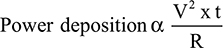Back to Journals » Journal of Pain Research » Volume 11
Enhanced expression of gene coding for β-endorphin in human monocytic cells exposed to pulsed radio frequency electric fields through thermal and non-thermal effects
Authors Azma T , Nishioka A, Ogawa S, Nagasaka H, Matsumoto N
Received 23 April 2018
Accepted for publication 4 September 2018
Published 16 November 2018 Volume 2018:11 Pages 2887—2896
DOI https://doi.org/10.2147/JPR.S171974
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Michael A Ueberall
Toshiharu Azma,1,2 Akira Nishioka,1 Saori Ogawa,3 Hiroshi Nagasaka,2 Nobuyuki Matsumoto2
1Department of Anesthesiology and Pain Medicine, Kohnodai Hospital, National Center for Global Health and Medicine, Ichikawa, Chiba 272-8516, Japan; 2Department of Anesthesiology, Saitama Medical University Hospital, Moroyama-cho, Iruma-gun, Saitama 350-0495, Japan; 3Department of Dental Anesthesiology, Matsumoto Dental University, Shiojiri, Nagano 399-0781, Japan
Background: The enhanced expression of endogenous opioid peptides, including β-endorphin, has been implicated in the mechanism of action of pulsed radio frequency (PRF) application in pain modulation. Because thermal effects cannot be separated from the physical property of PRF application to biological tissues, we evaluated whether temperatures higher than that of the normal body temperature (37°C) modulate mRNA expression for the precursor of β-endorphin, proopiomelanocortin (POMC) in human monocytic cells THP-1. We also attempted to examine whether mechanisms other than thermal effects also modulate such gene expression.
Methods and results: The mRNA for POMC in THP-1 cells increased by a 15-minutes incubation at 42°C, 45°C, or 70°C without PRF application as compared with that in cells incubated at 37°C. On the other hand, gene expression for POMC in cells incubated at 20°C as well as at 37°C with PRF application for 15 minutes increased as compared to that in cells incubated at 37°C without PRF application. Continuous radio frequency at 70°C but not PRF provoked apoptotic cell death at 1–2 hour, and necrotic cell death at 24 hours after the RF application.
Conclusion: A simple experimental system using human monocytic cells in culture demonstrated that a 15 minute elevation of temperature above 37°C enhanced gene expression for POMC in THP-1 cells, while a 15 minute application of PRF to these cells incubated at 37°C or lower, also enhanced gene expression, indicating that temperature-independent mechanisms as well as thermal effects may be involved in such gene expression.
Keywords: pulsed radio frequency electric field, human monocytic cells, THP-1, proopiomelanocortin, β-endorphin, necrosis, apoptosis, apoptotic vesicle
Introduction
A growing body of clinical evidence supports the concept that the application of pulsed radio frequency (PRF) current to peripheral nerves with conditions related to neuropathic pain is beneficial in improving pain.1 A systematic review using the meta-analysis procedure indicates that treatment with PRF decreases the intensity of pain in patients with post-herpetic neuralgia.2
PRF current is an alternative to continuous radio frequency (CRF) current that can be selected as a modality in RF generators for clinical use.1,3 These RF generators were originally developed to provoke thermal coagulation of neural tissues around the active tip of the needle-shaped RF probe.3–5 Therefore, a thermostat component which senses and regulates the temperature of the RF probe is essential for such RF generators.3
Using the CRF mode, a continuous current of approximately 500 kHz flows from the probe inserted in an insulated guiding needle with a 2–10 mm active tip.1,3,4 The temperature of tissues in an electric field around the active tip is elevated according to the power delivered, that is proportional to the square of voltage applied (V) and the time for RF current exposure (t), and inversely proportional to the tissue resistance (R).1
|
Therefore, the CRF mode is programmed to modulate (generally, to decrease) the voltage applied by the RF generator to maintain the temperature of the active tip at a pre-selected point during thermal coagulation.3 By contrast, in the PRF mode, 20 ms RF bursts are generated at a frequency of 2 Hz.1,3 This mode allows the RF generator to apply a higher voltage around the active tip than the CRF mode when the same tip temperature is selected because the power delivered by the PRF current is 4% of that of the CRF current at the same voltage.1,3
Especially, the PRF mode in the NeuroTherm system (St. Jude Medical, Saint Paul, MN, USA) is programmed to maintain the temperature at just below 43°C while keeping the voltage at the pre-selected level by decreasing the duration of RF bursts (NT500 system) or by increasing the interval of bursts (higher-end systems than NT500).3 This concept of the NeuroTherm system follows from the pioneering work of clinical investigators who suspected that higher electric fields around the active tip may modulate pain sensation through mechanisms other than thermal coagulation,3,5 although how electric fields work has not yet been clarified.6
The aim of this study is, thus, to evaluate whether temperatures higher than that of normal body (37°C) cause enhanced expression of gene coding for an endogenous opioid neuropeptide β-endorphin.7 We also attempted to examine whether mechanisms other than thermal effects also cause such biological change. We used a human monocytic cell line THP-1 as an experimental tool because: 1) it is known that β-endorphin is expressed in monocytes by some physiological stimuli and immune cell-derived β-endorphin is implicated in analgesia for certain conditions8 and 2) monocytes possess polymodal receptors that sense temperatures higher than that of the normal body.9,10 We evaluated the effects of temperatures higher than 37°C as well as those of the exposure to PRF electric fields with or without thermal effects on the modulation of gene expression for the precursor of β-endorphin, proopiomelanocortin (POMC),7 in THP-1 cells.
Methods
Materials
RPMI-1640 containing 2-mM L-alanyl-glutamine and 25-mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) was procured from Thermo Fisher Scientific (Waltham, MA, USA). Annexin V-FITC apoptosis detection kit, RPMI-1640 (with 25-mM HEPES, without L-glutamine) and fetal bovine serum were purchased from Sigma-Aldrich (St. Louis, MO, USA). The CountBright (CB) absolute counting beads (approximately 7 µm in diameter) were from Molecular Probes (Eugene, OR, USA). NucleoSpin RNA was from Macherey–Nagel (Düren, Germany). Primers for PCR, target specific fluorescent reporter probes, and other reagents used for reverse transcription (RT)-PCR were purchased from TaKaRa Bio (Kusatsu, Shiga, Japan). The quality of all chemicals used was certified for molecular biology or for cell culture.
THP-1
Human monocytic cells, provided by American Type Culture Collection as the cell line THP-1, were cultivated at densities of 1–9×105 cells/mL in the culture medium (RPMI-1640 containing 25-mM HEPES), supplemented with 2-mM L-alanyl-glutamine, 100 unit/mL penicillin, 100 µg/mL streptomycin, and 10% fetal bovine serum (complete medium) as previously described.11
General experimental protocol
THP-1 cells were washed three times with the culture medium by 200g centrifugation for 2 minutes. Cell count was performed twice by using the automated cell counter TC 20 (Bio-Rad, Hercules, CA, USA), and the average was used to re-suspend cells at 1×106 cells/mL. A 100-µL portion of the cell suspension was transferred to a PCR tube and was applied to a Veriti Thermal Cycler (Applied Biosystems, Foster City, CA, USA). PCR tubes containing such cell suspensions were incubated at 37°C for 10 minutes, followed by 15-minute incubation at various temperatures (37°C, 42°C, 45°C, or 70°C). The incubation of these PCR tubes was continued in the Thermal Cycler at 37°C for 30 minutes until total RNA was extracted using a nucleic acid purification kit (NucleoSpin RNA II) as previously described.11
On the other hand, a 500-µL portion of the suspension of THP-1 cells was transferred to a polypropylene microtube and was centrifuged at 1,000g for 5 minutes. The RF probe in a 10 cm length and 4 mm active tip guiding needle (22-gauge, Hakko, Chikuma, Nagano, Japan) was inserted into the microtube to place the active tip in the sedimented THP-1 cells. A counter electrode was tied on the plastic insulation of the guiding needle. During the application of RF current, the microtubes were incubated at 37°C in a water bath unless otherwise noted.
CRF or PRF current at a frequency of 480 kHz was applied to the cells using a NeuroTherm NT500 RF generator (St. Jude Medical, Sait Paul, MN, USA). The voltage generated in the CRF mode is automatically changed by the NT500 RF generator to stabilize the temperature of the RF probe at a selected point.3 The temperature of the RF probe was monitored by an LED indicator of the NT500 during the RF generation and was confirmed to be at the pre-selected level. By contrast, the PRF mode of the NT500 system (repeated 480-kHz RF bursts of duration less than 20 ms in 500 ms) is programmed for the voltage to be fixed at a pre-selected level during RF generation.3 We selected the maximum voltage (>45 V) for the PRF mode and confirmed that the probe temperature reached just below 43°C. On the other hand, in separate experiments to examine whether changes in the gene expression occur without temperature elevation, THP-1 cells in microtubes were incubated in an aluminium thermal block controlled at 20°C by the Peltier device. Special care was taken to ensure that the probe temperature did not reach 37°C during exposure to the PRF current.
After RF application, THP-1 cells were re-suspended by mild pipetting, and a 50-µL portion of the cell suspension was transferred to 96-multiwell culture plates. The incubation of 96-multiwell plates at 37°C was continued in a humidified atmosphere of 5% CO2 in air for 1 hour, 2 hours, or 24 hours until flow cytometric analysis. The remaining cell suspension was transferred to 24-multiwell plates filled with the same volume of the complete medium. The incubation of 24-multiwell plates at 37°C was continued in a humidified atmosphere of 5% CO2 in air for 24 hours until the extraction of total RNA as shown above.
Evaluation for the cellular viability and the shedding of apoptotic vesicles using flow cytometry
A 50-µL portion of the culture medium containing propidium iodide (PI) and fluorescein isothiocyanate isomer I (FITC)-conjugated Annexin V (1 µg/mL and 1% (v/v) of the provided stock solution, respectively, at final concentrations) was added to each dish of 96-multiwell plates with the same volume of the cell suspension incubated for a pre-selected time period after the RF application. Ten-minutes after adding these agents, a 50-µL portion of the mixture containing THP-1 cells (5×105 cells/mL) were transferred to a polystyrene tube after gentle pipetting. A fixed dose of the CB absolute counting beads was added to this tube and the culture medium was also added to fill up to 500 µL. Particles (1×104 counts) from each well were analyzed with a FACS Canto II flow cytometer (Becton Dickinson (BD), Franklin Lakes, NJ, USA), and the results were processed by the BD FACS DIVA software and the Kaluza flow cytometry analysis software (ver. 1.2, Beckman Coulter, Brea, CA, USA). FSC-A is the area of electric pulse for forward scatter (FSC) of particles detected in flow cytometry, indicative of the relative size of each particle (see Figure 1). SSC-A is the area of electric pulse for side scatter (SSC), which reflects the surface as well as the internal complexity of each particle (Figure 1). Total THP-1 cells were gated by a rectangle in the FSC-A/SSC-A plot according to the previous description of ours (FSC ≥7 µm CB calibration beads, SSC ≥ minimal value for normal THP-1 cells).11 Apoptotic cells were defined as the Annexin-bound particles (ie, PI/FITC=(+/+) or (−/+)) gated in the total THP-1. Necrotic cells were defined as the cell membrane-injured and thus PI-positive, and Annexin-not-bound particles (PI/FITC=(+/−)) in the total THP-1 gate. Apoptotic vesicles were defined as particles gated in a rectangle of FITC-positive (i.e., Annexin-bound) and with FSC values less than those for 7 µm CB beads in the FSC-A/FITC-A plot.
Evaluation for the gene expression
A 2-µL portion of the total RNA (<500 ng) was subjected to RT as previously described using TaKaRa PrimeScript RT Master Mix.11 A 1-µL portion from the resultant mixture containing cDNA was added to TaKaRa Premix Ex Taq (Probe qPCR) at a final volume of 10 µL containing the primer pair (200 nM each at the final concentration) and the target specific fluorescent reporter probe for POMC or GAPDH (200 nM each at the final concentration). The illumina Eco Real-Time PCR system (San Diego, CA, USA) was used for analysis. Thermal cycling conditions were designed as follows: initial denaturation at 95°C for 30 seconds followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Fluorescence was recorded during each annealing step. All amplification reactions were performed in duplicate, and average threshold cycle (Ct) numbers of the duplicates were recorded. The Ct value for POMC was normalized to Ct value for the housekeeping gene GAPDH (ie, ΔCt). The relative change in ΔCt was expressed as ΔΔCt, where ΔCt of interest was subtracted from that of the calibrator (i.e., control). The sequences of the primer pair used to amplify a part of the cDNA or the target specific fluorescent reporter probe are shown in Table 1.
  | Table 1 Sequences of PCR primers and the specific fluorescent reporter probes Abbreviation: POMC, proopiomelanocortin. |
Statistical analysis
The number of cells or vesicles was expressed as a percentage of the number of total THP-1 cells. The density of these cells or vesicles was also calculated from the ratio of particle count, as compared to that of the CB absolute counting beads; the density of the latter was measured by using the TC 20. The proportion of particles was expressed in a 95% CI, and the comparison with control was performed by using the chi-squared test. The values for ΔΔCt were expressed as mean ± SD. Comparison of multiple groups was performed by ANOVA followed by the Post-hoc Bonferonni’s correction. Statistical differences were considered to be significant for values of P less than 0.05.
Results
Cellular viability and the shedding of apoptotic vesicles
CRF currents applied to the sedimented THP-1 cells in a microtube for 3 minutes at a fixed temperature of 70°C caused significant apoptotic cell death evaluated at 1 hour, 2 hours, and 24 hours after the RF application by flow cytometry. The increase in necrotic cells was not observed at 1–2 hour after the CRF application but significant increase was observed at 24 hours after the application. By contrast, PRF currents applied for up to 15 minutes (41°C–42°C) failed to provoke apoptotic or necrotic cell death. CRF, but not PRF currents increased the number of apoptotic vesicles shed from THP-1 cells at 1 hour or 2 hours after RF application. Apoptotic vesicles decreased to the level of control (i.e., without RF) at 24 hours after CRF application (Figures 1 and 2, Table 2).
The expression of mRNA for POMC in THP-1 cells at 24 hours after RF application
CRF currents applied to the sedimented THP-1 cells in a microtube for 3 minutes at 70°C increased the gene expression for POMC in surviving cells at 24 hours after RF application (Tables 2 and 3). PRF currents applied to these cells for 3 minutes did not influence the gene expression for POMC observed at the same incubation period after RF application. However, prolonged PRF current for up to 15 minutes increased the gene expression for POMC in THP-1 cells at 24 hours after RF application (Table 3).
Effects of a 15-minute incubation at temperatures higher than 37°C on the expression of mRNA for POMC in THP-1 cells
The mRNA level for POMC in THP-1 cells at 30 minutes after incubation at temperatures higher than 37°C (42°C, 45°C or 70°C) for 15 minutes increased as compared to that in cells incubated at 37°C throughout (Table 3).
Effect of incubation temperatures on the expression of mRNA for POMC in THP-1 cells exposed to the PRF electric fields
To evaluate whether the elevation to temperatures higher than 37°C is the unique cause of the increased gene expression for POMC in THP-1 cells applied to the PRF currents, THP-1 cells in microtubes were incubated in a water bath at 37°C or in an alminium thermal block controlled at 20°C by the Peltier device during the exposure to the PRF electric field for 15 minutes. The mRNA level for POMC in THP-1 cells at 24 hours after exposure to the PRF electric field during incubation of the cells at 20°C as well as at 37°C was significantly increased as compared to that in cells without RF application incubated at 37°C (Table 3).
Discussion
Accumulated clinical experience following the most primary report from Sluijter et al5 indicates that considerable adverse effects should not occur after the application of PRF to peripheral nerves at below 43°C using clinically available RF generators.1,12 The lack of numbness or anesthesia dolorosa after PRF application, that are usually accompanied with CRF ablation at higher temperatures,5,13 implies that the mechanism of action of PRF in decreasing pain is different from the ablation of peripheral nerves by CRF applied at higher temperatures.6 However, it has not yet been clarified how PRF current modulates the sensory nerves with conditions related to neuropathic pain.
The therapeutic effects of PRF application to dorsal root ganglions (DRGs), where neuropathic pain was introduced by chronic constriction injury or related surgical techniques, on nociceptive responses in experimental animals were evaluated by several groups. Vallejo et al14 as well as Lin et al15 indicated that the production of acute proinflammatory cytokines TNF-α or IL-6 is suppressed by the PRF application to DRGs. Lin et al also showed that the phosphorylation of mitogen-activated protein kinases (MAPKs; p-ERK and p-p 38) in the spinal dorsal horn induced by spinal nerve ligation were suppressed by PRF application to DRGs.15 In addition to such therapeutic effects of PRF on the neuropathic pain model induced by spinal nerve ligation, Chen et al showed the beneficial roles of PRF in an inflammatory pain model induced by intradermal injection of complete Freund’s adjuvant (CFA) in the hind paw.16 They showed that the activation of c-Jun N-terminal kinases after the CFA injection was suppressed by PRF application to DRGs.16 These findings collectively suggest that the application of PRF to DRGs suppresses the central sensitization of pain through several pathways, while the mechanism of action of PRF currents in the modulation of such pathways could not be addressed by these studies.
Several investigators showed that the application of PRF alone to DRGs (i.e., without experimental intervention to establish neuropathic pain) induced the expression of markers for the activation of the nervous system, c-Fos17,18 or activating transcription factor 3 (ATF3),19 indicating that certain signal transduction pathways should be activated by PRF currents applied to DRGs. It is noteworthy that the expression of c-Fos in the dorsal horn was provoked by the application of PRF to DRGs, but not by CRF applied at the same temperature as PRF.18 This suggests that the higher electric fields generated by PRF currents than those generated by CRF currents have an advantage over the latter in activating DRGs. Hamann et al also indicated that the expression of ATF3 in DRGs was introduced by the application of PRF to DRGs, but not to peripheral nerve fibers.19 These collectively indicate that the therapeutic effect of PRF application is provoked by the exposure of DRGs to the electric field of PRF currents.
Although these studies showed that the exposure of DRGs to PRF electric fields activates spinal nerves,17–19 it has not yet been clarified what molecules were involved in such activation of nervous system. In this context, it is interesting to note that Jia et al showed increase in the expression of glial cell-derived neurotrophic factor (GDNF) by PRF in the neuropathic pain model provoked by spinal nerve ligation.20 GDNF is known to play important roles in post-injury reparation of neurons21,22 and has been reported to alleviate neuropathic pain in experimental animals.23–25 These reports indicate that GDNF is a possible candidate for the molecule involved in the modulation of pain by PRF application.
Several lines of experimental evaluation, including our present study, attempted to show the expression of endogenous opioids by the exposure of various types of cells to PRF currents. Wu et al showed that the level of met-enkephalin in the spinal cord was increased by the application of PRF to DRGs.26 Moffett et al demonstrated that the application of PRF currents to human dermal fibroblasts or epidermal keratinocytes in culture increased the level of precursor mRNAs for endogenous opioid, proenkephalin, proopiomelanocortin, and prodynorphin.27 The latter group also confirmed the increase in the production of these peptides by enzyme linked immunosorbent assay.27
The present study was planned to further examine the role of temperatures higher than that of the normal body (37°C) in gene expression for the precursor of β-endorphin in human monocytic cells provoked by PRF application, because thermal effects (i.e., power deposition) cannot be separated from the physical property of RF application to biological tissues.1,3 We demonstrated, firstly, that the incubation of THP-1 cells at temperatures higher than 37°C increased the gene expression for POMC in human monocytic cells. It is plausible that such thermal effects on the enhanced expression of POMC are caused by the activation of polymodal nociceptive receptors TRPV1,9 the expression of which has been demonstrated in monocytic cells.10 However, further evaluation is required to confirm the involvement of polymodal nociceptive receptors in the events observed in our experiments by using specific antagonists for these receptors or by using genome-edited THP-1 cells that lack expression for these receptors.
The secondary important finding of the present study was that the application of PRF currents to THP-1 cells under a condition that these cells are cooled to temperatures below those for the normal body (20°C) also increased the level of mRNA for POMC. Although the temperature around the active tip of the RF probe gradually increased due to power deposition1 (i.e., the Joule heating) in this set of experiments, we confirmed that it was less than 37°C by monitoring the temperature of the RF probe. This indicated that mechanisms other than thermal effects should be involved in the gene expression for POMC in human monocytic cells after the PRF application. The role of high electric fields around the active tip of the RF probe independent of the thermal effects is implicated from these interesting results while we cannot address the precise mechanisms for this phenomenon through the present study. Further study is also required to solve this issue.
Cytotoxicity or degeneration of neural/perineural tissues after the exposure to PRF electric fields
The present study demonstrated, using flow cytometry, that CRF application to human monocytic cells at 70°C for 3 minutes provoked apoptotic cell death within 1–2 hour after the application. The proportion of apoptotic cells decreased while that of necrotic cells increased at 24 hours after CRF at 70°C. By contrast, PRF application at temperatures below 43°C for up to 15 minutes did not provoke significant apoptotic or necrotic cell death during the same observation period. Such results of ours support an early study by Podhajsky et al using optical microscopy, showing that PRF and CRF applied at 42°C provoked no significant change in DRGs and sciatic nerves except for the extracellular edema of DRG cells and nerve fibers, or fibroblast activation causing the increase of collagen in subperineural and perivascular spaces.12 These changes, observed most obviously at 2 days after RF treatment, improved and returned to normal at 7 days and 21 days, respectively, after treatment. In contrast to such reversible changes caused by 42°C applications, CRF at 80°C provoked the Wallerian degeneration, consisting of swelling and degeneration of axons and disintegration of myelin.12
Several groups reported, using electron microscopy, histological changes after the application of PRF, including those of the enlargement of endoplasmic reticulum in DRG cells,28 the fusion of vacuoles in these cells,28 disruption of mitochondria in A and C-fibers,29 the separation of myelin of axons.30 But the relationship between these findings observed using electron microscopy and therapeutic effects of PRF are not clear. It is noteworthy here that these morphological changes provoked by PRF28–30 point to the potential risk of this modality of RF currents for the disruption of nervous system treated. Adherence to evidence-based settings for the frequency, amplitude, pulse width and interval, or the treatment time is required for the safe clinical practice using PRF application for interventional pain medicine.
The limitation and the strong point of this study
The primary outcome of this study is that enhanced gene expression for POMC was shown to be provoked after a 15-minute incubation of human monocytic cells at temperatures higher than 37°C. Because the thermal effect (<43°C) is an essential property of PRF application to biological tissues,1,3 it is likely that such enhanced gene expression occurs around the active tip of the RF probe within 30 minutes after a 15-minute exposure of THP-1 cells to the PRF electric field by clinically available RF generators through thermal effects. The secondary outcome is a demonstration that such enhancement occurred in PRF application even when the temperature of the RF probe was below 37°C. This could be demonstrated because we used a simple observational system consisting of a single type of cells in culture, that could be cooled to temperatures less than those for the normal body.
Although the secondary outcome is very interesting, we cannot address the precise mechanisms of the non-thermal effects in the modulation of gene expression for POMC because the present study was designed for the primary outcome and not for the secondary outcome. A possible explanation for the non-thermal effects of PRF application is the electroporation evoked by high electric fields of PRF currents.4 However, two unresolved issues exist for this concept: 1) there is no evidence for cell membrane disruption in histological studies using electron microscopy after the PRF application28–30 and 2) the mode of electric currents in commercially available electroporators is apparently different from those in PRF generators. The electroporator for experimental uses applies higher voltages than the PRF generator and applies direct current in contrast to the alternating RF current in the latter.31
Although several strongpoints can be addressed, it is not clear whether the enhanced gene expression for POMC, especially in monocytic cells, could explain the modulatory effects of PRF application on pain sensation in the whole animal body. While a human monocytic cell line THP-1 was demonstrated to be an excellent tool to examine the question in the present study, it is essential to perform animal behavior studies to solve the remaining issue. Further experimental studies as well as clinical observations are required to clarify the mechanism of action of the PRF application in the treatment of peripheral nerves and DRGs under neuropathic pain conditions.
Conclusion
Exposure of human monocytic cells to PRF currents using a clinically available RF generator increased the gene expression for POMC in these cells. Elevation to temperatures higher than 37°C appeared to be responsible for such PRF-provoked increase in the gene expression for POMC, while uncertain mechanisms, other than thermal effects, may also be involved in this phenomenon. PRF application to these cells failed to provoke significant apoptotic as well as necrotic cell death.
Research involving animal and human rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Acknowledgment
This work was supported in part by grants-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology in Japan (grant nos. 22591748, 25462451, 16K10979, 16K15684, and 18K08841).
Disclosure
The authors report no conflicts of interest in this work.
References
Chua NH, Vissers KC, Sluijter ME. Pulsed radiofrequency treatment in interventional pain management: mechanisms and potential indications-a review. Acta Neurochir. 2011;153(4):763–771. | ||
Shi Y, Wu W. Treatment of Neuropathic Pain Using Pulsed Radiofrequency: A Meta-analysis. Pain Physician. 2016;19(7):429–444. | ||
Gauci C, Cosman E, Cosman EJ. The physics of radiofrequency & pulsed radiofrequency. In: Gauci C, editor. Manual of RF Techniques: a Practical Manual of Radiofrequency Procedures in Chronic Pain Management. 2nd ed. Amsterdam: Flivo Press; 2008:12–34. | ||
Cosman ER Jr, Cosman ER Sr. Electric and thermal field effects in tissue around radiofrequency electrodes. Pain Med. 2005;6(6):405–424. | ||
Sluijter ME, Cosman ER, Rittman I, Vankleef ME. The effects of pulsed radiofrequency fields applied to the dorsal root ganglion: A preliminary report. Pain Clin. 1998;11:109–117. | ||
van Boxem K, Huntoon M, van Zundert J, Patijn J, van Kleef M, Joosten EA. Pulsed radiofrequency: a review of the basic science as applied to the pathophysiology of radicular pain: a call for clinical translation. Reg Anesth Pain Med. 2014;39(2):149–159. | ||
Raffin-Sanson ML, de Keyzer Y, Bertagna X. Proopiomelanocortin, a polypeptide precursor with multiple functions: from physiology to pathological conditions. Eur J Endocrinol. 2003;149(2):79–90. | ||
Machelska H, Stein C. Analgesic effects of immune-cell-derived opioids. In: DeLeo JA, Sorkin LS, Watkins LR, editors. Immune and Glial Regulation of Pain. Seattle: IASP Press; 2007:107–119. | ||
Clapham DE, Julius D, Montell C, Schultz G. International Union of Pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol Rev. 2005;57(4):427–450. | ||
Omari SA, Adams MJ, Geraghty DP. TRPV1 Channels in Immune Cells and Hematological Malignancies. Adv Pharmacol. 2017;79:173–198. | ||
Azma T, Ogawa S, Nishioka A, et al. Involvement of superoxide generated by NADPH oxidase in the shedding of procoagulant vesicles from human monocytic cells exposed to bupivacaine. J Thromb Thrombolysis. 2017;44(3):341–354. | ||
Podhajsky RJ, Sekiguchi Y, Kikuchi S, Myers RR. The histologic effects of pulsed and continuous radiofrequency lesions at 42 degrees C to rat dorsal root ganglion and sciatic nerve. Spine. 2005;30(9):1008–1013. | ||
Choi EJ, Choi YM, Jang EJ, Kim JY, Kim TK, Kim KH. Neural Ablation and Regeneration in Pain Practice. Korean J Pain. 2016;29(1):3–11. | ||
Vallejo R, Tilley DM, Williams J, Labak S, Aliaga L, Benyamin RM. Pulsed radiofrequency modulates pain regulatory gene expression along the nociceptive pathway. Pain Physician. 2013;16(5):E601–E613. | ||
Lin ML, Lin WT, Huang RY, et al. Pulsed radiofrequency inhibited activation of spinal mitogen-activated protein kinases and ameliorated early neuropathic pain in rats. Eur J Pain. 2014;18(5):659–670. | ||
Chen KH, Yang CH, Juang SE, et al. Pulsed radiofrequency reduced complete Freund’s adjuvant-induced mechanical hyperalgesia via the spinal c-Jun N-terminal kinase pathway. Cell Mol Neurobiol. 2014;34(2):195–203. | ||
van Zundert J, de Louw AJ, Joosten EA, et al. Pulsed and continuous radiofrequency current adjacent to the cervical dorsal root ganglion of the rat induces late cellular activity in the dorsal horn. Anesthesiology. 2005;102(1):125–131. | ||
Higuchi Y, Nashold BS, Sluijter M, Cosman E, Pearlstein RD. Exposure of the dorsal root ganglion in rats to pulsed radiofrequency currents activates dorsal horn lamina I and II neurons. Neurosurgery. 2002;50(4):850–856. | ||
Hamann W, Abou-Sherif S, Thompson S, Hall S. Pulsed radiofrequency applied to dorsal root ganglia causes a selective increase in ATF3 in small neurons. Eur J Pain. 2006;10(2):171–176. | ||
Jia Z, Ren H, Li Q, Ji N, Luo F. Pulsed Radiofrequency Reduced Neuropathic Pain Behavior in Rats Associated with Upregulation of GDNF Expression. Pain Physician. 2016;19(2):49–58. | ||
Bennett DL, Michael GJ, Ramachandran N, et al. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J Neurosci. 1998;18(8):3059–3072. | ||
Buj-Bello A, Buchman VL, Horton A, Rosenthal A, Davies AM. GDNF is an age-specific survival factor for sensory and autonomic neurons. Neuron. 1995;15(4):821–828. | ||
Sakai A, Asada M, Seno N, Suzuki H. Involvement of neural cell adhesion molecule signaling in glial cell line-derived neurotrophic factor-induced analgesia in a rat model of neuropathic pain. Pain. 2008;137(2):378–388. | ||
Wang C, Wang H, Pang J, et al. Glial cell-derived neurotrophic factor attenuates neuropathic pain in a mouse model of chronic constriction injury: possible involvement of E-cadherin/p120ctn signaling. J Mol Neurosci. 2014;54(2):156–163. | ||
Shi JY, Liu GS, Liu LF, et al. Glial cell line-derived neurotrophic factor gene transfer exerts protective effect on axons in sciatic nerve following constriction-induced peripheral nerve injury. Hum Gene Ther. 2011;22(6):721–731. | ||
Wu B, Ni J, Zhang C, Fu P, Yue J, Yang L. Changes in spinal cord met-enkephalin levels and mechanical threshold values of pain after pulsed radio frequency in a spared nerve injury rat model. Neurol Res. 2012;34(4):408–414. | ||
Moffett J, Fray LM, Kubat NJ. Activation of endogenous opioid gene expression in human keratinocytes and fibroblasts by pulsed radiofrequency energy fields. J Pain Res. 2012;5:347–357. | ||
Erdine S, Yucel A, Cimen A, Aydin S, Sav A, Bilir A. Effects of pulsed versus conventional radiofrequency current on rabbit dorsal root ganglion morphology. Eur J Pain. 2005;9(3):251–256. | ||
Erdine S, Bilir A, Cosman ER, Cosman ER. Ultrastructural changes in axons following exposure to pulsed radiofrequency fields. Pain Pract. 2009;9(6):407–417. | ||
Tun K, Cemil B, Gurcay AG, et al. Ultrastructural evaluation of Pulsed Radiofrequency and Conventional Radiofrequency lesions in rat sciatic nerve. Surg Neurol. 2009;72(5):496–500. | ||
Covello G, Siva K, Adami V, Denti MA. An electroporation protocol for efficient DNA transfection in PC12 cells. Cytotechnology. 2014;66(4):543–553. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.