment and consumption.6 Chemotactic substances released during sepsis or material obtained from inflammatory exudate have been shown to cause diffuse margination and sequestration of eosinophils at the site of infection, potentially contributing to initial peripheral eosinopenia.35,36 Further research is needed to test the assumption in COPD.
Another important finding of our study was that the eosinopenia group had significantly elevated CRP and procalcitonin. The CRP and procalcitonin were commonly used biomarkers for infection, and procalcitonin is a diagnostic marker of the presence of a bacterially induced systemic inflammatory reaction.37 Our finding indicated that eosinopenia was associated with more severe infection despite two groups having similar CURB65 and PSI scores at admission. This finding was consistent with previous reports conducted in various clinical settings. The study by Gil et al in a department of internal medicine demonstrated that an inflammatory syndrome associated with eosinopenia (<40/μL) was related to bacterial infectious diseases.38 Similarly, another study in ICU by Abidi et al showed that eosinopenia was a reliable marker of sepsis, and was associated with the severity of sepsis.39 Moreover the study in general internal medicine setting, excluding ICU and ED, revealed that eosinopenia was a useful predictor of bloodstream infection.7 In ED, eosinopenia (<10/μL) was reported to present a specificity of 94% for the diagnosis of infection. As mentioned above, eosinopenia could be caused by acute stress secondary to both infection and non-infection stimuli. The association between eosinopenia (<50/μL) and higher mortality found in this survey could, therefore, be partially explained both by more severe infection status and higher stress levels induced by infection.
The current study has a potentially important implication for physicians, especially in developing countries. As a cheap test for early identification of patients at high risk of death on admission, eosinopenia may help physicians in making management strategy. Once identified, a strategy of more aggressive diagnostic and therapeutic interventions may be warranted. The pros and cons of such a strategy need to be verified by prospective cohort studies.
Some limitations of the study merit consideration. First, the present study was a retrospective study, so there were inherent problems related to this design. Second, patients were identified as having AECOPD by medical history instead of pulmonary function, which has been demonstrated in past studies to be the same method as that used to identify other comorbid conditions included to create the PSI score. The severity of COPD between two groups could not be compared, although the PaO2 and PaCO2 was similar. Third, this study included patients only from January to June, so the seasonal variations and causes of CAP might be a concern. But in a study about seasonal variation of CAP, it was found that contrary to popular public opinion, winter with its low temperatures was not the main cause for CAP. Most of specific etiological causes of CAP, with the exception of respiratory viruses and Chlamydia pneumoniae, did not show significant seasonal variation.40
Conclusion
Eosinopenia (<50/μL) was a strong predictor of 18-month mortality in hospitalized AECOPD patients with CAP. Moreover eosinopenia was associated with more severe infection. Eosinophil count at admission can be used as a prognosis marker of mortality in hospitalized AECOPD patients with CAP. It may become a helpful clinical tool.
Data Sharing Statement
The datasets generated and/or analyzed during the current study are not publicly available due patients’ individual privacy could be compromised, but are available from the corresponding author on reasonable request.
Funding
This study is supported by National Natural Science Foundation of China (No. 81500061) and Beijing Bethune Charitable Foundation (BJ-RW2020025J).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Labaki WW, Rosenberg SR. Chronic obstructive pulmonary disease. Ann Intern Med. 2020;173(3):Itc17–Itc32. doi:10.7326/AITC202008040
2. MacLeod M, Papi A, Contoli M, et al. Chronic obstructive pulmonary disease exacerbation fundamentals: diagnosis, treatment, prevention and disease impact. Respirology. 2021;26(6):532–551. doi:10.1111/resp.14041
3. Bordon J, Slomka M, Gupta R, et al. Hospitalization due to community-acquired pneumonia in patients with chronic obstructive pulmonary disease: incidence, epidemiology and outcomes. Clin Microbiol Infect. 2020;26(2):220–226. doi:10.1016/j.cmi.2019.06.025
4. Cilli A, Erdem H, Karakurt Z, et al. Community-acquired pneumonia in patients with chronic obstructive pulmonary disease requiring admission to the intensive care unit: risk factors for mortality. J Crit Care. 2013;28(6):975–979. doi:10.1016/j.jcrc.2013.08.004
5. Kim YH, Park HB, Kim MJ, et al. Prognostic usefulness of eosinopenia in the pediatric intensive care unit. J Korean Med Sci. 2013;28(1):114–119. doi:10.3346/jkms.2013.28.1.114
6. Al Duhailib Z, Farooqi M, Piticaru J, Alhazzani W, Nair P. The role of eosinophils in sepsis and acute respiratory distress syndrome: a scoping review. Can J Anaesth. 2021;68(5):715–726. doi:10.1007/s12630-021-01920-8
7. Hirosawa T, Harada Y, Morinaga K, Takase H, Nin M, Shimizu T. Eosinopenia as a diagnostic marker of bloodstream infection in a general internal medicine setting: a cohort study. BMC Infect Dis. 2020;20(1):85. doi:10.1186/s12879-020-4814-5
8. Abidi K, Belayachi J, Derras Y, et al. Eosinopenia, an early marker of increased mortality in critically ill medical patients. Intensive Care Med. 2011;37(7):1136–1142. doi:10.1007/s00134-011-2170-z
9. Kolsum U, Donaldson GC, Singh R, et al. Blood and sputum eosinophils in COPD; relationship with bacterial load. Respir Res. 2017;18(1):88. doi:10.1186/s12931-017-0570-5
10. Holland M, Alkhalil M, Chandromouli S, Janjua A, Babores M. Eosinopenia as a marker of mortality and length of stay in patients admitted with exacerbations of chronic obstructive pulmonary disease. Respirology. 2010;15(1):165–167. doi:10.1111/j.1440-1843.2009.01651.x
11. Steer J, Gibson J, Bourke SC. The DECAF score: predicting hospital mortality in exacerbations of chronic obstructive pulmonary disease. Thorax. 2012;67(11):970–976. doi:10.1136/thoraxjnl-2012-202103
12. MacDonald MI, Osadnik CR, Bulfin L, et al. Low and high blood eosinophil counts as biomarkers in hospitalized acute exacerbations of COPD. Chest. 2019;156(1):92–100. doi:10.1016/j.chest.2019.02.406
13. Restrepo MI, Mortensen EM, Pugh JA, Anzueto A. COPD is associated with increased mortality in patients with community-acquired pneumonia. Eur Respir J. 2006;28(2):346–351. doi:10.1183/09031936.06.00131905
14. Fine MJ, Singer DE, Hanusa BH, Lave JR, Kapoor WN. Validation of a pneumonia prognostic index using the MedisGroups comparative hospital database. Am J Med. 1993;94(2):153–159. doi:10.1016/0002-9343(93)90177-Q
15. Cao B, Huang Y, She DY, et al. Diagnosis and treatment of community-acquired pneumonia in adults: 2016 clinical practice guidelines by the Chinese thoracic society, Chinese medical association. Clin Respir J. 2018;12(4):1320–1360. doi:10.1111/crj.12674
16. Müllerova H, Chigbo C, Hagan GW, et al. The natural history of community-acquired pneumonia in COPD patients: a population database analysis. Respir Med. 2012;106(8):1124–1133. doi:10.1016/j.rmed.2012.04.008
17. Cilli A. Community-acquired pneumonia in patients with chronic obstructive pulmonary disease. Curr Infect Dis Rep. 2015;17(1):444. doi:10.1007/s11908-014-0444-7
18. Lu Z, Cheng Y, Tu X, et al. Community-acquired pneumonia and survival of critically ill acute exacerbation of COPD patients in respiratory intensive care units. Int J Chron Obstruct Pulmon Dis. 2016;11:1867–1872. doi:10.2147/COPD.S113510
19. Molinos L, Clemente MG, Miranda B, et al. Community-acquired pneumonia in patients with and without chronic obstructive pulmonary disease. J Infect. 2009;58(6):417–424. doi:10.1016/j.jinf.2009.03.003
20. Shin B, Kim SH, Yong SJ, et al. Early readmission and mortality in acute exacerbation of chronic obstructive pulmonary disease with community-acquired pneumonia. Chron Respir Dis. 2019;16:1479972318809480. doi:10.1177/1479972318809480
21. Snijders D, van der Eerden M, de Graaff C, Boersma W. The influence of COPD on mortality and severity scoring in community-acquired pneumonia. Respiration. 2010;79(1):46–53. doi:10.1159/000213757
22. Loke YK, Kwok CS, Wong JM, Sankaran P, Myint PK. Chronic obstructive pulmonary disease and mortality from pneumonia: meta-analysis. Int J Clin Pract. 2013;67(5):477–487. doi:10.1111/ijcp.12120
23. Jiang HL, Chen HX, Liu W, Fan T, Liu GJ, Mao B. Is COPD associated with increased mortality and morbidity in hospitalized pneumonia? A systematic review and meta-analysis. Respirology. 2015;20(7):1046–1054. doi:10.1111/resp.12597
24. Mycroft K, Krenke R, Górska K. Eosinophils in COPD-current concepts and clinical implications. J Allergy Clin Immunol Pract. 2020;8(8):2565–2574. doi:10.1016/j.jaip.2020.03.017
25. Pascoe S, Locantore N, Dransfield MT, Barnes NC, Pavord ID. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med. 2015;3(6):435–442. doi:10.1016/S2213-2600(15)00106-X
26. Siddiqui SH, Guasconi A, Vestbo J, et al. Blood eosinophils: a biomarker of response to extrafine beclomethasone/formoterol in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192(4):523–525. doi:10.1164/rccm.201502-0235LE
27. Pavord ID, Lettis S, Locantore N, et al. Blood eosinophils and inhaled corticosteroid/long-acting β-2 agonist efficacy in COPD. Thorax. 2016;71(2):118–125. doi:10.1136/thoraxjnl-2015-207021
28. Bafadhel M, McKenna S, Terry S, et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am J Respir Crit Care Med. 2012;186(1):48–55. doi:10.1164/rccm.201108-1553OC
29. Rothenberg ME, Epstein FH. Eosinophilia. N Engl J Med. 1998;338(22):1592–1600. doi:10.1056/NEJM199805283382206
30. Bass DA. Behavior of eosinophil leukocytes in acute inflammation. II. Eosinophil dynamics during acute inflammation. J Clin Invest. 1975;56(4):870–879. doi:10.1172/JCI108166
31. Terradas R, Grau S, Blanch J, et al. Eosinophil count and neutrophil-lymphocyte count ratio as prognostic markers in patients with bacteremia: a retrospective cohort study. PLoS One. 2012;7(8):e42860. doi:10.1371/journal.pone.0042860
32. Yip B, Ho KM. Eosinopenia as a predictor of unexpected re-admission and mortality after intensive care unit discharge. Anaesth Intensive Care. 2013;41(2):231–241. doi:10.1177/0310057X1304100130
33. Linch SN, Danielson ET, Kelly AM, Tamakawa RA, Lee JJ, Gold JA. Interleukin 5 is protective during sepsis in an eosinophil-independent manner. Am J Respir Crit Care Med. 2012;186(3):246–254. doi:10.1164/rccm.201201-0134OC
34. Barnes PJ. Targeting cytokines to treat asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2018;18(7):454–466. doi:10.1038/s41577-018-0006-6
35. Bass DA, Gonwa TA, Szejda P, Cousart MS, DeChatelet LR, McCall CE. Eosinopenia of acute infection: production of eosinopenia by chemotactic factors of acute inflammation. J Clin Invest. 1980;65(6):1265–1271. doi:10.1172/JCI109789
36. Bass DA. Reproduction of the eosinopenia of acute infection by passive transfer of a material obtained from inflammatory exudate. Infect Immun. 1977;15(2):410–416. doi:10.1128/iai.15.2.410-416.1977
37. Titova E, Christensen A, Henriksen AH, Steinshamn S, Åsberg A. Comparison of procalcitonin, C-reactive protein, white blood cell count and clinical status in diagnosing pneumonia in patients hospitalized with acute exacerbations of COPD: a prospective observational study. Chron Respir Dis. 2019;16:1479972318769762. doi:10.1177/1479972318769762
38. Gil H, Magy N, Mauny F, Dupond JL. [Value of eosinopenia in inflammatory disorders: an “old” marker revisited]. Rev Med Interne. 2003;24(7):431–435. French. doi:10.1016/S0248-8663(03)00138-3
39. Abidi K, Khoudri I, Belayachi J, et al. Eosinopenia is a reliable marker of sepsis on admission to medical intensive care units. Crit Care. 2008;12(2):R59. doi:10.1186/cc6883
40. Lieberman D, Lieberman D, Porath A. Seasonal variation in community-acquired pneumonia. Eur Respir J. 1996;9(12):2630–2634. doi:10.1183/09031936.96.09122630
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
