Back to Journals » Infection and Drug Resistance » Volume 11
Entecavir add-on or switch-to pegylated interferon improves HBsAg clearance in HBe antigen negative chronic hepatitis B patients
Authors Yan L, Zhu C, Li J, Chen L, Ding Y, Cao Z, Liu K, Lin L, Tang W, Xie Q, Xu Y, Bao S, Wang H
Received 29 May 2018
Accepted for publication 15 August 2018
Published 29 October 2018 Volume 2018:11 Pages 2001—2009
DOI https://doi.org/10.2147/IDR.S175707
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Suresh Antony
Lei Yan,1,* Chuanwu Zhu,2,* Jing Li,3,* Liwen Chen,1 Yezhou Ding,1 Zhujun Cao,1 Kehui Liu,1,4 Lanyi Lin,1 Weiliang Tang,1 Qing Xie,1 Yumin Xu,1 Shisan Bao,5 Hui Wang1
1Department of Infectious Diseases, Rui Jin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200025, China; 2Department of Infectious Diseases, The Fifth People’s Hospital of Suzhou, Jiangsu 215007, China; 3Department of Infectious Diseases, Huai-An Fourth People’s Hospital, Jiangsu 223002, China; 4Department of Infectious Diseases, Rui Jin Hospital North, Shanghai Jiao Tong University School of Medicine, Shanghai 201801, China; 5Discipline of Pathology, School of Medical Sciences and Bosch Institute, Charles Perkin Centre, University of Sydney, Sydney, NSW, Australia
*These authors contributed equally to this work
Background and aims: Chronic hepatitis B (CHB) patients rarely achieve hepatitis B surface antigen (HBsAg) loss with nucleoside/nucleotide analog therapy.
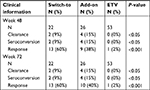
Methods: In this retrospective study, it was evaluated that the rate of HBsAg loss in the HBe antigen negative (HBeAg−) patients (n=101) treated with entecavir (ETV) for ≥24 weeks followed by switching to (n=22) or adding on (n=26) pegylated interferon (PEG-IFN), and continuing ETV (n=53).
Results: HBsAg clearance rate at week 48 was 9% (2/22), 15% (4/26), and 0% (0/53) (P<0.05), in switch-to or add-on, or ETV monotherapy CHB patients, respectively. HBsAg reduction at week 48 was 1.182, 0.6614, or 0.056 log IU/mL, in switch-to, add-on, and ETV patients, respectively (P<0.001). The response rate (HBsAg reduction >1 log IU/mL at week 48) in the switch-to, add-on, and ETV monotherapy CHB patients was 60%, 40%, and 2%, respectively (P<0.001). In the switch-to and add-on patients, HBsAg reduction and clearance were associated with HBsAg titers at week 0 and HBsAg reduction at week 24. Furthermore, HBsAg reduction at week 24 was associated with the response rate at week 48 in the switch-to and add-on patients, showing that the area under the receiver operating characteristic curve was 0.904. Positive predictive value and negative predictive value for response rate was 70% and 100% with cut-off value 0.2 log IU/mL, respectively.
Conclusion: In summary, we demonstrated that PEG-IFN enhanced HBsAg loss in HBeAg− CHB patients. High HBsAg clearance was achieved in the patients with HBsAg titers at baseline <1,000 IU/mL and HBsAg reduction >0.2 log IU/mL.
Keywords: chronic hepatitis B, HBsAg, PEG-IFN, entecavir, switch-to, add-on
Introduction
The primary goal in chronic hepatitis B (CHB) patients is to suppress hepatitis B virus (HBV) DNA replication. The definition of functional cure of CHB is the durable loss of serum hepatitis B surface antigen (HBsAg) with or without sustained HBs seroconversion, and appears to be the ultimate goal of antiviral therapy in HBe antigen negative (HBeAg−) CHB patients.1 Pegylated interferon (PEG-IFN) α and nucleoside (entecavir, lamivudine [LAM], and telbivudine) or nucleotide (tenofovir disoproxil fumarate [TDF] and adefovir dipivoxil) analogs (NAs) have been routinely administered to CHB patients.1 PEG-IFN is used as finite-duration treatment for 48 weeks; whereas NAs have to be used continuously to prevent relapse.
It has been reported that HBsAg seroconversion is only 3% and 6% of HBeAg− CHB patients treated with PEG-IFN for 48 and 96 weeks, respectively.2 The proportion of HBsAg clearance in PEG-IFN-treated HBeAg− CHB patients increases during follow-up, reaching 9% and 12% at 3 and 5 years, respectively.3,4 However, there is quite a low chance of achieving a sustained virological response in these HBeAg− CHB patients without decline in HBsAg titers and a <0.2 log decline in HBV DNA at 12 weeks of PEG-IFN. Subsequently, these HBeAg− CHB patients are warranted for discontinuation of PEG-IFN. On the other hand, NAs are very effective at suppressing HBV DNA in CHB patients within the first year of treatment, but long-term loss of HBsAg and anti-HBsAg antibody seroconversion are rarely achieved.5–8
It is well known that NAs partially restore adaptive immunity, whereas PEG-IFN enhances innate immunity via preventing formation of HBV proteins, depleting intrahepatic cccDNA pool. PEG-IFN finally leads to more HBsAg loss than NAs.5,9 Furthermore, naïve HBeAg− CHB patients with PEG-IFN or PEG-IFN-added LAM 48 weeks, following up to 24 weeks response rate (HBV DNA <400 copy/mL) is better than LAM monotherapy.2 In addition, HBsAg loss is 5.1% and 1.3% among naïve HBeAg− CHB patients with TDF combined with PEG-IFN or PGE-IFN monotherapy 48 weeks, respectively.10
Therefore, it is desirable to enhance HBsAg loss by addition of PEG-IFN to NAs in CHB patients with long period of HBV DNA suppression following NAs monotherapy. It has been demonstrated that an early PEG-IFN add-on is better than monotherapy in terms of sustained HBsAg reduction in HBeAg+ CHB patients.11–13 Moreover, few case reports and uncontrolled pilot studies observe that there is decline in HBsAg titers, HBsAg loss, and HBs seroconversion in HBeAg− CHB with PEG-IFN add-on.14–16
Therefore, we designed a multicenter retrospective case–control study to evaluate the effect on HBsAg clearance of add-on or switch-to PEG-IFN in initial ETV treated patients, compared with continuous ETV in HBeAg− CHB patients.
Methods
Patients
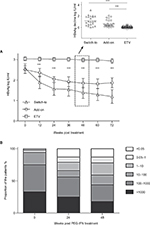
In this retrospective study, we had identified 101 HBeAg− patients (n=101) treated with ETV for ≥24 weeks in the Department of Infectious Diseases, Shanghai Rui Jin Hospital, The Fifth People’s Hospital of Suzhou, and Huai-An Fourth People’s Hospital from January 2012 to November 2016. The selection criteria for the current study included the following: 1) patients aged between 18 and 65 years, 2) CHB patients defined by serum HBsAg positive for at least 6 months, HBeAg negative, and 3) ETV treatment for ≥24 weeks with HBV DNA undetectable and without PEG-IFN treatment within 2 years. In our current retrospective study, these CHB patients identified had high HBsAg and HBV DNA initially, and the levels of HBsAg and HBV DNA were reduced following initial ETV treatment. Thus, PEG-IFN was not the first selection for these high HBsAg and HBV DNA patients. Subsequently, the optimal option(s) were explored in the patients with low HBsAg and HBV DNA negative for switch-to, add-on, or continue with ETV. There was no criteria for the decision of switch or add to PEG-IFN or ETV monotherapy at the time of treatment. Thus, the current study was to collect more information, which may be useful to contribute and to generate a guideline in our future medical practice. In this retrospective study, we had identified HBeAg− patients (n=101) treated with ETV for ≥24 weeks followed by switching to (n=22) or adding on (n=26) PEG-IFN, and continuing ETV (n=53). (Figure 1A).
The exclusion criteria were as follows: 1) neutropenia (neutrophils count <1.5×109/L), 2) thrombocytopenia (platelet count of <70×109/L), 3) co-infection with HIV, hepatitis C virus, or hepatitis D virus, 4) decompensated cirrhosis (defined as a Child–Pugh score of ≥7 or episodes of ascites, edema, hepatic encephalopathy, or gastrointestinal bleeding), 5) other chronic liver diseases (eg, hemochromatosis, auto-immune hepatitis, Wilson’s disease or alcoholic or toxic liver disease), 6) allergy to interferon α or a component of the tested product, psychiatric disorders, a history of seizures, 7) cardiovascular disease, a history of cancer in the last 5 years, uncontrolled thyroid disorders or autoimmune disorders, renal dysfunction, and 8) treatment with immunosuppressive or immunomodulatory drugs, treatment for >4 weeks consecutively with systemic corticosteroid therapy, a reported daily alcohol intake of greater than 30 g (women) or 40 g (men).
The study has been approved by the Human Ethics Committees, Rui Jin Hospital, Shanghai Jiaotong University School of Medicine, Huai-An Fourth People’s Hospital and The Fifth People’s Hospital of Suzhou. Written informed consent was obtained from every participant.
Procedures
Patients in the PEG-IFN add-on or switch-to had received subcutaneous injections of 180 µg PEG-IFN α -2a (Pegasys, Roche, Switzerland) per week for at least 48 weeks; whereas the ETV monotherapy patients had continuously taken 0.5 mg ETV daily.
Routine blood biochemistry, hematology, anti-HBsAg, and HBV DNA were performed in the Central Clinical Laboratory, RuiJin Hospital every 3 months. Fibroscan (Echosens, Paris, France) was utilized as a non-invasive approach to determine the severity of liver stiffness,17 correlating with liver fibrosis and/or cirrhosis at baseline and 48 weeks post treatment. HBV DNA was measured, using Roche COBAS TaqMan version 2.0 (limit of detection 20 IU/mL). Undetectable HBV DNA was defined as HBV DNA less than the limit of detection. Quantification of serum HBsAg titers was performed as described previously.18 Titers of HBsAg in serum were quantified by a standardized electrochemiluminescent carbonylmetalloimmunoassay (Architect HBsAg; Abbott, Frisco, TX, USA), with a lower detection limit of 0.05 IU/mL. Loss of HBsAg was defined as HBsAg titer lower than the detection limit. Samples with titers of more than the upper linearity limit of the assay (250 IU/mL) were retested after being diluted as recommended by the manufacturer. The CHB patients achieved HBsAg negative following the treatment, and then were confirmed again 1 month later. Anti-HBs seroconversion was defined as an anti-HBs antibodies titer of >10 mIU/mL.
Study end-points
The primary outcome was to evaluate the rate of HBsAg loss in these HBeAg− CHB patients at week 48. Secondary outcomes were to obtain the kinetics of HBsAg titers, response at week 48, and assessment of predictive factors associated with HBsAg clearance and response (consisting of treatments, age, sex, HBsAg titer at baseline, and HBsAg reduction at week 24). We defined that the response was HBsAg reduction >1 log IU/mL.
Statistical analyses
Continuous variables were analyzed using one-way ANOVA. Categorical data were analyzed with chi-squared and Fisher’s exact tests. A p-value of <0.05 was considered significant. Univariate analyses were performed to identify variables that were significantly different between response and non-response patients. Multivariate analysis was further performed for selecting and eliminating variables. Receiver operating characteristic (ROC) curves were constructed and the area under the ROC curves (AUROC) was calculated. Optimal cut-off values were selected to maximize specificity and sensitivity, and diagnostic accuracy. Positive predictive value (PPV) and negative predictive value (NPV) were calculated, using the optimal cut-off value obtained by ROC curves. SPSS software version 22.0 (SPSS, Inc. Chicago, IL, USA) was used for statistical analyses.
Results
Baseline characteristics
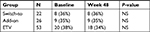
Baseline characteristics of switch-to, add-on, and ETV groups in the current study are presented in Table 1. There were totally 101 HBeAg− CHB patients enrolled. Male vs female was 82 (81.18%) vs 19 (18.81%). There was no significant difference of body mass index, alanine aminotransferase, aspartate aminotransferase, albumin, white blood cell, platelet, liver stiffness, mean HBsAg titer, and HBV DNA among these three groups, except the age and number of male vs female.
  | Table 1 Characteristic of patients at baseline Notes: Continuous data are expressed as the mean ± SD, age shown with the mean (range). Categorical data, male, BMI, liver stiffness, shows with number and proportion. Liver stiffness >9 kPa refers to sever liver fibrosis or cirrhosis.17 Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALB, albumin; BMI, body mass index; ETV, entecavir; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; WBC, white blood cell; PLT, platelet; NS, non-significant. |
Among these 48 patients treated with PEG-IFN (both add-on and switch-to), there were 67% (32/48) patients with HBsAg titer under 1,000 IU/mL at the baseline. More specifically, there were 68% (15/22) and 65% (17/26) in switch-to and add-on patients, respectively (Figure 1B). The number of patients in ETV with HBsAg titers <1,000 IU/mL was 16 (30%).
HBsAg clearance/reduction in ETV, PEG-IFN switch-to, and PEG-IFN add-on groups
There was no obvious alteration of HBsAg titers over 48 weeks with ETV continuous-treated group (Figure 2A). However, HBsAg titers were decreased significantly in either PEG-IFN switch-to and add-on treated patients from 12 to 72 weeks following initial 24 weeks ETV (Figure 2A). More substantial difference was detected at 48-weeks with subsequent treatment, showing that mean decline of HBsAg titer from baseline in PEG-IFN switch-to (1.182 log IU/mL) and add-on (0.6614 log IU/mL) group was 21- and 12-fold higher, respectively, compared with that in the ETV group (0.056 log IU/mL) (P<0.001) (Figure 2A, inset). Furthermore, HBsAg clearance in PEG-IFN switch-to, PEG-IFN add-on and ETV continuous-treated group was 9%, 15%, and 0%, respectively, with significant difference between PEG-IFN switch-to and ETV monotherapy (P<0.001) or between PEG-IFN add-on and ETV monotherapy (P<0.001). Similar patterns were also observed in anti-HBs seroconversion among these three groups (Table 2).
Over the 48 weeks PEG-IFN treatment period, proportion of patients with high HBsAg titer (>1,000 and 100–1,000) gradually decreased, whereas the proportion of low HBsAg titer (<0.05, 1, 10, 100) patients gradually increased (Figure 2B). Thus, such data suggest that HBsAg titer was effectively controlled by PEG-IFN.
The response rate of PEG-IFN switch-to, PEG-IFN add-on, and ETV monotherapy was, 60%, 40%, and 2%, respectively (Table 2). Consistently, there was significant difference of response rate between PEG-IFN switch-to and ETV (P<0.05) or PEG-IFN add-on and ETV (P<0.05).
HBsAg reduction in PEG-IFN add-on and switch-to groups
There was no significant difference of mean HBsAg titer reduction between combination and sequential groups. The response rate was 40% and 60% in add-on and switch-to group, respectively; whereas clearance rate was 15% and 9%, respectively in add-on and switch-to group (Table 2).
Liver stiffness from baseline to week 48 in three groups
Fibroscan has been used as non-invasive approach to evaluate the liver structure, as described previously.19 There was no significant correlation between liver stiffness and these three treatments (among ETV, PEG-IFN add-on, and switch-to groups) (Table 3).
  | Table 3 Liver stiffness change Notes: The data shown in the table is the number and proportion of patients with liver stiffness >9 kPa. Abbreviation: ETV, entecavir; NS, non-significant. |
Correlation between HBsAg reduction and associate factors
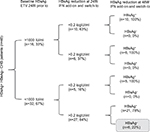
Univariate and Multivariate analysed illustrated that titer of baseline HBsAg, the age of patients, reduction of HBsAg at 24 weeks significantly contributed to HBsAg reduction at 48 week. (Table 4). HBsAg reduction was associated with the response rate at week 24 (Table 4), showing AUROC was 0.904, suggesting that there was a significant correlation between the response at 48 week post treatment and HBsAg reduction of 24 week (Figure 3). PPV for response was 70% and NPV was 100% with the cut-off value 0.2 log IU/mL. ROC analysis of HBsAg at baseline with response showed AUROC =0.769, PPV was 70%, NPV was 81% with cut-off value of 3 log IU/mL. The series test of both HBsAg at baseline and reduction at week 24 was much better to identify patients with AUROC 0.974. The patients with HBsAg at baseline <1,000 IU/mL and HBsAg reduction >0.2 log IU/mL had significantly higher proportion of HBsAg clearance (22%) (Figure 4).
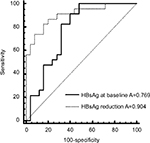  | Figure 3 ROC analysis of HBsAg reduction at week 24 with response at week 48 (HBsAg reduction>1 log IU/mL). Abbreviations: HBsAg, hepatitis B surface antigen; ROC, receiver operating characteristic. |
Discussion
In the present study, we demonstrated that PEG-IFN add-on or switch-to in HBeAg− patients enhanced HBsAg reduction significantly compared with the patients with the continuation of ETV, following initial 24-week ETV. Such data suggest that PEG-IFN add-on or switch-to is a better strategy for the management of HBeAg− patients, particularly in promoting HBsAg loss.
It has been reported that there is a close correlation between HBsAg loss/reduction and prognosis, including development of hepatocellular carcinoma and subsequent CHB-related mortality in HBeAg+ patients.1,20 In addition, good prognosis is expected in long-term follow-up after sequential therapy in the sequential group patients. In line with such findings, we observed that both PEG-IFN add-on and switch-to following ETV 24 weeks initial therapy substantially increased HBsAg loss and HBs seroconversion among HBeAg− CHB patients with undetectable HBV DNA. Moreover, HBsAg loss was significantly higher in patients who received a full 48-week course of PEG-IFN. More interestingly, among 48 PEG-IFN (combining add-on and switch-to) patients, 6/48 (12.5%) patients with PEG-IFN had lost HBsAg at week 24 achieved anti-HBs seroconversion, suggesting PEG-IFN is effective in battling hepatitis B virus.
Interestingly, we detected that HBsAg clearance was 15% and 9% in HBeAg− CHB patients with initial 24-week ETV followed by add-on and switch-to PEG-IFN, respectively. However, it has been reported that HBsAg loss is only 5% in the naive HBeAg−CHB patients with TDF/PEG-IFN 48-week treatment.10 Furthermore, HBsAg clearance is only 1% in the naive CHB patients with PEG-IFN 48-week treatment.10 Thus, our finding suggests that PEG-IFN switch-to or add-on in initial ETV-treated HBeAg−CHB patients may be more effective for HBV viral clearance than initial PEG-IFN monotherapy or PEG-IFN combined with TDF. In previous study by Marcellin et al (2016), the mean (SD) of HBsAg baseline was 3.8 (09)–3.9 (0.8) log IU/mL in TDF+ PEG-IFN (48W), TDF+ PEG-IFN (16W)+ TDF (32W), TDF (48W), or PEG-IFN (48W); whereas the baseline in our current study was 2.5 (1.0)–3.0 (0.6) log IU/mL. Thus, the baseline HBsAg in our current study was lower compared with that from the studies from Marcellin et al (2016), which may also be a contributing factor in HBsAg level.10 In addition, our data also suggest that PEG-IFN switch-to or add-on in initial ETV may be more acceptable in the developing countries/regions, due to lower financial burden.
We realize that not all HBeAg− CHB patients reached HBsAg clearance in our current study; nevertheless, the benefit of reduced HBsAg titer in HBeAg− CHB patients is to improve prognosis in long term. We further illustrated that the mean HBsAg reduction in PEG-IFN add-on or switch-to group was significantly higher than ETV group in HBeAg− CHB patients. Our data are consistent with other studies which found that HBsAg reduction in HBeAg− CHB patients is detected in PEG-IFN switch-to only; however, no add-on was investigated in their patients.21
Our finding is further supported by others, showing that the usefulness of this PEG-IFN in HBeAg− CHB patients appears to be meaningful only among patients with an HBsAg titer at baseline of <1,000 IU/mL, and HBsAg decline during treatment is a strong predictor of responses.18
There is also difference in HBsAg clearance among the CHB patients treated with PEG-IFN combined with TDF and PEG-IFN-only-treated patients.10 However, in our current study, it was demonstrated that there was no significant difference of HBsAg clearance between PEG-IFN add-on and switch-to groups. The discrepancy between the findings of others and ours maybe due to different races (ie, Caucasians vs Asians), different treatments (naïve vs experienced), which will be further investigated.
We acknowledge that the sample size in the current study was not very big. We will recruit more patients as well as more different races for our future study. Furthermore, we also realize that the current study was focusing on HBeAg− patients with PEG-IFN therapy retrospective investigation, but will have a prospective well-controlled study in future.
We acknowledge that the number of patients in total and with HBsAg loss or clearance was rather small, which cannot make a final conclusive statement at this stage. We will identify more CHB patients from multiple centers and different races in our future study. Furthermore, the CHB patients with HBsAg loss/clearance at weeks 48 and 72 will also be extended in future study. We also realize that the age of switch-to was younger than add-on or ETV monotherapy, although there was no significant difference in age between ETV and add-on groups. We acknowledge that these findings were within 24 weeks post treatment. To fix this problem, we will recruit more patients with longer time period and reduce the age difference among the different groups in our future study.
It is well known that genotype contributes to HBV DNA production. However, the absence of HBV genotype is a potential shortfall of our study. Lack of HBV genotype in the current study was mainly due to undetectable HBV DNA after 24 weeks ETV treatment. Although there was no genotype in the current study, most genotype was B or C in Chinese population, who have demonstrated less response.20,22 There was no significant correlation between liver stiffness and PEG-IFN treatment in the current study. The explanation for this finding may be due to relative short term of PEG-IFN treatment, and possibly due to small number of patients.
Ideally long-term use of PEG-IFN in HBeAg− CHB could achieve better HBsAg clearance and seroconversion. In our current study, some of the patients had 72 weeks PEG-IFN treatment, but treatment in a number of other HBeAg− CHB patients had to be stopped at week 48 due to serious side effects. In our future study, we will recruit more patients with long-term usage of PEG-IFN to achieve high proportion of HBsAg clearance and seroconversion.
In summary, we demonstrated that PEG-IFN enhanced HBsAg loss in HBeAg− CHB population. HBeAg− CHB patients with HBsAg titers <1,000 IU/mL at baseline and reduction >0.2 log IU/mL at week 24 could have high chance of achieving HBsAg loss and anti-HBs seroconversion.
Acknowledgments
We acknowledge all the patients, their families, and all the staff from department of infectious disease in Shanghai Rui Jin Hospital, The Fifth People’s Hospital of Suzhou, and Huai-An Fourth People’s Hospital. This work was supported by grants from the National Natural Science Foundation of China (81570560, 81670569), Technology Supporting Project of the Science and Technology Commission Shanghai Municipality (16411960300), The Shanghai Three-year Plan of the Key Subjects Construction in Public Health-Infectious Disease and Pathogenic Microorganism (15GWZK0102), The Medical Science Research Foundation (YWJKJJHKYJJ-B17503), The Suzhou Expert Team of Clinical Medicine (SZYJTD201717), and National Thirteen-Five Project (2018Z × 10205504–001–002, 2018Z × 10303501). YX, SB and HW are equal senior authors.
Disclosure
The authors report no conflicts of interest in this work.
References
EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. Journal of hepatology. 2017;67(2):370–398. | ||
Marcellin P, Lau GK, Bonino F, et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. The New England journal of medicine. 2004;351(12):1206–1217. | ||
Marcellin P, Bonino F, Lau GK, et al. Sustained response of hepatitis B e antigen-negative patients 3 years after treatment with peginterferon alpha-2a. Gastroenterology. 2009;136(7):2169–2179.e2161–2164. | ||
Brunetto MR, Marcellin P, Cherubini B, et al. Response to peginterferon alfa-2a (40KD) in HBeAg-negative CHB: on-treatment kinetics of HBsAg serum levels vary by HBV genotype. J Hepatol. 2013;59(6):1153–1159. | ||
Wursthorn K, Lutgehetmann M, Dandri M, et al. Peginterferon alpha-2b plus adefovir induce strong cccDNA decline and HBsAg reduction in patients with chronic hepatitis B. Hepatology. 2006;44(3):675–684. | ||
Chang TT, Lai CL, Kew Yoon S, et al. Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology. 2010;51(2):422–430. | ||
Chevaliez S, Hézode C, Bahrami S, Grare M, Pawlotsky JM. Long-term hepatitis B surface antigen (HBsAg) kinetics during nucleoside/nucleotide analogue therapy: finite treatment duration unlikely. J Hepatol. 2013;58(4):676–683. | ||
Ono A, Suzuki F, Kawamura Y, et al. Long-term continuous entecavir therapy in nucleos(t)ide-naïve chronic hepatitis B patients. J Hepatol. 2012;57(3):508–514. | ||
Swiecki M, Colonna M. Type I interferons: diversity of sources, production pathways and effects on immune responses. Curr Opin Virol. 2011;1(6):463–475. | ||
Marcellin P, Ahn SH, Ma X, et al. Combination of tenofovir disoproxil fumarate and peginterferon alpha-2a increases loss of hepatitis B surface antigen in patients with chronic hepatitis B. Gastroenterology. 2016;150(1):134–144.e110. | ||
Brouwer WP, Xie Q, Sonneveld MJ, et al. Adding pegylated interferon to entecavir for hepatitis B e antigen-positive chronic hepatitis B: A multicenter randomized trial (ARES study). Hepatology (Baltimore, Md). 2015;61(5):1512–1522. | ||
Ning Q, Han M, Sun Y, et al. Switching from entecavir to PegIFN alfa-2a in patients with HBeAg-positive chronic hepatitis B: a randomised open-label trial (OSST trial). J Hepatol. 2014;61(4):777–784. | ||
Li GJ, Yu YQ, Chen SL, et al. Sequential combination therapy with pegylated interferon leads to loss of hepatitis B surface antigen and hepatitis B e antigen (HBeAg) seroconversion in HBeAg-positive chronic hepatitis B patients receiving long-term entecavir treatment. Antimicrob Agents Chemother. 2015;59(7):4121–4128. | ||
Mangano C, Squadrito G, Cacciola I, Carpentieri M, Foti G, Raimondo G. Effectiveness of add-on pegylated interferon alfa-2a therapy in a lamivudine-treated patient with chronic hepatitis B. Ann Hepatol. 2011;10(1):84–87. | ||
Barone M, Iannone A, di Leo A. HBsAg clearance by Peg-interferon addition to a long-term nucleos(t)ide analogue therapy. World J Gastroenterol. 2014;20(26):8722–8725. | ||
Ouzan D, Pénaranda G, Joly H, Khiri H, Pironti A, Halfon P. Add-on peg-interferon leads to loss of HBsAg in patients with HBeAg-negative chronic hepatitis and HBV DNA fully suppressed by long-term nucleotide analogs. J Clin Virol. 2013;58(4):713–717. | ||
European Association for Study of L, Asociacion Latinoamericana para el Estudio del H. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63(1):237–264. | ||
Bourliere M, Rabiega P, Ganne-Carrie N, et al. Effect on HBs antigen clearance of addition of pegylated interferon alfa-2a to nucleos(t)ide analogue therapy versus nucleos(t)ide analogue therapy alone in patients with HBe antigen-negative chronic hepatitis B and sustained undetectable plasma hepatitis B virus DNA: a randomised, controlled, open-label trial. Lancet Gastroenterol Hepatol. 2017;2(3):177–188. | ||
Cao Z, Li Z, Wang H, et al. Algorithm of Golgi protein 73 and liver stiffness accurately diagnoses significant fibrosis in chronic HBV infection. Liver Int. 2017;37(11):1612–1621. | ||
Hou J, Wang G, Wang F, et al. Guideline of Prevention and Treatment for Chronic Hepatitis B (2015 Update). J Clin Transl Hepatol. 2017;5(4):297–318. | ||
Tamaki N, Kurosaki M, Kusakabe A, et al. Hepatitis B surface antigen reduction by switching from long-term nucleoside/nucleotide analogue administration to pegylated interferon. J Viral Hepat. 2017;24(8):672–678. | ||
Moucari R, Martinot-Peignoux M, Mackiewicz V, et al. Influence of genotype on hepatitis B surface antigen kinetics in hepatitis B e antigen-negative patients treated with pegylated interferon-alpha2a. Antivir Ther. 2009;14(8):1183–1188. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.