Back to Journals » Vascular Health and Risk Management » Volume 14
Endothelin-1, nitric oxide, serotonin and high blood pressure in male adolescents
Authors Aflyatumova GN , Nigmatullina R , Sadykova DI, Chibireva MD, Fugetto F , Serra R
Received 6 April 2018
Accepted for publication 17 June 2018
Published 18 September 2018 Volume 2018:14 Pages 213—223
DOI https://doi.org/10.2147/VHRM.S170317
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Takashi Kajiya
Gulfiia Nagimovna Aflyatumova,1 Razina Ramazanovna Nigmatullina,2 Dinara Ilgizarovna Sadykova,3 Mariia Dmitrievna Chibireva,4 Francesco Fugetto,5 Raffaele Serra6,7
1Department of Pediatrics, Children’s Republican Clinical Hospital of the Ministry of Health of the Republic of Tatarstan, Kazan, Republic of Tatarstan, Russian Federation; 2Department of Normal Physiology, Kazan State Medical University, Kazan, Republic of Tatarstan, Russian Federation; 3Department of Hospital Pediatrics, Kazan State Medical Academy, Kazan, Republic of Tatarstan, Russian Federation; 4Koltsov Institute of Developmental Biology, Russian Academy of Sciences, Moscow, Russian Federation; 5Department of Vascular Surgery, Interuniversity Center of Phlebolymphology (CIFL), International Research and Educational Program in Clinical and Experimental Biotechnology, Headquarters: University Magna Graecia of Catanzaro, Catanzaro, Italy; 6Department of Vascular Surgery, Interuniversity Center of Phlebolymphology (CIFL), International Research and Educational Program in Clinical and Experimental Biotechnology, Headquarters: University Magna Graecia of Catanzaro, Catanzaro, Italy; 7Department of Medical and Surgical Sciences, University of Catanzaro, Catanzaro, Italy
Background: Essential arterial hypertension (EAH) in adolescents represents a social burden. The endothelium is involved in the pathogenesis of EAH. Imbalance of key vasoactive factors – namely nitric oxide (NO) and endothelin-1 (ET-1) – is observed, and serotonin (5-HT) release is also impaired. The relationship between the factors and high blood pressure (BP) has been established mainly in preclinical studies and in the adult age. The aim of the present manuscript is to establish the association between plasma ET-1, serum NO and 5-HT, platelet 5-HT levels and BP in male adolescents, analyzing their concentrations in controls, prehypertensive and hypertensive children. Consequently, we want to evaluate ET-1, NO and 5-HT levels as preclinical biomarkers of EAH.
Methods: Outpatient adolescents, examined at Children’s Republican Clinical Hospital of the Ministry of Health of the Republic of Tatarstan, were recruited between 26th of May and 25th of September 2016. Predictor variables identified were plasma ET-1, serum NO and 5-HT levels and were evaluated in serum and platelets of case and control groups.
Results: Plasma ET-1 and serum 5-HT concentrations in prehypertensive and hypertensive children were higher than in controls, with hypertensive adolescents showing higher levels of both factors compared with prehypertensive adolescents. Platelet 5-HT levels were lower in prehypertensive and hypertensive children compared with controls, while serum NO levels were higher in prehypertensive children than in hypertensive children.
Conclusion: Measurable ET-1, NO and 5-HT are related to BP in adolescents and may serve as diagnostic biomarkers of EAH. Furthermore, they could help to better define prehypertensive and hypertensive children.
Keywords: endothelin-1, serotonin, nitric oxide, pediatric arterial hypertension, biomarkers, pre-hypertension, monoamines, endothelial dysfunction
Introduction
Background
Since hypertension-associated cardiovascular events usually do not occur in childhood, the definition of hypertension in children is statistical rather than functional. According to the fourth report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents released by The National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents in 2004,1 pediatric hypertension (PH) is defined as average systolic blood pressure (SBP) and/or diastolic blood pressure (DBP) that is ≥95th percentile for age, sex and height on three or more occasions. The same report also established the definition of pediatric prehypertension (PPH), ie, average SBP or DBP that is ≥90th percentile but <95th percentile. In adolescents beginning at age 12 years, prehypertension is defined as blood pressure (BP) between 120/80 mm Hg and the 95th percentile. This condition represents a category of patients at a higher risk for developing overt hypertension. Finally, the working group suggested that in case of BP ≥95th percentile, the hypertension should be staged. Children with BP between the 95th and 99th percentile plus 5 mm Hg are categorized as stage I hypertension, while children with BP above the 99th percentile plus 5 mm Hg should be staged as II hypertension.
Although more common in adulthood, essential (primary) arterial hypertension (EAH) is also detectable in children and adolescents. The prevalence of primary essential hypertension, mostly in older school-age children and adolescents, has increased in prevalence in parallel with the obesity epidemic. When stratified by age, the prevalence of hypertension is 0.9/1,000 in patients aged 2–11 years, compared with 5.4/1,000 in those aged 12–18 years.2 Other studies suggest that verified hypertension and prehypertension can be found in more than 3% of asymptomatic children and adolescents.3 It is now widely accepted that a significant part of adult patients with EAH represents a continuum of a pre-existent condition of high BP in childhood and adolescence.4
Hypertension represents a major risk factor for myocardial infarction, stroke and renal failure and is the leading cause of premature death among adults throughout the world.5 While late hypertension-related cardiovascular events from EAH do not usually occur in childhood, hypertensive children, who remain usually asymptomatic, already manifest evidence of target organ damage, with 8.0% showing cardiac complications (particularly left ventricular hypertrophy) at echocardiographic studies and early atherosclerosis in autopsy series.2 Thus, pediatric EAH is a serious epidemiological and clinical problem, and represents an important point to which population-based health care strategies should be focused.
By definition, EAH is a form of high BP where no identifiable cause can be found. However, many factors, including familiar history (with a polygenic type of inheritance), diet, stress, obesity and other lifestyle factors may play a role in the development of primary hypertension. One of the most crucial and earliest pathogenic mechanisms of EAH is endothelial dysfunction.6 Endothelial dysfunction is characterized by a shift of the actions of the endothelium toward reduced vasodilation, a proinflammatory state and prothrombotic properties.7 The balance of key vasoactive factors – namely, nitric oxide (NO) and endothelin-1 (ET-1) – is altered. NO inhibits the expression of adhesion molecules and platelet aggregation, and acts as a vasodilative, antiproliferative and antithrombotic agent.8 ET-1 is a potent vasoconstrictor peptide mainly produced by endothelium. Its effects are principally mediated by endothelin receptor type A and type B, both found in vascular smooth muscle (VSM) with substantial effects in the systemic, renal, pulmonary, coronary and cerebral circulation; they induce profound vasoconstriction, pro-inflammatory actions, mitogenic and proliferative effects, stimulation of free radical formation and platelet activation.9,10
There is also an established relationship between the serotonergic system and endothelium.11 The monoamine serotonin (5-HT) is one of the main vasoconstrictors and exerts its effect through 5-HT1B and 5-HT2A receptors located on the membrane of VSM cells.12 In particular, 5-HT is locally released via serotonin transporter (SERT) by activated platelets, which serve as storage site, and significantly changes the function of both endothelial cells and VSM,13 inducing smooth muscle cell proliferation and migration via 5-HT2A receptors.14 High plasma 5-HT concentration can lead to thrombosis and vasoconstriction in animal models.15 Finally, in vitro and ex vivo analyses have showed interactions between 5-HT and ET-1 at physiological concentrations, with the former contributing to vascular growth and contractile responses induced by the latter.12
To date, no studies have investigated the relationship between NO, ET-1 and 5-HT levels and BP in pediatric population. Understanding the pathophysiological mechanisms underlying endothelial dysfunction and PH as well as finding diagnostic and prognostic biomarkers is essential for early recognition and better managing a disease with high costs in childhood, adolescence and adulthood.
Objectives
The primary aim of this study was to establish the association between plasma ET-1, serum NO, serum 5-HT and platelet 5-HT concentrations and BP in male outpatient adolescents (two-sided alternative hypothesis).
The secondary aims of the study were to evaluate if NO, ET-1 and 5-HT are correlated and if they may be considered as preclinical diagnostic and prognostic biomarkers of endothelial dysfunction and PPH/PH in male adolescents.
Methods
Standard protocol approvals, registrations and patient consents
The study was approved by the research ethics committee of the Children’s Republican Clinical Hospital of the Ministry of Health of the Republic of Tatarstan (protocol number 4, 24th of May 2016). Each participant and respective legal representative provided written informed consent prior to study enrollment. The present work has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans16 and with the International Committee of Medical Journal Editors’ guidelines.17
Study and manuscript design
The main research question was conceived as feasible, interesting, novel, ethical and relevant.
First, a comprehensive study plan was created. It included 1) outline, with a clear and univocal one-sentence research question; 2) study protocol; and 3) operations manual, ie, the collection of specific procedural instructions in order to ensure a uniform and standardized approach to carry out the research with a good quality control.
To meet the already exposed hypotheses, a case–control design was chosen. Adolescents with high BP – as specified by The National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents – were included in case group and comprised newly or previously diagnosed adolescents without current antihypertensive treatment; normotensive adolescents were included in control group.
A standard questionnaire was ideated for the collection of demographic, anamnestic (both personal and familiar) and auxological (ie, sex, age, height, weight, body mass index) data of both potential cases and controls. An anonymous database was predisposed.
The present manuscript was written according to STROBE statement guidelines for observational, case–control studies (fourth version).18
Setting
Outpatient adolescents, examined at Children’s Republican Clinical Hospital of the Ministry of Health of the Republic of Tatarstan, were recruited between 26th of May and 25th of September 2016. All the patients were pre-screened for eligibility by the attending physicians. If diagnostic and inclusion criteria were met, a research assistant introduced the study and obtained informed consent.
Participants
For the cases, the inclusion criteria were 1) male sex, 14–17-year old (adolescents); 2) diagnosis of PPH and PH, according to the fourth report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents released by The National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents1 ; 3) patients attending the clinic at Children’s Republican Clinical Hospital of the Ministry of Health of the Republic of Tatarstan and 4) exclusion of any condition causing high BP (ie, secondary hypertension). The exclusion criteria were 1) patients at high likelihood of being lost to follow-up (eg, severe co-pathologies or planning to move out from the State); 2) prescription of drugs known to alter or interact with sexual endocrine and monoaminergic systems (eg, anti-depressants) as well as blood pressure; 3) contraindications to ambulatory blood pressure monitoring (ABPM) (ie, atrial fibrillation, coagulation disorders and, for some brands of equipment, latex allergy).
For the controls, the inclusion criteria were 1) male sex, 14–17-year old (adolescents); 2) normal BP and 3) patients attending the clinic at Children’s Republican Clinical Hospital of the Ministry of Health of the Republic of Tatarstan. The exclusion criteria were 1) patients at high likelihood of being lost to follow-up; 2) prescription of drugs known to alter or interact with sexual endocrine and monoaminergic systems as well as BP and 3) contraindications to ABPM.
A 1:1 ratio between case and controls was chosen.
Variables
Predictor variables (continuous): plasma ET-1, serum NO and 5-HT levels in serum and platelets.
Outcome variables (categorical, ordinal): normal BP/high BP (PPH and PH).
Potential confounders were addressed as illustrated in the section “bias”. No effect modifiers were considered.
Data sources/measurement
Blood pressure
In order to assess BP in both cases and controls, ABPM was performed using automatic measurement of the oscillometric-based, wearable A&D TM-2430 monitor (accuracy: BP±3 mmHg; pulse±5%) validated by Association for the Advancement of Medical Instrumentation.19,20 The monitor was applied on non-dominant arm as specified by the manufacturer only by personnel with specific training in the application of the device in pediatric patients. According to American Heart Association guidelines,21 1) the monitors were programmed to record every 20 minutes throughout the day and every 30 minutes during sleep; 2) after application, BP measured with the device was compared with resting, clinic BP using oscillometric technique (agreement of an average of three clinic and three ambulatory BP levels within 5 mm Hg was considered adequate calibration); 3) a sufficient number of valid BP recordings were collected and analyzed (ie, minimum of one reliable reading per hour, including during sleep) and 4) two well trained, independent specialists interpreted the results.
Blood sampling
After overnight fasting, two blood samples were drawn from the cubital vein of each subject in EDTA-coated tubes (BD Vacutainer® Blood Collection Tube, Becton Dickinson, Broken Bow, NE, USA) between 7.30 and 9.00 a.m. and then immediately centrifuged at 4°C (2500 rpm for 15 minutes). Platelet-depleted plasma and serum were aspirated and duplicate samples were stored at –80°C until assay for ET-1 NO, NO metabolites and 5-HT. All samples were analyzed blind to subject status.
ET-1 assessment
Plasma ET-1 was assessed using an enzyme-linked immunosorbent assay (Endothelin 1–21, catalog no. 442–0052, BCM, Biomedica, Vienna, Austria. Features of the ELISA system are as follows: sensitivity=0.02 fmol/mL (0 fmol/mL+3 SD); standard range=0.02–10 fmol/mL; inter-assay and intra-assay coefficients of variation ≤4 and ≤6% respectively.
NO assessment
Serum NO concentrations were quantified via Total NO/Nitrite/Nitrate Assay Kit (catalog no. KGE001; R&D Systems, Inc., Minneapolis, MN, USA), with the following features: sensitivity=0.78 μmol/L; assay range=3.1–200 μmol/L (cell culture supernates, serum, EDTA plasma, heparin plasma, citrate plasma, urine); specificity=total NO, nitrite and nitrate levels measured in various samples; cross-reactivity=≤0.5% cross-reactivity observed with available related molecules and <50% cross-species reactivity observed with species tested.
5-HT assessment
Platelet-depleted serum concentration of 5-HT was measured in duplicate using Serotonin FAST Kit (catalog no. EIA5061; DRG instrument GmbH, Marburg, Germany). Its characteristics are as follows: detection range=15–2,500 ng/mL; sensitivity=5 ng/mL.
An aliquot of aspirated platelet rich serum was then used to assess platelet count via Advia Centaur Analyser (Bayer Ltd., Berks, UK). Additional duplicate samples were used for 5-HT analysis of platelet-rich serum, while platelet-depleted serum 5-HT concentrations were measured following centrifugation.
Auxological measurements
Both cases and controls underwent physical examination, including anthropometric measurements that were performed by well-trained examiners according to the Anthropometric Standardization Reference Manual.22 Height was measured to the nearest 0.1 cm using a wall-mounted stadiometer (Harpenden, Crymych, UK). Body weight was measured to the nearest 0.1 kg, and body mass index was calculated as weight in kg/height in meters squared.
Bias
With a particular regard for the optimization of the recruitment and preservation of generalizability, in order to minimize the sampling bias, participants (both cases and controls) were enrolled from the eligible clinical-based population and sampled through a simple probability sampling method (ie, enumeration of the units of the population and selection of a subset at random via a table of random numbers).
In order to copy with confounders from the early design phase, the authors applied two strategies: 1) specification, including in the study only male adolescents because cyclic changes in estrogen levels observed during the menstrual cycle in females would potentially lead to fluctuations of endothelial vasodilation, hormones imbalance and changes of biogenic amines blood level acting as confounder and 2) frequency matching for the constitutional variables collected (namely sex, age, height, weight, body mass index) and platelet count (Table 1).
Measurement bias of blood samples was limited via repeating the measurement and using the mean of the two readings obtained.
All personnel assessing BP and levels of ET-1, NO/NO metabolites and 5-HT were blinded to case–control assignment of patients.
Other sources of error
The strategies for enhancing precision and accuracy of measurements were 1) standardizing the measurement methods in an operation manual for both observers and subjects and 2) training and certifying the observer.
Study size
The sample size was estimated based on extensive search for previous and related findings on the topic, discussion between investigators, clinically meaningful values and educated guess. The following data were considered: 1) two-sided alternative hypothesis; 2) effect size: difference of 15% (0.10 fmol/mL calculated basing on physiologic plasma ET-1 levels – 1 fmol/mL ca); 3) SD: 0.1 fmol/mL; 4) standard effect size: 1.0; and 5) a (two-sided)=0.05; b=0.10; power=0.90. Sample size required per group when using the Student’s t-test to compare means of continuous variables was 23.
In order to deal with dropouts and subjects with missing data, the authors planned to stop recruitment only when 100 potential participants for each group were enrolled.
Statistical methods
Statistical data processing was performed on a personal computer using Excel spreadsheets and software package “Statistic for Windows” version 6.0. Variation of predictor variables was calculated and expressed as follows: arithmetic mean (M), median (Me), standard error of arithmetic mean (m) and frequency table. A two-sided Student’s t-test was applied for comparison of continuous predictor variables between the different groups. The significance of differences in qualitative characteristics was tested using the Pearson c2 criterion. Statistical significance was set at P-value <0.05 (P<a). Pearson correlation coefficient was calculated in order to check for correlations between the predictor variables.
No statistical strategies were needed to copy with confounders in the analysis phase.
Results
Participants
A total of 200 potentially eligible male adolescents with and without elevated BP were identified, and 177 were screened. In total, 60 cases and 60 controls were finally included in the study (Figure 1). Reasons for non participation and quit were mainly of logistic nature, and two authors independently verified the lack of severe pathologies or other potentially confounding reasons.
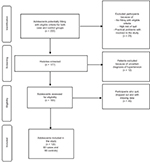  | Figure 1 Flow diagram of enrolled patients. |
Descriptive data
Demographic and laboratory characteristics of cases and controls are provided in Table 1. Of note, no statistically significant difference was found between the two groups when constitutional characteristics other than BP and platelet count were considered.
No participants with missing data were present in the last phase of this clinical research.
According to the results of ABPM, two subgroups of adolescents with high BP were formed: 1) adolescents with PPH (group 1, n=33, 41.3%) and 2) adolescents with PH (group 2, n=27, 33.7%).
Outcome data and main results
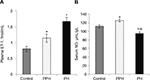
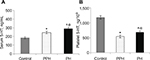
Cases and controls levels of plasma ET-1, serum NO, serum 5-HT and platelet 5-HT, along with their comparison, are reported in Table 2 and Figures 2 and 3. Healthy participants were identified as group A, while patients were identified as group B.
Patients’ plasma ET-1 levels (expressed in fmol/mL) appeared to be significantly higher than controls (1.38±0.13 vs 0.8±0.08, respectively; P-value: <0.0001; 95% CI: 0.54–0.62). When stratified according to the severity of high BP, adolescents with hypertension showed significantly higher levels of plasma ET-1 compared to those with prehypertension (1.67±0.12 vs 1.14±0.13, respectively; P-value: <0.0001; 95% CI: 0.46–0.60).
Serum NO concentrations (expressed in µmol/mL) did not significantly differ between cases and controls (111.63±3.09 vs 111.71±3.52, respectively; P-value: 0.89; 95% CI: –1.28–1.12): However, when stratified for the severity of high BP and compared with control group, patients with prehypertension had significantly higher levels of NO (125.0±3.13; P-value: <0.0001; 95% CI: 11.83–14.75), while adolescents with hypertension showed significantly lower levels of the same parameter (95.29±3.04; P-value: <0.0001; 95% CI: –17.98–14.86). A statistically significant difference in serum NO concentrations between children with prehypertension and those with hypertension was also appreciated (P-value: <0.0001; 95% CI: –31.32–28.10).
Patients’ serum 5-HT levels (expressed in ng/mL) appeared to be significantly higher than controls (266.62±14.14 vs 186.15±12.54, respectively; P-value: <0.0001; 95% CI: 76.64–86.30). When stratified for the severity of high BP, adolescents with hypertension showed significantly higher levels of serum 5-HT than those with prehypertension (291.98±13.25 vs 245.87±14.87; P-value: <0.0001; 95% CI 38.75–53.47).
Platelet 5-HT levels (expressed in ng/109 platelets) were significantly lower in patients than in controls (606.77±46.95 vs 1177.37±54.88, respectively; P-value: <0.0001; 95% CI: –589.06–552.14). When stratified for BP, children with hypertension had significantly higher content of platelet 5-HT than prehypertensive children (684.26±47.41 vs 543.37±46.57, respectively; P-value: <0.0001; 95% CI: 116.50–165.28).
Pearson correlation coefficient referred to serum 5-HT and plasma ET-1 levels in children with PPH and PH was r=0,019 (P=0.885; 95% confidence interval=−0.2361 to 0.2716).
Discussion
Key results
Owing to the well-established relationship with childhood the obesity epidemic, high BP in the young is rapidly increasing and now represents a social burden. Even if death and cardiovascular disability, typical of adults, do not usually occur in hypertensive children, left ventricular hypertrophy, early atherosclerosis and other intermediate markers of target organ damage such as retinal vascular changes and even subtle cognitive changes can be appreciated in this population,3 without overt symptoms in the majority of cases. Although unknown for definition, EAH etiology appears to be linked to a positive family history of hypertension, obesity and lifestyle factors. Several experimental models suggested that an excess of vasoconstrictive ET-1, vasoconstrictive and prothrombotic 5-HT along with a reduction of vascular NO concentrations could represent main actors in the complex process at the origin of high BP. This condition is known as “endothelial dysfunction”, and is evidently characterized by a shift of the actions of the endothelium toward reduced vasodilation, a proinflammatory state and prothrombotic properties.7 With such premises, the present research group hypothesized that the previously mentioned mediators (namely ET-1, NO, and 5-HT) could be elected as biomarkers of high BP in humans.
ET-1 is a powerful vasoconstrictor peptide and regulator of blood flow that plays an important role in BP elevation in some models of experimental hypertension. The present study showed that plasma levels of ET-1 in children with hypertension were significantly higher than in normotensive children (ratio: 2.1:1). This agrees with the data of Głowin´ska et al23 who demonstrated increased ET-1 plasma concentrations in adolescents with hypertension compared to healthy subjects (0.63 pg/mL vs 0.40 pg/mL, P<0.05; mean age: 15.3±2.4 years vs 14.7±2.9 years). They also found a significant correlation between ET-1 plasma concentrations and SBP (P=0.02) levels. In the paper published by Katona et al,24 it was found that NO plasma concentrations were significantly lower (27.7±13.7 vs 35.8±7.0 μmol/L, respectively, P<0.001), while ET-1 concentrations were higher (3.11±3.9 vs 1.09±1.07 fmol/mL, respectively, P<0.001) in hypertensive adolescents than that in controls. NO and ET-1 correlated, respectively, negatively and positively with BP values, especially with SBP.24 In another study involving adults, Schneider et al found that there is a correlation between elevated plasma ET-1 concentrations and arterial hypertension. In particular, they showed significantly higher plasma ET-1 levels (0.35±0.26 fmol/mL, P<0.0001) in hypertensive patients compared to controls (0.08±0.13 fmol/mL). They did not consider ET-1 as a marker of endothelial dysfunction but supposed that changes in plasma ET-1 levels may precede vascular complications associated with hypertension and diabetes.25 What the present study adds is the evaluation of ET-1 plasma concentrations in children with prehypertension, which we found to be significantly higher than controls (ratio: 1.43:1). This finding represents new data in scientific literature and opens new speculations about pathophysiology of PH. With such evidences, we could firmly support the role of continuous ET-1 increase in the development of PH and state its altered levels already in PPH.
NO plays an important role in maintaining cardiovascular homeostasis.26 In humans, it regulates vascular tone inducing an endothelium-dependent vasodilation.27 Additionally, endothelium-derived NO exerts antioxidant, antiproliferative, antithrombotic and anti-inflammatory properties.28 It was found that NO-mediated vasodilation is inhibited in hypertension by an increase in arginase activity in endothelial cells, which limits l-arginine availability to endothelial NO synthase (eNOS) for NO production.29 In our paper, prehypertensive adolescents had higher levels (+11%) of NO when compared to normotensive children, representing a novelty as NO levels have never been studied in children with prehypertension. On the contrary, patients with hypertension showed lower levels of NO (–15%) with respect to the control group; this is in concordance with what was found by Katona et al24 and Forte et al,30 the latter demonstrating that urinary NO metabolite content was lower in adults with essential hypertension. Although in both cases we did not find those differences to be statistically significant, increased NO levels in adolescents with prehypertension may represent a compensatory response to reduced endothelium-dependent vascular relaxation, which is NO dependent. This mechanism may be associated with upregulation of inducible NO synthase (iNOS) as was mentioned by Oliveira-Paula et al.31 Furthermore, while relatively small amounts of NO produced by eNOS are important to cardiovascular homeostasis, high NO levels produced by activated iNOS may contribute to higher BP values as these excessive amounts of NO can react with superoxide anions forming peroxynitrite, thereby promoting nitrosative stress and endothelial dysfunction. In addition, abnormal iNOS activity can upregulate arginase activity, allowing it to compete with eNOS for l-arginine, thereby resulting in reduced NO bioavailability. This may also lead to eNOS uncoupling with enhanced production of superoxide anions instead of NO. While all these data could explain the relationship between prehypertension and higher NO concentrations, a decrease of NO levels in adolescents with hypertension could merely represent a gradual exhaustion of endothelium’s compensatory vasodilating capacity, potentially representing a poor prognostic sign for the development of overt hypertension. Camilletti et al32 described a significant decrease in platelet NO levels are observed in hypertensive subjects, which may confirm the link between hypertension and altered platelets function and suggest a role for NO in cardiovascular events.32
5-HT is a VSM cell mitogen and contributes to arterial’s smooth muscle cell contractility and growth, both appreciated in hypertension.33 Similarly to ET-1, in our study cases presented significantly higher levels of serum 5-HT than controls (ratio: 1.43:1), with hypertensive children showing the highest rates (+18% in children with hypertension). Conversely, platelet 5-HT concentrations in patients with hypertension were lower than controls (–48%), with this issue needing further deepening as literature lacks of studies evaluation this relationship in the pediatric population. Fetkovska et al34 demonstrated that 5-HT plasma concentrations increased with BP in adult hypertensive patients, which is in accordance with our results of pediatric population. SERT is widely presented in cardiovascular tissues such as pulmonary arteries, heart, systemic arteries and endothelial cells; it is also present in platelets, where it is highly efficient and enables them to take up 5-HT from the gut and lung and store it.35 Some studies showed that 5-HT content and uptake velocity in blood platelets were significantly lower in adult hypertensive patients, with an increase in 5-HT efflux and an accompanying increase in plasma 5-HT and 5-HIAA levels.34,36,37 This is in accordance with our data on pediatric population and may explain decreased platelet 5-HT content. Isbister et al38 showed that when selective serotonin reuptake inhibitors are used, platelet 5-HT is not depleted, but extracellular 5-HT is higher as 5-HT reuptake is prevented. The role of SERT in cardiovascular diseases appears to be related also with its vascular isoforms. In particular, SERT’s activity on peripheral arteries is enhanced in hypertension39 and in pulmonary hypertension pulmonary artery’s SERT dysfunction contribute to increased pulmonary BP by dual mechanisms, promoting vasoconstriction and pulmonary artery remodeling via 5-HT.40 All the presented data support the hypothesis that SERT is inhibited in the pathogenesis of cardiovascular diseases as EAH. It can be concluded that altered 5-HT metabolism, platelet hyperactivity and SERT dysfunction dramatically change the function of VSM and endothelial cells and increase the risk of occurrence of cardiovascular events.38,41
Limitations
Due to a careful design of the present research, the authors exclude the presence of important confounding variables or biases. Random errors were copied though a punctual conduction of the various assessments and measurements. Population size was appropriately achieved and patients were randomly enrolled.
Unfortunately, in order to address the best statistical consistency, only male patients were included. The authors are already planning to conduct a similar study, with appropriate laboratory and analytic adjustments limiting the influence of physiological fluctuation of estrogens in female adolescents.
Interpretation and generalizability
The obtained results suggest that plasma ET-1, serum NO, serum 5-HT and platelet 5-HT are related to BP in male adolescents, answering to the primary hypothesis of the present study.
Furthermore, it can be asserted that these factors are eligible biomarkers of EAH in the studied population. In particular, their combination could serve not only as a diagnostic tool, but also as a tool for stratification of severity of high BP in children and maybe could serve as preclinical biomarkers of hypertension. Compatible with the presented results is also the fact that in the early stages of hypertension compensatory mechanisms aimed to maintain optimal BP are triggered; however, their action has physiological limits and a chronic hyper-activation of vasoregulatory systems finally leads to vasoconstriction, platelet aggregation and vascular inflammation, configuring the condition of “endothelial dysfunction”. Thus, it can be concluded that the prespecified hypothesis of the present research is verified.
The presented methods of BP, ET-1, NO and 5-HT assessment are widely available in the Developed World; thus, the study findings of the present manuscript are generalizable.
Given the importance of the presented clinical condition, the fact that ET-1, NO and 5-HT levels appear to change in children with high BP fairly before the clinical manifestations of overt hypertension, as well as the statistical strength of the associations found, the authors suggest to consider the triad “ETNOS” as an useful tool for preventing irreversible cardiovascular changes and high socio-economical costs.
Additionally, these data may be useful in hypertension treatment improvement. New classes of antihypertensive drugs that can increase serum NO levels and decrease plasma ET-1 and serum 5-HT levels in order to reestablish balance of vasoactive factors and to recover endothelial function can be considered for further studies.
Acknowledgments
The authors thank William Edward James Jenner – BSc (Hons), MA, RN(A), Associate Clinical Education Specialist, B\Braun Medical UK Ltd. – for his editorial assistance. This work was not funded.
Disclosure
The authors report no conflicts of interest in this work.
References
National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 Suppl 4th Report):555–576. | ||
Dobson CP, Eide M, Nylund CM. Hypertension Prevalence, Cardiac Complications, and Antihypertensive Medication Use in Children. J Pediatr. 2015;167(1):92–97. | ||
Falkner B. Hypertension in children and adolescents: epidemiology and natural history. Pediatr Nephrol. 2010;25(7):1219–1224. | ||
Anyaegbu EI, Dharnidharka VR. Hypertension in the teenager. Pediatr Clin North Am. 2014;61(1):131–151. | ||
Chobanian AV, Bakris GL, Black HR, et al. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 Report. JAMA. 2003;289:2560–2572. | ||
Taddei S, Virdis A, Ghiadoni L, Versari D, Endothelium SA. aging, and hypertension. Curr Hypertens Rep. 2006;8(1):84–89. | ||
Endemann DH, Schiffrin EL. Endothelial dysfunction. J Am Soc Nephrol. 2004;15(8):1983–1992. | ||
Akcaboy M, Kula S, Göktas T, et al. Effect of plasma NOx values on cardiac function in obese hypertensive and normotensive pediatric patients. Pediatr Nephrol. 2016;31(3):473–483. | ||
Böhm F, Pernow J. The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc Res. 2007;76(1):8–18. | ||
Mazzuca MQ, Khalil RA. Vascular endothelin receptor type B: structure, function and dysregulation in vascular disease. Biochem Pharmacol. 2012;84(2):147–162. | ||
Machida T, Iizuka K, Hirafuji M. 5-hydroxytryptamine and its receptors in systemic vascular walls. Biol Pharm Bull. 2013;36(9):1416–1419. | ||
Bhaskaran S, Zaluski J, Banes-Berceli A. Molecular interactions of serotonin (5-HT) and endothelin-1 in vascular smooth muscle cells: in vitro and ex vivo analyses. Am J Physiol Cell Physiol. 2014;306(2):143–151. | ||
Mercado CP, Kilic F. Molecular mechanisms of SERT in platelets: regulation of plasma serotonin levels. Mol Interv. 2010;10(4):231–241. | ||
Gamoh S, Hisa H, Yamamoto R. 5-hydroxytryptamine receptors as targets for drug therapies of vascular-related diseases. Biol Pharm Bull. 2013;36(9):1410–1415. | ||
Callebert J, Esteve JM, Hervé P, et al. Evidence for a control of plasma serotonin levels by 5-hydroxytryptamine(2B) receptors in mice. J Pharmacol Exp Ther. 2006;317(2):724–731. | ||
World Medical Association. WMA Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects. World Medical Association. Available from: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/. Published March 2018. Accessed May 22, 2018. | ||
Uniform requirements for manuscripts submitted to biomedical journals: writing and editing for biomedical publication. J Pharmacol Pharmacother. 2010;1(1):42–58. | ||
Institute of Social and Preventive Medicine (ISPM). STROBE Statement. ISPM – University of Bern. Available from: https://strobe-statement.org/index.php?id=available-checklists. Published November 2007. Accessed May 23, 2018. | ||
Palatini P, Frigo G, Bertolo O, Roman E, Da Cortà R , Winnicki M. Validation of the A&D TM-2430 device for ambulatory blood pressure monitoring and evaluation of performance according to subjects’ characteristics. Blood Press Monit. 1998;3(4):255–260. | ||
Yip GW, So HK, Li AM, Tomlinson B, Wong SN, Sung RY. Validation of A&D TM-2430 upper-arm blood pressure monitor for ambulatory blood pressure monitoring in children and adolescents, according to the British Hypertension Society protocol. Blood Press Monit. 2012;17(2):76–79. | ||
Urbina E, Alpert B, Flynn J, et al. Ambulatory blood pressure monitoring in children and adolescents: recommendations for standard assessment: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee of the council on cardiovascular disease in the young and the council for high blood pressure research. Hypertension. 2008;52(3):433–451. | ||
Cheikh Ismail L, Knight HE, Ohuma EO, Hoch L, Chumlea WC. International Fetal and Newborn Growth Consortium for the 21st Century. Anthropometric standardisation and quality control protocols for the construction of new, international, fetal and newborn growth standards: the INTERGROWTH-21st Project. BJOG. 2013;120(Suppl 2):48–55. | ||
Głowin´ska B, Urban M, Hryniewicz A, Peczyn´ska J, Florys B, Al-Hwish M. Endothelin-1 plasma concentration in children and adolescents with atherogenic risk factors. Kardiol Pol. 2004;61(10):329–338. | ||
Katona E, Settakis G, Varga Z, et al. Target-organ damage in adolescent hypertension. Analysis of potential influencing factors, especially nitric oxide and endothelin-1. J Neurol Sci. 2006;247(2):138–143. Available from: https://www.ncbi.nlm.nih.gov/pubmed/16737713. Accessed May 21, 2018. | ||
Schneider JG, Tilly N, Hierl T, et al. Elevated plasma endothelin-1 levels in diabetes mellitus. Am J Hypertens. 2002;15(11):967–972. | ||
Godo S, Shimokawa H. Divergent roles of endothelial nitric oxide synthases system in maintaining cardiovascular homeostasis. Free Radic Biol Med. 2017;109:4–10. | ||
Vanhoutte PM, Shimokawa H, Feletou M, Tang EH. Endothelial dysfunction and vascular disease - a 30th anniversary update. Acta Physiol. 2017;219(1):22–96. | ||
Gkaliagkousi E, Ferro A. Nitric oxide signalling in the regulation of cardiovascular and platelet function. Front Biosci. 2011;16(16):1873–1897. | ||
Zhang C, Hein TW, Wang W, et al. Upregulation of vascular arginase in hypertension decreases nitric oxide-mediated dilation of coronary arterioles. Hypertension. 2004;44(6):935–943. Available from: https://www.ncbi.nlm.nih.gov/pubmed/15492130. Accessed May 22, 2018. | ||
Forte P, Copland M, Smith LM, Milne E, Sutherland J, Benjamin N. Basal nitric oxide synthesis in essential hypertension. Lancet. 1997;349(9055):837–842. | ||
Oliveira-Paula GH, Lacchini R, Tanus-Santos JE. Inducible nitric oxide synthase as a possible target in hypertension. Curr Drug Targets. 2014;15(2):164–174. | ||
Camilletti A, Moretti N, Giacchetti G, et al. Decreased nitric oxide levels and increased calcium content in platelets of hypertensive patients. Am J Hypertens. 2001;14(4 Pt 1):382–386. | ||
Watts SW. 5-HT in systemic hypertension: foe. friend or fantasy? Clin Sci. 2005;108(5):399–412. | ||
Fetkovska N, Pletscher A, Ferracin F, Amstein R, Buhler FR. Impaired uptake of 5 hydroxytryptamine platelet in essential hypertension: clinical relevance. Cardiovasc Drugs Ther. 1990;4(Suppl 1):105–159. | ||
Ni W, Watts SW. 5-hydroxytryptamine in the cardiovascular system: focus on the serotonin transporter (SERT. Clin Exp Pharmacol Physiol. 2006;33(7):575–583. | ||
Kamal LA, Le Quan-Bui KH, Meyer P. Decreased uptake of 3H-serotonin and endogenous content of serotonin in blood platelets in hypertensive patients. Hypertension. 1984;6:568–573. | ||
Nityanand S, Tekwani BL, Chandra M, Shanker K, Singh BN. Kinetics of serotonin in platelets in essential hypertension. Life Sci. 1990;46(5):367–372. | ||
Isbister GK, Bowe SJ, Dawson A, Whyte IM. Relative toxicity of selective serotonin reuptake inhibitors (SSRIs) in overdose. J Toxicol Clin Toxicol. 2004;42(3):277–285. | ||
Ni W, Thompson JM, Northcott CA, Lookingland K, Watts SW. The serotonin transporter is present and functional in peripheral arterial smooth muscle. J Cardiovasc Pharmacol. 2004;43(6):770–8071. | ||
Ni W, Watts SW. 5-hydroxytryptamine in the cardiovascular system: focus on the serotonin transporter (SERT. Clin Exp Pharmacol Physiol. 2006;33(7):575–583. | ||
Haszon I, Papp F, Kovács J, et al. Platelet aggregation, blood viscosity and serum lipids in hypertensive and obese children. Eur J Pediatr. 2003;162(6):385–390. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.