Back to Journals » Local and Regional Anesthesia » Volume 13
Enlarged Brachial Plexus Nerve Found During Ultrasound-Guided Peripheral Nerve Block Diagnosed as Charcot-Marie-Tooth Disease: A Case Report
Authors Shiraishi T , Masumoto K, Nakamura M, Hidano G
Received 1 July 2020
Accepted for publication 5 October 2020
Published 19 October 2020 Volume 2020:13 Pages 141—146
DOI https://doi.org/10.2147/LRA.S270189
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Stefan Wirz
Toshie Shiraishi, Kentaro Masumoto, Mitsuyo Nakamura, Gumi Hidano
Minimally Invasive Surgery Center, Department of Anesthesiology, Yotsuya Medical Cube, Tokyo, Japan
Correspondence: Toshie Shiraishi Minimally Invasive Surgery Center, Department of Anesthesiology
Yotsuya Medical Cube, 7-7 Nibancho, Chiyoda-Ku, Tokyo 102-0084, Japan
Tel +8130-3261-0401
Fax +8130-3261-0402
Email [email protected]
Abstract: Ultrasound-guided peripheral nerve block (PNB) has become a popular anesthetic procedure. We report a case of an enlarged brachial plexus nerve noted on ultrasonographic images, as part of PNB, which was diagnosed postoperatively as Charcot-Marie-Tooth disease (CMTD), an inherited neurological disorder of the peripheral nerves. Although nerve enlargement is characteristic of demyelinating diseases such as CMTD, the use of ultrasonography in the diagnosis of neurological disorders is a developing area for neurologists and anesthesiologists can lack knowledge in this emerging field. Unusual nerve presentation on ultrasonographic images during PNB anesthetic procedures should be recognized as being indicative of underlying neurologic disorders. This case highlights that increased awareness of the diagnosis of underlying neurologic disorders by ultrasonography would assist the general practice of PNB in anesthetic medicine. This is especially important as underlying neurological conditions can have important consequences for patient-appropriate anesthesia and may inform best anesthetic practice. A new category, “neurological disorder on ultrasound image”, should be introduced to PNB knowledge in anesthetic field.
Keywords: ultrasonography, local anesthesia and analgesia, neurological disorders, demyelinating diseases
Introduction
Charcot-Marie-Tooth disease (CMTD) is one of the most commonly encountered inherited sensory-motor neuropathic disorders. Demyelination, followed by nerve enlargement, is well-known as a characteristic feature of this disease. Ultrasonographic techniques have been developed for use in elucidating nerve changes, such as the enlargement in CMTD, and can help diagnose neurological disorders.1
In anesthesiology, ultrasonography has also become an important tool, especially for ultrasound-guided peripheral nerve block (PNB) when used as an analgesic treatment. There are, however, specific concerns for patients with CMTD undergoing anesthesia.2 Depolarizing muscle relaxants may cause hyperkalemia, while non-depolarizing ones may result in prolongation of the analgesic effect. Moreover, local anesthetics can have toxic effects on demyelinated nerves.3
Herein we report a case of a patient with an enlarged brachial plexus that was discovered during ultrasound-guided PNB and was diagnosed postoperatively as CMTD. This case report highlights the need to recognize the significance of nerve changes in ultrasonographic images in PNB procedures to ensure that anesthesiologists are able to provide safe anesthesia in similar cases.
Case Description
A 56-year-old woman (height 161 cm, weight 74 kg, body mass index 28) was scheduled to undergo neurolysis performed by a hand surgeon to treat median nerve palsy. She had suffered from numbness in her right thumb with increasing severity for six months. Her medical history included depression and osteoarthritis of the left knee and right hip. She had no abnormalities in the routine preoperative examinations that included blood tests, electrocardiography, and respiratory function tests. No apparent anatomical anomalies were declared. However, electrophysiological examination, which is conducted preoperatively in all hand surgery patients, revealed slow nerve conduction.
Total intravenous anesthesia was administered as a single bolus of 5 mg midazolam and 20 mg ketamine, followed by 2 mg/kg/hour propofol. Subsequently, the brachial plexus was scanned by an ultrasonographic scanner with a 15–6 MHz linear array transducer to assist in performing a nerve block for intraoperative and postoperative analgesia. Unexpectedly, ultrasonographic images revealed enlarged brachial plexus nerves (Figure 1). Three nerve trunks were evident in the supraclavicular region, with cross sectional dimensions of approximately 6.3 x 4.4 mm (upper trunk), 6.3 x 5.3 mm (middle trunk), and 7.8 x 6.3 mm (lower trunk), with their respective cross-sectional areas (CSAs) being 21.8 mm2, 26.2 mm2, and 38.6 mm2.
Following consultation with the surgeon, it was decided to operate as scheduled. A PNB at the supraclavicular level was performed, and local anesthetics were injected as initially planned. After 10 mL 0.75% ropivacaine and 10 mL 1% lidocaine were injected around the nerves under ultrasonographic guidance, a good sensory and motor block ensued. Spontaneous respiration was maintained with a face mask (5 L/min oxygen). During the operation, anesthetic maintenance was straightforward, and the surgery proceeded uneventfully. The total surgery time was 57 minutes, and the total anesthesia time was 78 minutes. The postoperative course was uneventful, and the analgesic effects remained for 10 hours. The patient was discharged the following day.
Postoperatively, we consulted a neurologist, who further investigated the potential presence of an underlying neurological disorder. The neurologist suspected CMTD based on the characteristic nerve enlargement and foot deformity, which is pathognomonic of CMTD (Figure 2). The patient’s foot exhibited high arches and claw toes, but were unnoticed by the patient.
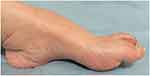 |
Figure 2 The patient’s feet as a pathognomonic characteristic of CMTD, showing high arches and claw toes. The patient had not noticed these abnormalities. |
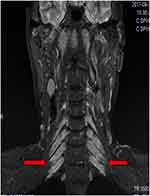
Cervical magnetic resonance imaging (MRI) was performed without contrast medium (1.5 T, repetition time 3500 ms, echo time 127 ms). Gigantic bilateral brachial nerves were observed on a coronal T2-weighted image (Figure 3). Genetic testing was performed, identifying duplication of the peripheral myelin protein 22 (PMP22) gene. This mutation is consistent with CMTD type 1A.
Discussion
Ultrasound-guided PNB has become a common anesthetic procedure, and consequently, anesthesiologists are familiar with the appearances of nerves on ultrasonographic images. Occasionally, the nervous system may present with unusual anatomy. The objective of anesthesiologists is to perform PNB while being aware of possible uncommon features. In the present case, the nerves were clearly enlarged, but the anesthesiologists were unfamiliar with this finding. Consultation with a neurologist regarding the abnormality led to the diagnosis of CMTD.
CMTD is one of the most commonly encountered inherited sensory-motor neuropathic disorders, with an estimated incidence of approximately 1 in 2500.4–6 Traditionally, these neuropathies have been diagnosed by MRI, nerve conduction tests, and pathological tests, following the European Federation of Neurological Societies/Peripheral Nerve Society guidelines.7 Ultrasonography has recently become a prevalent complementary examination tool for neurologists, performed in conjunction with MRI. It is non-invasive and easy-to-perform at the bedside, but the results can be dependent to a degree on the examining practitioner, and it can be difficult to obtain clear imaging of deep structures.1 As a result, it is not recommended in the relevant guidelines. In the current case, CMTD was suspected based on clinical symptoms, MRI, and nerve conduction testing. Genetic testing led to a definitive diagnosis of CMTD.
Demyelination is a characteristic pathological feature of CMTD. A repeated cycle of demyelination and remyelination leads to the enlargement of some nerves due to the formation of myelin layers called “onion bulbs.”8 The enlarged nerves observed in our patient were consistent with this “onion bulb” type.
In previous studies, the sizes of peripheral nerves have been determined using ultrasonography.9–11 Sugimoto et al12 evaluated and compared enlargement of peripheral nerves in cases of CMTD and chronic inflammatory demyelinating polyneuropathy (CIDP), reporting that ultrasonography was useful for nerve enlargement diagnosis in both diseases. Moreover, the median and ulnar nerves in CMTD were significantly larger than those in CIDP. Won et al9 measured the CSAs of the brachial plexus nerves by ultrasonography and reported that the mean bilateral areas were 16.70 ± 2.88, 14.01 ± 2.70, and 13.75 ± 2.57 mm2 in the upper, middle, and lower trunk segments, respectively. In our patient, the corresponding CSAs were substantially larger, at 21.8, 26.2, and 38.6 mm2, respectively. The difference suggests that a nerve swelling of more than 30% is sufficient for diagnosing the neuropathy by ultrasonography. Yoon et al have evaluated nerve swelling for ulnar neuropathy diagnosis and suggested that a ratio of >1.5 might be informative.13
We have some concerns regarding the general practice of anesthesia in CMTD patients.2 The biggest foreseeable complication relates to the use of muscle relaxants. Depolarizing muscle relaxants are of major concern because muscle wasting in CMTD is considered to increase extracellular potassium, leading to hyperkalemia. A combination of the relaxants and hyperkalemia could trigger malignant hyperthermia.14 The demyelinating nerve in CMTD may also be sensitive to non-depolarizing muscle relaxants, resulting in prolonged neuromuscular blocking.14 Inhalational agents may also present some risks to CMTD patients. However, Isbister et al15 suggested that nitrous oxide would be safe. Intravenous anesthetics, including propofol, fentanyl, alfentanil, and thiopental, are also considered relatively safe since they do not appear to elicit high sensitivity.14 In the current case, only intravenous anesthetics were used.
Some studies have concluded that PNB is efficient and has no adverse outcomes, such as nerve injury.16,17 Other reports have warned about possible nerve injury after neuraxial anesthesia. Hebl et al evaluated the neurologic complications after neuraxial anesthesia in patients with peripheral sensorimotor neuropathy.18 They reported that 0.4% of the 567 patients had postoperative neurologic deficits.18 Bui et al reported a case of a CMTD patient who underwent shoulder surgery and suggested that PNB should only be considered in cases in which the potential benefits outweigh the potential risks.19 Regional anesthesia in CMTD patients remains controversial, and anesthesiologists have not been able to dispel concerns about PNB. The current consensus is that the toxic effects of direct local anesthetic injections around the demyelinated nerves are a cause for concern,3 but the inference that this might lead to future nerve degeneration is invalid.
In retrospect, when enlarged nerves were observed after ultrasonography, we should have conducted further examinations before the operation to identify the patient’s underlying disease. If we had done so, induction of anesthesia could have been planned more cautiously and could be tailored to the underlying condition. Notably, few studies have performed accurate measurements of enlarged nerves in patients with CMTD or other demyelinating diseases, and therefore, “enlargement” could not be assessed clearly. Another limitation is that anesthesiologists do not observe the patient over a prolonged period. As a result, they cannot know whether the patient will go on to develop any adverse symptoms because of the general anesthetic procedure or resulting from PNB over time. In this patient, an anesthesiologist made a follow-up phone call after one year to check that no neurological problems had occurred.
Nerve enlargement is a characteristic feature of CMTD, but anesthesiologists do not frequently encounter it. Furthermore, ultrasonographic diagnosis of neurological disorders is a new, developing, and increasingly utilized technique. Some examples of its emerging use are in the diagnosis of amyotrophic lateral sclerosis to visualize the atrophic changes in cervical nerves, in polymyositis or dermatomyositis where muscle atrophy and fascial thickening can be detected, and thirdly, in compressive neuropathy, particularly carpal tunnel syndrome and ulnar neuropathy at the elbow, where enlargement of the nerve proximal to the site of compression, decreased echogenicity, and increased vascularity can be identified.20 With more detailed knowledge about neurological disorders, their symptoms, and ultrasonographic presentation, anesthesiologists will be better prepared to identify underlying neurological conditions before undertaking surgical procedures.
There have been some arguments regarding the fragmentation of medical care, where a patient’s missing information across multiple departments can lead to medical errors, adverse events, and increased medical costs.21 Some reports emphasized the importance of the integration of clinical information technology.21,22 This case is indicative of the problems associated with this type of fragmentation of medical care. Although multidisciplinary approaches that integrate collaboration between different specialist areas can be difficult, it is nonetheless still the task of anesthesiologists to judge the general physical status of a patient. As many textbooks on PNB are available, we suggest that a chapter on “neurological disorders on ultrasound images” should be included in them. This should be considered a new category of knowledge in the PNB field for anesthesiologists.
Conclusions
We have described a case in which enlarged brachial plexus nerves were detected by ultrasonography and subsequently diagnosed as CMTD by genetic testing, which revealed a duplication of the PMP22 gene. Enlargement of the brachial plexus nerve was objectively determined by measuring the CSAs of the nerves. Nerve enlargement is a characteristic feature of demyelinating diseases, but there is insufficient knowledge among anesthesiologists, especially with respect to diagnosis by ultrasonography. This case report highlights the importance that anesthesiologists are equipped with the capacity to recognize the presentation of abnormal nerves using ultrasound, especially for the potential identification of underlying undiagnosed neurologic disorders. This is particularly important since undiagnosed neurologic disorders bear relevance for the best anesthetic procedure.
Abbreviations
CIDP, chronic inflammatory demyelinating polyneuropathy; CMTD, Charcot-Marie-Tooth disease; CSA, cross-sectional area; MRI, magnetic resonance imaging; PMP, peripheral myelin protein; PNB, peripheral nerve block.
Ethics Approval and Informed Consent
Approval from the institutional review board is not required for a single patient case report.
Consent for Publication
Written informed consent was obtained from the patient for publication of all data including images.
Acknowledgments
Editorial support, in the form of medical writing based on authors’ detailed directions, collating author comments, copyediting, fact-checking, and referencing, was provided by Cactus Communications.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This study received no funding.
Disclosure
The authors declare that they have no competing interests.
References
1. Noto Y. Ultrasound diagnosis of Charcot-Marie-Tooth disease. Brain Nerv. 2014;66(3):237–246.
2. Alzaben KR, Samarah OQ, Obeidat SS, Halhouli O, Al Kharabsheh M. Anesthesia for Charcot-Marie-Tooth disease: case report. Middle East J Anaesthesiol. 2016;23(5):587–590.
3. Yang S, Abrahams MS, Hurn PD, Grafe MR, Kirsch JR. Local anesthetic schwann cell toxicity is time and concentration dependent. Reg Anesth Pain Med. 2011;36(5):444–451. doi:10.1097/AAP.0b013e318228c835
4. Timmerman V, Strickland AV, Züchner S. Genetics of Charcot-Marie-Tooth (CMT) disease within the frame of the human genome project success. Genes (Basel). 2014;5(1):13–32. doi:10.3390/genes5010013
5. Pareyson D, Marchesi C. Diagnose, natural history, and management of Charcot-Marie-Tooth disease. Lancet Neurol. 2009;8(7):654–667. doi:10.1016/S1474-4422(09)70110-3
6. Pareyson D, Scaioli V, Laura M. Clinical and electrophysiological aspects of Charcot-Marie-Tooth disease. Neuromolecular Med. 2006;8(1–2):3–22. doi:10.1385/NMM:8:1-2:3
7. Joint Task Force of the EFNS and the PNS. European federation of neurological societies/peripheral nerve society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European federation of neurological societies and the peripheral nerve society—first revision. J Peripher Nerv Syst. 2010;15(1):1–9.
8. Thomas PK. Overview of Charcot-Marie-Tooth disease type 1A. Ann N Y Acad Sci. 1999;883(1):1–5. doi:10.1111/j.1749-6632.1999.tb08560.x
9. Won SJ, Kim BJ, Park KS, Kim SH, Yoon JS. Measurement of cross-sectional area of cervical roots and brachial plexus trunks. Muscle Nerve. 2012;46(5):711–716. doi:10.1002/mus.23503
10. Kerasnoudis A, Pitarokoili K, Behrendt V, Gold R, Yoon MS. Cross sectional area reference values for sonography of peripheral nerves and brachial plexus. Clin Neurophysiol. 2013;124(9):1881–1888. doi:10.1016/j.clinph.2013.03.007
11. Lapegue F, Faruch-Bilfeld M, Demondion X, et al. Ultrasonography of the brachial plexus, normal appearance and practical applications. Diagn Interv Imaging. 2014;95(3):259–275. doi:10.1016/j.diii.2014.01.020
12. Sugimoto T, Ochi K, Hosomi N, et al. Ultrasonographic nerve enlargement of the median and ulnar nerves and the cervical nerve roots in patients with demyelinating Charcot-Marie-Tooth disease: distinction from patients with chronic inflammatory demyelinating polyneuropathy. J Neurol. 2013;260(10):2580–2587. doi:10.1007/s00415-013-7021-0
13. Yoon JS, Walker FO, Cartwright MS. Ultrasonographic swelling ratio in the diagnosis of ulnar neuropathy at the elbow. Muscle Nerve. 2008;38(4):1231–1235. doi:10.1002/mus.21094
14. Ohshita N, Oka S, Tsuji K, et al. Anesthetic management of a patient with Charcot-Marie-Tooth disease. Anesth Prog. 2016;63(2):80–83. doi:10.2344/15-00010R1.1
15. Isbister GK, Burns J, Prior F, Ouvrier RA. Safety of nitrous oxide administration in patients with Charcot-Marie-Tooth disease. J Neurol Sci. 2008;268(1–2):160–162. doi:10.1016/j.jns.2007.12.004
16. Barbary JB, Remérand F, Brilhault J, Laffon M, Fusciardi J. Ultrasound-guided nerve blocks in the Charcot-Marie-Tooth disease and Friedreich’s ataxia. Br J Anaesth. 2012;108(6):1042–1043. doi:10.1093/bja/aes160
17. Dhir S, Balasubramanian S, Ross D. Ultrasound-guided peripheral regional blockade in patients with Charcot-Marie-Tooth disease: a review of three cases. Can J Anaesth. 2008;55(8):515–520. doi:10.1007/BF03016671
18. Hebl JR, Kopp SL, Schroeder DR, Horlocker TT. Neurologic complications after neuraxial anesthesia or analgesia in patients with preexisting peripheral sensorimotor neuropathy or diabetic polyneuropathy. Anesth Analg. 2006;103(5):1294–1299. doi:10.1213/01.ane.0000243384.75713.df
19. Bui AH, Marco AP. Peripheral nerve blockade in a patient with Charcot-Marie-Tooth disease. Can J Anaesth. 2008;55(10):718–719. doi:10.1007/BF03017751
20. Wiesler ER, Chloros GD, Cartwright MS, et al. The use of diagnostic ultrasound in carpal tunnel syndrome. J Hand Surg. 2006;31(5):726–732. doi:10.1016/j.jhsa.2006.01.020
21. Bourgeois FC, Olsonv KL, Mandl KD. Patients treated at multiple acute health care facilities: quantifying information fragmentation. Arch Intern Med. 2010;170(22):1989–1995. doi:10.1001/archinternmed.2010.439
22. Enthoven AC. Integrated delivery systems: the cure for fragmentation. Am J Manag Care. 2009;15(10 Suppl):S284–90.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.