Back to Journals » Drug Design, Development and Therapy » Volume 12
Enhanced gastric therapeutic effects of Brucea javanica oil and its gastroretentive drug delivery system compared to commercial products in pharmacokinetics study
Authors Zhang Y , Zhang L, Zhang Q, Zhang X, Zhang T, Wang B
Received 26 October 2017
Accepted for publication 30 January 2018
Published 13 March 2018 Volume 2018:12 Pages 535—544
DOI https://doi.org/10.2147/DDDT.S155244
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sukesh Voruganti
Yue Zhang,1,2,* Liying Zhang,3,* Qi Zhang,1,2 Xitong Zhang,4 Tong Zhang,1,2 Bing Wang1,2
1Experiment Center for Teaching and Learning, Shanghai University of Traditional Chinese Medicine, Pudong New District, Shanghai, People’s Republic of China; 2School of Pharmacy, Shanghai University of Traditional Chinese Medicine, Pudong New District, Shanghai, People’s Republic of China; 3Foreign Languages Teaching Center, Shanghai University of Traditional Chinese Medicine, Pudong New District, Shanghai, People’s Republic of China; 4Department of Pharmacy, Shanghai Xiangshan Hospital of Traditional Chinese Medicine, Huangpu District, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Background: Brucea javanica oil (BJO), a traditional Chinese herbal medicine, has a variety of pharmacological activities and several BJO-related patent drugs have been widely used in China.
Purpose: The objective of this study was to evaluate the gastric therapeutic effects of self-made BJO and its pharmaceutical potential to formulate novel BJO gastroretentive floating bead by comparing with commercial products.
Methods: BJO was extracted from the seeds of B. javanica, and its therapeutic effects were evaluated by comparing with commercial products in the treatment of human gastric cancer and gastric ulcer. Furthermore, the developed gastroretentive drug delivery system was evaluated by in vivo tests. A high-performance liquid chromatography–tandem mass spectrometry (HPLC-MS/MS) method for detecting the concentration of glycerol trioleate in the pharmacokinetic study was applied.
Results: The antitumor activity of BJO was stronger than that of the marketed preparation; the 50% inhibitory concentration (IC50) values of BJO extracts on HGC27, SGC7901 and BGC823 gastric carcinoma were 0.3091, 1.736 and 2.743 μg/mL, respectively, whereas the values of marked BJO preparation were 15.26, 32.60 and 7.456 μg/mL, respectively. Histopathological studies demonstrated the ability of BJO to locally prevent and treat absolute ethanol-induced gastric ulcer. Developed BJO gastroretentive floating bead showed a satisfactory in vivo study. The highest glycerol trioleate concentration in the stomach after taking BJO gastroretentive floating bead was nearly two times higher when compared to the marketed BJO soft capsule.
Conclusion: Self-made BJO has a strong therapeutic effect on the stomach, and gastroretentive drug delivery system can be a promising approach to prolong and enhance its therapy ability when treating gastric diseases.
Keywords: BJO, gastric tumor, gastric cancer, gastroretentive floating bead
Introduction
Brucea javanica (B. javanica [L.] Merr.) is a shrub mostly originated in India, Southeast Asia and Northern Australia.1 It is widely used in Traditional Chinese Medicine. Brucea javanica oil (BJO), extracted from B. javanica seeds, is a mixture of glycerol trioleate (85%) and other saturated and unsaturated fatty acids (15%), such as oleic, linoleic and stearic acids. It is believed that BJO can reduce tumor cell growth2 and has activities in anti-inflammation and anti-malaria.3 In China, two BJO-contained marketed products, javanica oil emulsion injection and BJO soft capsule, are widely used in association with chemotherapy for cancer patients. Almost all the studies demonstrated that the side effects caused by chemotherapy were mitigated and patients’ quality of life were improved significantly by jointly using javanica oil emulsion injection and BJO soft capsule. One of the commonly adjunctive therapeutic areas of these two BJO marketed products is gastric cancer.
Oleic acid is BJO’s main activity component, so it is commonly used as the essential indicator for the determination of BJO.4 However, oleic acid exists not only as the free one as mentioned earlier but also in the glycerol trioleate as well. Actually, oleic acid and linoleic acid amount to 63.3% and 21.2%, respectively, in BJO. After orally taking glycerol trioleate, gastric lipase will initiate the digestion of glycerol trioleate. Later in the small intestine, glycerol trioleate is broken down by pancreatic lipase, which acts primarily at the sn-1 and sn-3 positions of glycerol trioleate to produce 2-monoglyceride and free fatty acid.5 Hence, most commonly used methods for the analysis of oleic acid in pharmacokinetic and bioavailability studies of BJO preparations involve hydrolysis6 by specific lipase or methyl esterification by an acid or a base catalyst,7,8 through which the total oleic acid could be calculated including those on the sn-2 position. However, such a preprocess is highly impacted by the specificity of lipase and completion of the reaction; any inappropriate procedure will lead to the loss of starting glycerol trioleate and will fail to obtain the whole oleic acid. After the preprocess, gas chromatography (GC) or gas chromatography–mass spectrometry (GC–MS) plays an indispensable role in the final analysis of oleic acid as oleic acid has mainly ultraviolet end absorption, which makes it hard to be detected by the ordinary high-performance liquid chromatography (HPLC) analytical method.7 Nevertheless, GC has its drawbacks when detecting fatty acids, such as instability, low accuracy, tailing peaks and interferences.9 Therefore, compared to taking the oleic acid as the detective component, glycerol trioleate is a preferred indicated marker for the pharmacokinetic study of BJO preparation due to high content of glycerol trioleate in BJO and slow digestion rate and extent of glycerol trioleate. Glycerol trioleate in BJO is constituted by the long-chain monounsaturated fatty acid,10 oleic acid, which takes a slower uptake route.11
The objective of this study was to investigate laboratory self-prepared BJO’s therapeutic effect against gastric cancer and gastric ulcer by comparing with commercial products. In addition, to better achieve BJO’s therapeutic effects in stomach, gastroretentive drug delivery system (GRDDS) was investigated. As conventional systems do not possess the capacity to face gastric emptying, the incomplete release of drugs and the concomitant reduction of dose effectiveness are the consequences as conventional systems cannot be retained in the stomach.12 Hence, we applied a novel oral gastroretentive preparation as a drug-control pattern with in vivo pharmacokinetic study performed. A high-performance liquid chromatography–tandem mass spectrometry (HPLC-MS/MS) method by detecting the concentration of glycerol trioleate in pharmacokinetic study was also established so as to increase the accuracy, convenience and repeatability of detection and shed light on the possible alternative in BJO quantification.
Materials and methods
Materials
The seeds of B. javanica were purchased from Guangdong Province, China, and were identified by Dr Hongmei Zhang (School of Pharmacy, Shanghai University of Traditional Chinese Medicine). The reference standards of glycerol trioleate and tolbutamide (internal standard [IS]) were obtained from Sigma Chemicals (Perth, Australia). Marketed javanica oil emulsion injection (Lot No 13022012, BJO content at 10% [w/w]) was manufactured by Shenyang Yaoda Leiyunshang Pharmaceutical Co., Ltd (Shenyang, China). BJO soft capsule (Lot No 1402231, BJO content at 90% [w/w]) was produced by Jiangsu Vanguard Pharmaceutical Co., Ltd (Haimen, China). Reference drug ranitidine (RAN) hydrochloride capsules (Lot No 131211) was manufactured by Yunnan PanLong YunHai Pharmaceutical Co., Ltd. (Kunming, China). Pharmaceutical excipients for GRDDS including sodium alginate, carrageenan (FMC Corporation, Philadelphia, PA, USA), Polysorbate 80 (Shanghai Chemical Reagent Co., Shanghai, China), calcium carbonate and calcium chloride (Sinopharm Chemical Reagent Co., Shanghai, China) were of analytical grade. Other chemicals such as absolute ethanol, petroleum ether and acetonitrile were of HPLC or analytical grade.
Preparation of BJO
As we mentioned in the previous study,13 the seeds of B. javanica were crushed, pulverized and screened by 60# mesh and put into the 250 mL conical flask with cover; 100% ethanol as the solvent was added at the solid to liquid ratio of 24:1 (mL/g) and extracted in an ultrasound cleaner (Ningbo Xinzhi Bioscience Co., Ningbo, China) at 70 kHz for 40 min. The extracts were collected by vacuum filtration and concentrated using a rotary evaporator under vacuum. Then a liquid/liquid extraction was performed on the concentrated extracts with petroleum ether; after that, the supernatant was taken out. BJO was available after the evaporation of the solvent.
Gastric cancer cell lines and cell culture
The human gastric cancer cell lines BGC823, HGC27 and SGC7901 were obtained from Shanghai Baili Biological Technology Co., Ltd. (Shanghai, China). Cells were incubated in complete DMEM (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 5% heat-inactivated fetal bovine serum (Thermo Fisher Scientific) at 37°C in an atmosphere containing 5% CO2.
Cell viability analysis of BJO
Cells (3.5 × 104/mL) were cultured in 96-well chamber slides for 24 h before use. The culture medium was replaced with the fresh medium containing BJO for 72 h. Cell number was determined using an MTT assay kit. In all, 20 μL MTT (Sigma Chemicals) at a concentration of 5 mg/mL was added into each well and cultured for another 3 h, and the supernatant of each well was discarded and replaced with 100 μL DMSO (Sigma Chemicals). Finally, all absorbance values were read on a universal microplate spectrophotometer (Donghua Electronics, Nanjing, China) at 492 nm. Marketed javanica oil emulsion injection was chosen as the negative control. The 50% inhibitory concentration (IC50) was calculated by GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA, USA). All experiments were made in triplicate.
Preventative and therapeutic effects of BJO against ethanol-induced gastric ulcer in rats
Animals
Sprague-Dawley (SD) rats of either gender weighing 200 ± 20 g were used for the present study. The animals were obtained from Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). They were maintained at 23°C ± 2°C with a relative humidity of 65% ± 5% in a 12-hour light/dark cycle. The animals were allowed water ad libitum throughout the study. Before experiments, all rats were allowed to acclimate for 1 week. All animal experiments were performed according to the guiding principles of the Guide for the Care and Use of Laboratory Animals of Shanghai University of Traditional Chinese Medicine (Shanghai, China). The protocol approval number is SYXK (Shanghai) 2014-0008.
Experimental procedure
Preventative effect of BJO on gastric ulcer
The animals (six per group) were pretreated with BJO (0.2, 0.4 and 0.8 g/kg/d, i.g.) and reference drug RAN (30 mg/kg/d, i.g.) once daily for 3 days before ethanol challenge. A control group was only challenged by ethanol with no drug administered. Gastric ulcer was induced in mice by administering absolute ethanol (1 mL/rat, i.g.) 1 h after the last administration of drugs. After 2 h, the animals were euthanized.
Therapeutic effect of BJO on gastric ulcer
Gastric ulcer induction was undertaken on the rats of all the groups (six per group) by using absolute ethanol (1 mL/rat, i.g.). After 2 h of ethanol challenge, the animals were posttreated with BJO (0.2, 0.4 and 0.8 g/kg/d, i.g.) and reference drug RAN (30 mg/kg/d, i.g.) once daily for 3 days. No treatment was given to the control group. Animals were euthanized 2 h after the last administration of drugs.
Histopathological evaluation
Samples of gastric tissue were fixed in 4% paraformaldehyde, embedded in paraffin, sectioned (thickness, 5 μm), mounted on glass slides and then stained with H&E and examined under a light microscope (BX51; Olympus, Tokyo, Japan).
Preparation of BJO gastroretentive floating bead
BJO gastroretentive floating bead was prepared by ionotropic gelation technology in a previous study.14 Briefly, mixture of sodium alginate (1.7%) and carrageenan solid (10:6, w/w) was prepared in warm deionized water. BJO was added to polysorbate 80 to obtain emulsion. The prepared alginate–carrageenan solution was mixed with oil emulsion as well as calcium carbonate (1.4%) and used for preparation of beads. This solution was then dropped through the fine needle of a disposable syringe into a 100 mL CaCl2 solution (pH = 0.8). The formed beads were left over in the CaCl2 solution for 30 min; afterward, they were washed with distilled water and dried at 40°C for 2 h.
Modeling of drug-release profiles
Drug in vitro release was already performed previously by using the Ch.P apparatus (II) paddle dissolution method.14 In the present study, drug-release data were analyzed according to zero-order, first-order and Higuchi kinetic equations. The model with the highest coefficient of determination (R2) was considered to be the best fitting one.
HPLC-MS/MS system and operating conditions
Chromatographic analysis was performed on Shimadzu LC20AD (Shimadzu Corporation, Kyoto, Japan), consisting of a binary pump solvent management system, an online degasser and an auto-sampler. Gimini C18 (4.6 × 50 mm, 5 μm; Phenomenex, Cheshire, UK) was used, and the column temperature was maintained at the room temperature. The mobile phase was composed of dichloromethane and acetonitrile (35:65, v/v), and the flow rate was set at 1.0 mL/min. The injection volume was 5 μL. Mass spectrometer (API 4000 triple quadrupole; AB Sciex, Framingham, MA, USA) equipped with an atmospheric pressure chemical ionization source was used. The analytic detection was operated in the positive ion mode using a multiple reaction monitoring approach at m/z transitions of 603.5 → 271.0 for glycerol trioleate and 271.0 → 74.2 for IS. Data were acquired and processed using Analyst 1.6.1 software (AB Sciex).
Preparation of standard solution and quality control (QC) samples
A series of standard samples of glycerol trioleate were prepared by diluting the stock solution (8.0 mg/mL) in dichloromethane-acetonitrile (35:65, v/v) to obtain the following concentrations: 2, 1, 0.5, 0.4, 0.2, 0.1, 0.04 and 0.02 mg/mL. The high, medium and low concentration levels of QC samples were prepared in the same manner as the standards at 1.6, 0.4 and 0.06 mg/mL, respectively. In all, 6 μg/mL working solution of IS was prepared by adding 600 μL, 1.0 mg/mL tolbutamide stock solution of IS into appropriately labeled 100-mL volumetric flask and volume adjustment with acetonitrile.
Sample preparation
Stomach tissue samples were thawed and then homogenized in 100 mM phosphate-buffered saline (pH = 7.4; 1:3, w/v) before analysis. All the plasma samples were thawed at room temperature before analysis as well. In all, 50 μL tissue homogenate and plasma samples were added to 20 μL IS working solution. After vortexing for 30 s, all the tissue and plasma samples were extracted using trichloromethane (600 μL) to precipitate protein. Following centrifugation at 3,000 rpm for 10 min at 4°C, 220 μL of subnatant was transferred to the 96-well plate and evaporated to dryness under nitrogen flow. The residue was reconstituted with the mobile phase (a mixture of dichloromethane and acetonitrile with ration of 35:65, v/v), vortex mixed for 10 min and 5 μL of the extracted plasma or tissue sample was injected into the LC-MS/MS system for analysis.
Pharmacokinetics of BJO gastroretentive floating bead and BJO soft capsule
Male SD rats (obtained from Shanghai SIPPR-Bk Laboratory Animal Co., Shanghai, China; certificate number for approval: SCXK [Shanghai] 2013-0016) with body weights of 290 ± 20 g were used for the study. The SD rats were randomly divided into two groups (n = 3 per group), BJO gastroretentive floating bead (at 0.14 mg/g of BJO) or BJO soft capsule (at 0.14 mg/kg of BJO) was dosed by oral administration. Approximately 0.1 mL blood samples were collected into heparinized glass tubes via eye sockets at various time points (0.25, 0.5, 1, 2, 4 and 6 h) after administration and immediately centrifuged for 6 min at 8,000 rpm/min to obtain plasma. The harvested plasma samples were stored at -20°C for subsequent analysis. Pharmacokinetic parameters were calculated using Drug and Statistics software (version 3.0; Mathematical Pharmacology Professional Committee of China, Shanghai, China) in the noncompartmental model.
Tissue distributions of BJO gastroretentive floating bead and BJO soft capsule
A total of six rats were randomly divided into two groups (n = 3 per group). After oral administration of a single dose of BJO gastroretentive floating bead (at 0.14 mg/g of BJO) or BJO soft capsule (at 0.14 mg/g of BJO), three rats at each time point (2, 4 and 6 h) were euthanized by CO2 and gastric tissues were collected. Tissue samples were weighed rapidly, rinsed with physiological saline to remove the blood or content, blotted on filter paper and then stored at -20°C. Each weighed tissue sample was thawed and then homogenized in ice-cold physiological saline by the methods described before.
Results
Cytotoxic effects of BJO extract
The effects of BJO extract and marketed javanica oil emulsion injection on the growth of human gastric carcinoma (BGC823, HGC27 and SGC7901) cells were examined using an MTT-based assay. Among tested extracts, as shown in Figure 1, the BJO extract appeared to exert the most potent effects on HGC27. The IC50 values were 0.3091 ± 0.0498 μg/mL for HGC27, followed by 1.736 ± 0.221 μg/mL for SGC7901 and 2.743 ± 0.116 μg/mL for BGC823. The IC50 value of marketed javanica oil emulsion injection on abovementioned three gastric cancer cells was calculated as follows: HGC27, 15.26 ± 0.134 μg/mL; SGC7901, 32.60 ± 3.978 μg/mL and BGC823, 7.456 ± 1.735 μg/mL. The results showed that javanica oil emulsion injection obtained from the market was far less potent than the BJO extract we produced, which means that the extract method we used has future application value.
Histopathological results of BJO’s preventative and therapeutic effects against the gastric ulcer
Compared to the normal group that was not challenged by absolute ethanol (Figure 2A), administration of ethanol-induced striking histopathological changes such as blurry gastric muscle layers, disruption of gastric glands, exfoliation of the superficial gastric epithelium and infiltration of the inflammatory cell with lymphocytes the most. Moreover, there was an apparent extensive elongated band of necrosis in gastric mucosa as well as lots of lymphocytes in the lamina propria and submucosa (Figure 2B).
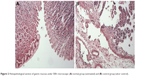  | Figure 2 Histopathological section of gastric mucosa under 500× microscope: (A) normal group (untreated) and (B) control group (ulcer control). |
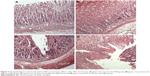
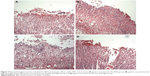
The preventative effects of the oral administration of BJO and RAN on gastric ulcer induced by ethanol are shown in Figure 3A–D. Rats pretreated with BJO exhibited less gastric lesions than those found in the control group in a dose-related manner. The high-dose BJO group presented the most active gastric damage prevention ability with reduced gastric mucosal damage, more regular mucosal gland arrangement and decreased infiltration of inflammatory cells. The high-dose BJO (0.8 g/kg/d) also showed a similar preventative activity to the reference RAN on gastric lesion formation.
The therapeutic effects of BJO and RAN on gastric ulcer induced by ethanol are shown in Figure 4A–D. Rats posttreated with BJO exhibited less gastric lesions than those found in the control group in a dose-related manner. In the group treated with the higher dose of BJO (0.8 g/kg), there was an evident recovery in mucosal epithelium, the glandular structure was well organized and the gastric epithelium was intact and showed a similar therapeutic activity to the reference RAN on gastric lesion formation (Figure 4A and B). Rats administered with BJO at 0.4 g/kg presented a slight sign of lesion; however, serious destruction in the glands of the gastric mucous and damaged mucosal epithelium were not observed (Figure 4C). In the group treated with 0.2 g/kg BJO, epithelial cell loss in part of the gastric mucous could be seen, but epithelium of the submucosa was intact (Figure 4D). The presence of inflammatory cells, lymphocytes infiltrating into the gastric mucosal tissues and defective gastric epithelium, which was found in the control group, did not exist in the high-dose BJO group, indicating that BJO had a therapeutic effect against ethanol-induced gastric ulcer, when administered at oral doses, particularly at the dose of 0.8 g/kg.
Modeling of drug-release profiles of BJO gastroretentive floating bead
The percentage of BJO released was found to be a little bit quick for the initial 1 h but maintained a slow release afterward and reached >90% release after 6 h.14 Similar drug-release behaviors among the three batches could be found, which indicated that the formulation and technology used to develop BJO gastroretentive floating bead had a good reproducibility.
Sodium alginate and carrageenan are hydrophilic, colloidal substances. They are expected to undergo hydration and form a protective gel layer upon reaching the simulated gastric fluid (SGF). With more gel structure formed, the diffusion hindrance in SGF was increased. Thus, the release of BJO in SGF was slower. Besides, with the penetration of water into the beads, the presence of carrageenan formed a large number of pores along the matrices of beads, leading to the formation of channels that brought the complete release of BJO at the end. Thus, the drug-release mechanism was classified as a combination of gel diffusion and erosion controlling.
Table 1 lists the values for the correlation coefficient (R2), which was used to determine the best-fit kinetic model. Criteria for selecting the most appropriate model was based on best fit indicated by the value of coefficient of determination (R2) nearer to 1.15 It was observed that regression coefficient was highest for the Higuchi model, indicating an anomalous (non-Fickian) diffusion with both diffusion- and erosion-controlled drug release. The results were in accordance with our previous finding14 of bead morphologies using scanning electron microscope.
  | Table 1 Kinetic parameters of BJO gastroretentive floating bead |
Pharmacokinetic study of BJO gastroretentive floating bead
Following oral administration of BJO gastroretentive floating bead and marketed BJO soft capsule at the same concentration to healthy rats, plasma concentration of glycerol trioleate of both groups was measured. However, no glycerol trioleate was detected in the blood at 0.25, 0.5, 1, 2, 4 and 6 h after administration. This could attribute to the slow uptake rate in the stomach of BJO gastroretentive floating bead and nearly completed digestion and absorption of glycerol trioleate in the small intestine16 of BJO soft capsule.
Tissue distribution of BJO gastroretentive floating bead
After orally administrating BJO gastroretentive floating bead and marketed BJO soft capsule at the same concentration, distribution of glycerol trioleate in animals’ stomachs was observed. Glycerol trioleate in tissue could still be spotted after the original affiliated glycerol trioleate in the stomach was offset. As shown in Figure 5, the concentration of glycerol trioleate in the stomach was 41.1 ± 16.6 μg/mL, 177.8 ± 153.6 μg/mL and 189.5 ± 125.4 μg/mL at 2, 4 and 6 h, respectively, after administration of BJO gastroretentive floating bead, whereas the concentration of glycerol trioleate in the stomach after taking the marketed BJO soft capsule displayed a rapid decline, with 104.3 ± 41.3 μg/mL, 7.5 ± 12.9 μg/mL and 5.3 ± 4.6 μg/mL at 2, 4 and 6 h, respectively. BJO gastroretentive floating bead exhibited a prolonged release capability and nearly two times higher glycerol trioleate concentration in the stomach when compared to the marketed BJO soft capsule. The result indicated the advantages of BJO gastroretentive floating bead with enhanced and prolonged local effects.
  | Figure 5 Tissue concentration–time profiles of glycerol trioleate. |
Discussion
Javanica oil emulsion injection is a marketed product that uses petroleum ether extracts of B. javanica seeds as raw materials and purified soybean lecithin as an emulsifier.17 It is reported to be used as adjunctive therapy in the treatment of advanced lung adenocarcinoma,17 nonsmall-cell lung cancer18 and gastric cancer.19 There are also other methods of extracting BJO, such as extracting with ether and refined with 10% ethyl alcohol, or purifying the crude oil, after crude extraction, by adding NaOH alcoholic solution and activated carbon afterwards.4,20 In a previous study,13 we developed a new BJO extraction method by using 100% ethanol extracted in an ultrasound cleaner and refining it with petroleum ether. In this study, we investigated the potency and efficacy of the self-prepared BJO against gastric cancer cell lines. Our findings clearly demonstrate that the BJO that we produced presented a stronger anti-gastric cancer activity compared to the commercial product javanica oil emulsion injection.
We also investigated the self-made BJO’s effects on gastric ulcer. The animal models were induced by ethanol administration since ethanol could produce necrotic damage penetrating deeply into the mucosa,21 disappearance of the mucus cells,22 hemorrhagic erosion23 and inflammatory cell infiltration.21 As expected, ethanol caused a marked gastric ulceration in the present study. Yet pretreated and posttreated BJO exhibited dose-dependent preventative and therapeutic effects against gastric mucosal damage. Severe gastric submucosal lesions, irregular arrangement of glands, epithelial cell loss and lymphocyte infiltration in mice with ulcer were alleviated. The high dose of BJO exhibited a similar preventative effect with RAN, a histamine H2-receptor antagonist, that inhibits stomach acid production,24 when acting against stomach injury.
Although BJO has evidently strong pharmacological action, its clinical use is restricted on account of its nonspecific distribution, low therapeutic index25 and poor systemic bioavailability.26 Besides, the current BJO preparations available in the market are frequently administrated with large dosage (oral emulsion: 2 mL BJO/20 mL, 20 mL/time, two to three times/day; soft capsule: 0.5 mL/capsule, four capsules/time, two to three times/day).27 Therefore, different delivery systems have been investigated for improvement of BJO’s absorption and bioavailability when treating cancer, such as liposomes,28 nanocarriers,29 emulsion28 and microencapsule.30 However, as far as we are concerned, no particular efforts were made on expanding the narrow absorption window as well as strengthening local activity in stomach since BJO’s prominent role was showed in this segment. Hence, we developed a GRDDS to improve BJO’s bioavailability after oral administration in a previous study.14 Also in that study,14 we investigated in vitro release profile of BJO gastroretentive floating bead. It was found that even under the agitation at 100 rpm, the beads still delivered stable drug release for 6 h. Combing with the kinetic parameters, in the present study, we concluded that quick release was observed at first in the system due to part of the drug on the surface of the beads. Then, the embedding of rest of the drug into the gel matrix beads controlled the drug release. As we mentioned, the system formed a gel layer upon reaching the SGF so that the gel layer could control the drug-release rate effectively. Later on, with the water penetrated into the beads, the drug release from gel matrices was controlled by erosion of the polymers.
In order to investigate whether BJO gastroretentive floating bead could really improve the bioavailability of BJO, pharmacokinetic evaluation was applied. We studied drug concentration in both blood and stomach of rats. First, the concentration of oleic acid was measured; unfortunately no oleic acid, as we expected, was detected at each time point after correcting by the original oleic acid concentration in blank plasma. This might be because gastric lipase has a greater activity against short-chain than long-chain triacylglycerol,31 while glycerol trioleate in BJO is a long chain as mentioned. Therefore, glycerol trioleate in stomach was cleaved very slowly. Besides, as free acid bound in position sn-2 of the glycerol is much better absorbed in the form of monoacylglycerols32 and the content of oleic acid on sn-2 is ~74.9%,33 suggesting that the concentration of oleic acid could be hardly measured directly unless a preprocess is applied. In this regard, we took glycerol trioleate as the indicative component to see whether our gastroretentive floating bead could increase the therapeutic effect of BJO. After removing the endogenous concentration of glycerol trioleate, we found that although the drug concentration in blood cannot be detected after 6 h due to the slow uptake rate in stomach and almost complete digestion and absorption of glycerol trioleate in the small intestine as mentioned before, the drug concentration in the stomach was detectable up to 6 h and the value was much higher than that of marketed BJO soft capsule, whose tissue concentration was almost declined to zero. This result, showing a slower drug release that BJO gastroretentive floating bead possessed, which corresponded to the finding of in vitro drug-release profile14 and was also evidenced by the SPET/CT study, which demonstrated that all the floating beads remained in the stomach and no signal was observed in the small intestine.14
Conclusion
In the present study, the anti-gastric tumor effect of self-prepared BJO was investigated. The results showed that the BJO we extracted had more effective action on cancer cells compared with commercial formulation. Self-made BJO also showed a paralleled capability with RAN in preventing and treating gastric ulcer. We developed a BJO gastroretentive floating bead to increase the bioavailability. The in vitro drug release study showed an excellent sustained release characteristic. Pharmacokinetic studies by HPLC-MS/MS indicated BJO gastroretentive floating bead’s enhanced absorption and oral bioavailability with longer drug residence time in the stomach compared to the BJO marketed production. Hence, BJO gastroretentive floating bead would be a promising sustained and controlled drug delivery system for BJO.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Project No 81303233) and Shanghai Committee of Science and Technology (Project No 17401902300).
Disclosure
The authors report no conflicts of interest in this work.
References
Kamperdick C, Sung TV, Thuy TT, Van Tri M, Adam G. (20R)-O-(3)-alpha-L-arabinopyransyl-pregn-5-en-3-beta, 20-diol from Brucea javanica. Phytochemistry. 1995;38(3):699–701. | ||
Cui Y, Wu Z, Liu X, et al. Preparation, safety, pharmacokinetics, and pharmacodynamics of liposomes containing Brucea javanica oil. AAPS PharmSciTech. 2010;11(2):878–884. | ||
Kim IH, Takashima S, Histotsuyanagi Y, Hasuda T, Takeya K. New quassinoids, javanicolides C and D and javanicosides B – F, from seeds of Brucea javanica. J Nat Prod. 2004;67(5):863–868. | ||
Yu YL, Lu Y, Tang X, Cui FD. Formulation, preparation and evaluation of an intravenous emulsion containing Brucea javanica oil and Coix seed oil for anti-tumor application. Biol Pharm Bull. 2008;31(4):673–680. | ||
Kalepu S, Manthina M, Padavala V. Oral lipid-based drug delivery systems – an overview. Acta Pharm Sinica B. 2013;3(6):361–372. | ||
Yu YL, Tang X, Cui FD. Pharmacokinetics of oleic acid in compound Brucea javanica oil submicron emulsions for injection in rats. J Shenyang Pharm Univ. 2008;25(5):337–341. (in Chinese). | ||
Wang Y, Li Y, Wu J, Shen Q. Characterisation and evaluation of self-microemulsifying drug delivery system of Brucea javanica oil. Micro Nano Lett. 2012;7(3):256–261. | ||
Icchihara K, Kohsaka C, Tomari N, et al. Fatty acid analysis of triacylglycerols: preparation of fatty acid methyl esters for gas chromatography. Anal Biochem. 2016;495:6–8. | ||
Zeng Z, Ji ZY, Hu N, Bai B, Wang H, Suo YR. A sensitive pre-column derivatization method for the analysis of free fatty acids by RP-HPLC with fluorescence detector and its application to Caragana species. J Chromatogr B. 2017;1064:151–159. | ||
Youzbachi N, Trabelsi H, Elfalleh W, Khaldi A, Nasri N, Tlili N. Fatty acids and triacylglycerols composition from Tunisian Acacia species seed oil. Arab J Chem. In press. | ||
Zhou SM, Wang YQ, Jiang YR, Yu LL. Safety assessment of medium- and long-chain triacylglycerols containing 30% (W/W) medium-chain fatty acids in mice and rats. Regul Toxicol Pharmacol. 2017;86:42–48. | ||
Lopes CM, Bettencourt C, Rossi A, Buttini F, Barata P. Overview on gastroretentive drug delivery systems for improving drug bioavailability. Int J Pharm. 2016;510(1):144–158. | ||
Wu ZN, Li L, Li N, et al. Optimization of ultrasonic-assisted extraction of fatty acids in seeds of Brucea javanica (L.) Merr. from different sources and simultaneous analysis using high-performance liquid chromatography with charged aerosol detection. Molecules. 2017;22(6):E931. | ||
Zhang Y, Zhang XT, Zhang Q, Wang B, Zhang T. Formulation development and evaluation of gastroretentive floating beads with Brucea javanica oil using ionotropic gelation technology. Chin J Nat Med. In press 2018. | ||
Prabakaran D, Singh P, Kanaujia P, Vyas SP. Effect of hydrophilic polymers on the release of diltiazem hydrochloride from elementary osmotic pumps. Int J Pharm. 2003;259(1–2):173–179. | ||
Osaki N, Meguro S, Yajima N, Matsuo N, Tokimitus I, Shimasaki H. Metabolites of dietary triacylglycerol and diacylglycerol during the digestion process in rats. Lipids. 2005;40(3):281–286. | ||
Lu YY, Huang XE, Cao J, et al. Phase II study on Javanica oil emulsion injection (Yadanzi®) combined with chemotherapy in treating patients with advanced lung adenocarcinoma. Asian Pac J Cancer Prev. 2013;14(8):4791–4794. | ||
Xu W, Jiang XC, Xu ZY, Ye T, Shi QH. The Efficacy of Brucea javanica oil emulsion injection as adjunctive therapy for advanced non-small-cell lung cancer: a meta-analysis. Evid Based Complement Alternat Med. 2016;2016:5928562. | ||
Liu J, Huang XE, Tian GY, et al. Phase II study on safety and efficacy of Yadanzi® (Javanica oil emulsion injection) combined with chemotherapy for patients with gastric cancer. Asian Pac J Cancer Prev. 2013;14(3):2009–2012. | ||
Shi WR, Liu Y, Wang XT, Huang QY, Cai XR, Wu SR. Antitumor efficacy and mechanism in hepatoma H22-bearing mice of Brucea javanica oil. Evid Based Complement Alternat Med. 2015;2015:217494. | ||
Liu JM, Wang FY, Luo HH, et al. Protective effect of butyrate against ethanol-induced gastric ulcers in mice by promoting the anti-inflammatory, anti-oxidant and mucosal defense mechanisms. Int Immunopharmacol. 2016;30:179–187. | ||
Barka ZB, Tlili M, Alimi H, et al. Protective effects of edible Rhus tripartita (Ucria) stem extract against ethanol-induced gastric ulcer in rats. J Func Foods. 2017;30:260–269. | ||
Jabri MA, Aissani N, Tounsi H, Sakly M, Marzouki L, Sebai H. Protective effect of chamomile (Matricaria recutita L.) decoction extract against alcohol-induced injury in rat gastric mucosa. Pathophysiology. 2017;24(1):1–8. | ||
Hesari Z, Shafiee A, Hooshfar S, Mobarra N, Mortazavi SA. Formulation and taste masking of ranitidine orally disintegrating tablet. Iran J Pharm Res. 2016;15(4):677–686. | ||
Yang F, Yu XH, Qiao F, et al. Formulation and characterization of Brucea javanica oil microemulsion for improving safety. Drug Dev Ind Pharm. 2014;40(2):266–277. | ||
Shao A, Chen G, Jiang N, et al. Development and evaluation of self-microemulsifying liquid and granule formulations of Brucea javanica oil. Arch Pharmacal Res. 2013;36(8):993–1003. | ||
Liu TT, Mu LQ, Dai W, Wang CB, Liu XY, Xiang DX. Preparation, characterization, and evaluation of antitumor effect of Brucea javanica oil cationic nanoemulsions. Int J Nanomed. 2016;11:2515–2529. | ||
Ye HX, Liu XJ, Sun JC, Zhu S, Zhu Y, Chang SF. Enhanced therapeutic efficacy of LHRHa-targeted Brucea javanica oil liposomes for ovarian cancer. BMC Cancer. 2016;16:831–843. | ||
Zou A, Li Y, Chen Y, et al. Self-assembled stable sponge-type nanocarries for Brucea javanica oil delivery. Colloids Surf B Biointerfaces. 2017;153(1):310–319. | ||
Hu LD, Zhang JL, Hu QF, Yang XN. Microencapsulation of Brucea javanica oil: characterization, stability and optimization of spray drying conditions. J Drug Deliv Sci Technol. 2016;36:46–54. | ||
Cohen M, Morgan RG, Hofmann AF. Lipolytic activity of human gastric and duodenal juice against medium and long chain triglycerides. Gastroenterology. 1971;60(1):1–15. | ||
Rezanka T, Lukavsky J, Nedbalova L, Sigler K. Production of structured triacylglycerols from microalgae. Phytochemistry. 2014;104:95–104. | ||
Bi YL. Analysis on oil quality of Brucea javanica (L.) Merr. J Zhengzhou Inst Technol. 2001;22(4):72–74. (in Chinese). |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.