Back to Journals » International Journal of Nanomedicine » Volume 11
Enhanced chondrocyte culture and growth on biologically inspired nanofibrous cell culture dishes
Authors Bhardwaj G, Webster T
Received 8 August 2015
Accepted for publication 20 December 2015
Published 4 February 2016 Volume 2016:11 Pages 479—483
DOI https://doi.org/10.2147/IJN.S94000
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Lei Yang
Garima Bhardwaj,1 Thomas J Webster1,2
1Department of Chemical Engineering, Northeastern University, Boston, MA, USA; 2Center of Excellence for Advanced Materials Research, King Abdulaziz University, Jeddah, Saudi Arabia
Abstract: Chondral and osteochondral defects affect a large number of people in which treatment options are currently limited. Due to its ability to mimic the natural nanofibrous structure of cartilage, this current in vitro study aimed at introducing a new scaffold, called XanoMatrix™, for cartilage regeneration. In addition, this same scaffold is introduced here as a new substrate onto which to study chondrocyte functions. Current studies on chondrocyte functions are limited due to nonbiologically inspired cell culture substrates. With its polyethylene terephthalate and cellulose acetate composition, good mechanical properties and nanofibrous structure resembling an extracellular matrix, XanoMatrix offers an ideal surface for chondrocyte growth and proliferation. This current study demonstrated that the XanoMatrix scaffolds promote chondrocyte growth and proliferation as compared with the Corning and Falcon surfaces normally used for chondrocyte cell culture. The XanoMatrix scaffolds also have greater hydrophobicity, three-dimensional surface area, and greater tensile strength, making them ideal candidates for alternative treatment options for chondral and osteochondral defects as well as cell culture substrates to study chondrocyte functions.
Keywords: chondrocytes, XanoMatrix™, cell culture, substrates, biomimetic scaffolds
Introduction
People of all ages are affected by joint pain due to chondral and osteochondral defects.1 These problems are often triggered by cartilage lesions caused by trauma or due to age-related degeneration.2 Although many therapeutic and surgical techniques are used to treat this affliction, there is no optimal solution due to the limited vascularity and nonregenerative nature of this tissue.3–6 It is clear that new biomaterials are badly needed, which possess unprecedented abilities to promote cartilage cell functions.
Existing options include symptom management by chondrocyte transplantation to the cartilage lesion initiating native repair processes, but that often leads to the formation of a soft hyaline-like tissue with suboptimum mechanical properties.7 Also, factors such as limited transplant sites and donor site morbidity lead to various other complications, including bleeding, infection, inflammation, and chronic pain. Collagen I, chitosan, and hyaluronic acid-based scaffolds and gels have proven to be good carriers for chondrocytes.8–13 These scaffolds often promote the release of certain cytokines and growth factors from cells that promote the healing and regeneration of cartilage lesions.14 However, they have the disadvantage of poor mechanical properties and fast degradation, thus, they are not a stable platform for cellular proliferation and extracellular matrix generation and deposition.7,15
The current study introduces a brand-new scaffold for cartilage regeneration (termed XanoMatrix™) for enhancing the growth and proliferation of chondrocytes and the deposition of cartilage extracellular matrix proteins while possessing appropriate mechanical properties. XanoMatrix is currently made with biocompatible materials such as polyethylene terephthalate (PET) and cellulose acetate (CA). PET, also known as Dacron®, has been widely used as a prosthetic vascular graft material, and has excellent mechanical strength and good biocompatibility. CA is an industrially important cellulose ester with good mechanical and wetting properties. CA nanofibers have also increasingly been used in tissue engineering. XanoMatrix scaffolds offer the advantage of mimicking the natural cell growth environment while combining the advantage of nanofibered tortuous beds and supports. Moreover, XanoMatrix scaffolds can be easily cut into desired cartilage defects at the site of surgery. The objective of this in vitro study was to determine for the first time the cytocompatibility properties of XanoMatrix for cartilage applications and assess their use as suitable cell culture inserts for chondrocyte research. Traditional cell culture dishes are polymers which do not resemble the structure of cartilage and, thus, results (such as chondrocyte signaling pathways) obtained with such materials should be suspect.
Materials
XanoMatrix scaffolds were fabricated by Xanofi (Raleigh, NC, USA) by XanoShear technology. These scaffolds were then placed into 96-well plates and chondrocyte adhesion and proliferation were studied over a period of 7 days as described in the methods section. Sterile traditional plasma-treated polystyrene cell culture dishes were obtained from Corning and Falcon (Thermo Fisher Scientific, Waltham, MA, USA) for comparative analysis.
Methods
Cell culture
Human chondrocytes (C-12750) obtained from PromoCell (Heidelberg, Germany) were cultured in chondrocyte basal media (C-27111) and a chondrocyte growth media supplement mix (C-39635) with 10% fetal bovine serum and 1% penicillin (HyClone; Thermo Fisher Scientific, Waltham, MA, USA). The samples were sterilized with 70% ethanol for 20 minutes and then rinsed thrice with phosphate-buffered saline. 3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assays were used to determine cell adhesion and proliferation after 1, 3, 5, and 7 days. The cells were seeded at 5,000 cells/cm2 for the proliferation assays. The media was changed every other day. The MTS (CellTiter 96® AQueous One Solution Cell Proliferation Assay, G3581 Promega Corporation, Fitchburg, WI, USA) reagent (1:5 ratio with cell culture media) was added to each well and was incubated for 3 hours on the day of the measurement. Absorbance from each well was measured by a SpectraMax M3 (MT05412; Molecular Devices LLC, Sunnyvale, CA, USA) at 490 nm and a color change from pink to dark brown was seen. Northeastern University Institutional Review board approved the use of human cells during this research.
Confocal microscopy
The data gathered by the MTS assays were verified using confocal microscopy after fixing the cells after 5 days with glutaraldehyde followed by successive dehydration with 50%, 70%, 90%, and 100% ethanol and then staining with 20 nm SYTO9 dye.
Surface characterization
Contact angle analysis
Wettability of the samples was determined using the Pioneer (Succasunna, NJ, USA) contact angle 300 goniometer. The tests were performed using distilled water.
Scanning electron microscopy
A Hitachi 4800-S (Tokyo, Japan) scanning electron microscopy with a voltage of 5.0 kV and a magnification of ×250 was used to visualize the surfaces of the samples.
Mechanical tensile testing
Mechanical tensile testing was performed using a uniaxial tensile tester equipped with a 10 lb load cell and material analysis software (ADMET Materials Testing Systems, Norwood, MA, USA). Samples were cut into 10×30 mm rectangular strips and secured with grips, such that the initial gauge length was 10 mm. The grips were moved apart at a rate of 0.1 mm/s at room temperature to simulate biological conditions. This arrangement was used to obtain stress–strain curves, as well as the elastic modulus, material elongation, and maximum load endured for each sample.
Statistics
All experiments were conducted in triplicate and repeated at least three times each. A Student’s t-test was used to determine whether the differences in cell numbers over the different time periods were significant.
Results and discussion
In this study, CA- and PET-based nanofibrous scaffolds were used to test the adhesion and proliferation of chondrocytes over a period of 7 days. The scaffolds were subjected to contact angle analysis where it was seen that the XanoMatrix surface was more hydrophobic as compared with traditional Corning and Falcon cell culture surfaces (Figure 1). The contact angles were: Corning (90.51°), Falcon (88.371°), and XanoMatrix (122.8°).
  | Figure 1 Contact angle images of the surfaces of (A) Corning (90.51°), (B) Falcon (88.371°), and (C) XanoMatrix™ (122.8°). |
The surface of the scaffold was also visualized using scanning electron microscopy and the presence of a three-dimensional, fibrous, extracellular matrix-like structure was observed as shown in Figure 2. The scaffold was subjected to tensile testing and the data obtained showed that the maximum load that the scaffold could withstand without breaking was 17.4652 N and the modulus of elasticity was found to be 5.0875×108 Pa. The extension at the end of test was observed to be 1.9204 mm as shown in Figure 3. For normal human cartilage, the Young’s modulus at the microscale varies in a narrow size range of 0.6–0.7MPa.11 For nanoscale measurements, this value varies during the loading process almost for an order of magnitude from 0.5 MPa to 1.8MPa.11 Even though the XanoMatrix scaffold can bear more tensile stress than normal cartilage, the increased growth of chondrocytes on the surface as compared with normal cell culture choices (such as Corning and Falcon) and its natural extracellular matrix-like structure still make it a suitable choice for performing in vitro experiments where cartilage tissue is concerned.
  | Figure 2 Scanning electron microscopy images of the surfaces of (A) Corning, (B) Falcon, and (C) XanoMatrix™. Scale bar =500 μm. |
  | Figure 3 Graph depicting the stress developed in a XanoMatrix™ material when applying a constantly increasing load. |
The data obtained from the cell adhesion and proliferation assays showed that there was increased chondrocyte adhesion and proliferation on the XanoMatrix surface as compared with the Corning and Falcon petri dishes as shown in Figure 4. Specifically, there was a fivefold increase in cell density from day 1 to day 7 on the XanoMatrix surfaces and the cell population almost doubled between day 1 and day 3. Confocal microscopy was used to obtain a closer look at the interaction between cells and the scaffold as shown in Figure 5. The cells aligned along the fibers as shown in the figure also resembles natural cartilage. An image of the scaffold without cells was used as reference. Because of all these properties observed, it was concluded that this surface is desirable and deserves further consideration for cartilage applications. Moreover, the results from the study show that the XanoMatrix could be a more suitable substrate for chondrocyte studies than typical cell culture substrates, improving fundamental studies on chondrocyte cell biology.
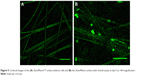  | Figure 5 Confocal images of the (A) XanoMatrix™ surface without cells and (B) the XanoMatrix surface with chondrocytes at day 5 at ×40 magnification. |
Conclusion
Cartilage-associated defects are difficult to treat and the search for an ideal biomaterial scaffold is a pressing issue. The current study shows that XanoMatrix scaffolds with their composition, structure, and mechanical properties offer a great surface for chondrocyte growth and proliferation and hence should be used for in vitro studies involving chondral and osteochondral defects.
Acknowledgment
The authors would like to thank Xanofi for providing all the material and Northeastern University for funding.
Disclosure
The authors report no conflicts of interest in this work.
References
Klangjorhor J, Phitak T, Pruksakorn D, Pothacharoen P, Kongtawelert P. Comparison of growth factor adsorbed scaffold and conventional scaffold with growth factor supplemented media for primary human articular chondrocyte 3D culture. BMC Biotechnol. 2014;14:108. | ||
Ueblacker P, Wagner B, Vogt S, et al. In vivo analysis of retroviral gene transfer to chondrocytes within collagen scaffolds for the treatment of osteochondral defects. Biomaterials. 2007;28(30):4480–4487. | ||
Jia L, Duan Z, Fan D, Mi Y, Hui J, Chang L. Human-like collagen/nano-hydroxyapatite scaffolds for the culture of chondrocytes. Mater Sci Eng C Mater Biol Appl. 2013;33(2):727–734. | ||
Peterson L, Minas T, Brittberg M, Lindahl A. Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: results at two to ten years. J Bone Joint Surg Am. 2003; 85-A(Suppl 2):17–24. | ||
Aigner T, Kim HA, Roach HI. Apoptosis in osteoarthritis. Rheum Dis Clin North Am. 2004;30:639–653. | ||
Peretti GM, Xu JW, Bonassar LJ, Kirchhoff CH, Yaremchuk MJ, Randolph MA. Review of injectable cartilage engineering using fibrin gel in mice and swine models. Tissue Eng. 2006;12:1151–1168. | ||
Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889–895. | ||
Nürnberger S, Meyer C, Ponomarev I, et al. Equine articular chondrocytes on MACT scaffolds for cartilage defect treatment. Anat Histol Embryol. 2013;42(5):332–343. | ||
Huard J, Li Y, Peng H, Fu FH. Gene therapy and tissue engineering for sports medicine. J Gene Med. 2003;5(2):93–108. | ||
Moutos F, Freed LE, Guilak F. A biomimetic three-dimensional woven composite scaffold for functional tissue engineering of cartilage. Nat Mater. 2007;6:162–167. | ||
Raghunath J, Rollo J, Sales KM, Butler PE, Seifalian AM. Biomaterials and scaffold design: key to tissue-engineering cartilage. Biotechnol Appl Biochem. 2007;46:73–84. | ||
Shortkroff S, Yates K. Alteration of matrix glycosaminoglycans diminishes articular chondrocytes’ response to a canonical Wnt signal. Osteoarthr Cartil. 2007;15:147–154. | ||
Steinert A, Ghivizzani SC, Rethwilm A, Tuan RS, Evans CH, Nöth U. Major biological obstacles for persistent cell-based regeneration of articular cartilage. Arthritis Res Ther. 2007;9(3):213. | ||
Gough JE, Scotchford CA, Downes S. Cytotoxicity of glutaraldehyde crosslinked collagen/poly(vinyl alcohol) films is by the mechanism of apoptosis. J Biomed Mater Res. 2002;61(1):121–130. | ||
Wakitani S, Kawaguchi A, Tokuhara Y, Takaoka K. Present status of and future direction for articular cartilage repair. J Bone Miner Metab. 2008;26(2):115–122. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

