Back to Journals » Vascular Health and Risk Management » Volume 13
Endothelial dysfunction assessment by flow-mediated dilation in a high-altitude population
Authors Calderón-Gerstein WS , López-Peña A, Macha-Ramírez R, Bruno-Huamán A , Espejo-Ramos R, Vílchez-Bravo S, Ramírez-Breña M, Damián-Mucha M, Matos-Mucha A
Received 17 September 2017
Accepted for publication 20 October 2017
Published 21 November 2017 Volume 2017:13 Pages 421—426
DOI https://doi.org/10.2147/VHRM.S151886
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Amudha Kadirvelu
Walter S Calderón-Gerstein,1,2 Antonio López-Peña,3 Raúl Macha-Ramírez,3 Astrid Bruno-Huamán,2 Roxana Espejo-Ramos,2 Stephany Vílchez-Bravo,2 María Ramírez-Breña,2 Milagros Damián-Mucha,2 Adriana Matos-Mucha2
1Department of Medicine, National Hospital Ramiro Prialé Prialé, Essalud Junín, Huancayo, Junín, Peru; 2Faculty of Medicine, Continental University, Huancayo, Junín, Peru; 3Department of Radiology, National Hospital Ramiro Prialé Prialé, Essalud Junín, Huancayo, Junín, Peru
Introduction: Endothelial function at high altitude has been measured only in populations that are genetically adapted to chronic hypoxia. The objective of this study was to evaluate endothelial dysfunction (ED) in a nongenetically adapted high-altitude population of the Andes mountains, in Huancayo, Peru (3,250 meters above sea level).
Methods: Participants included 61 patients: 28 cases and 33 controls. The cases were subjects with hypertension, diabetes mellitus, obesity, or a history of stroke or coronary artery disease. Flow-mediated vasodilation (FMD) of the brachial artery was measured in the supine position, at noon, after 5 minutes of resting. The brachial artery was identified above the elbow. Its basal diameter was measured during diastole, and FMD was tested after 5 minutes of forearm ischemia. Intima–media complex in the right carotid artery was also determined. An increase in the artery’s baseline diameter <10% indicated a positive test. Endothelium-independent vasodilation was evaluated with sublingual nitrate administration. The intima–media complex in the right carotid artery was also measured.
Results: 100% of diabetics had ED; ED was also found in 68.8% of obese individuals, 55% of hypertensive patients, and 46.5% of controls. Age, height, body mass index, and waist diameter were higher in the cases as compared with the controls. A total of 57.9% (n=11) of the cases and 45.2% (n=19) of the controls presented ED. Patients without ED had a mean increase in brachial artery diameter of 23.16%, while in those with ED it was only 3.84%. Individuals with diabetes or hypertension had a greater thickness of the carotid artery intima media layer (1.092 versus 0.664 cm) (p=0.037). A positive test for ED was associated with a greater basal diameter of the brachial artery (4.66±0.62 versus 4.23±0.59 cm) (p=0.02). A total of 7 patients presented paradoxical response, developing posthyperemia vasoconstriction.
Discussion: The proportion of ED was high among controls and among patients with risk factors. Controls showed better FMD profiles than subjects studied in Tibet and the Himalayas.
Keywords: endothelial dysfunction, vasoreactivity, brachial artery, high altitude, flow-mediated vasodilation
Introduction
The endothelium possesses an extraordinary variety of functions, such as arterial tone control, coagulation, fibrinolysis, and vascular growth.1 Impairment of these functions or “endothelial dysfunction (ED)” has been implicated as a key event in the pathogenesis of atherosclerosis, coronary vasoconstriction, and myocardial ischemia.2
Endothelial function is usually impaired after acute hypoxia exposure,3 especially in lowlanders who arrive to a high-altitude region. This physiologic feature has been measured also in the chronic scenario, but only in Tibetans and Sherpas,3,4 populations that are genetically adapted to chronic hypoxia.5
According to genetic studies,6,7 Sherpas derive from Tibetan lineage7 and both populations share a set of “high altitude adaptation” genes that give these subjects some especial characteristics that differentiate them from other populations: they have larger spirometric volumes, higher arterial oxygen saturation, more physical endurance, lower hemoglobin values, lower respiratory rate, and faster heart rate. Time seems to have been critical for genetic adaptation to high altitude: Tibetans have lived for more than 25,000 years in that environment (approximately for 1,100 generations),6 while Andeans only around 10,000 years, that is around 600 generations.8 Andeans have higher hemoglobin levels, enlarged thoracic cage, faster respiratory rate, and higher pulmonary pressures and can develop acute or chronic mountain sickness.8
Individuals regularly living at high altitude are exposed to chronic hypobaric hypoxia, a physiologic state that increases pulmonary arteriolar resistance.9 Most humans are not genetically adapted to high altitude and develop mild pulmonary hypertension, a condition that can lead to right ventricular failure and arryhthmias.
At Mantaro valley, in the Central Andes mountains, at 3,250 meters above sea level, no research has addressed this issue, despite the knowledge that chronic hypoxia modifies the endothelial response by increasing nitric oxide (NO) and nitric oxide synthase producing vasodilation.6
Even though Andeans are phenotypically adapted to high altitude, they do not share the genetic adaptation of Tibetan and Sherpas, being more representative of the physiological changes that an individual who does not live at this environment may have to endure in order to survive. If ED is higher between high-altitude dwellers, measures can be taken to prevent cardiovascular events, and if the opposite is true, more research can be done to fully disclose the underlying mechanisms of this adaptation, which may be useful for other populations.
The objective of this study was to assess the postischemic vasodilator response of the brachial artery in a nongenetically adapted high-altitude population of the Andean region, using flow-mediated dilation (FMD). FMD is currently the most frequently used method to evaluate ED, as it is a noninvasive and relatively nonexpensive method10 with a high reproducibility rate.11 The influence of age, gender, anthropometric measures, and risk factors on endothelial function in a high-altitude environment was evaluated.
Materials and methods
An analytical, case–control study was performed. The main hypothesis to be tested was that individuals with risk factors may have a higher prevalence of ED as compared with healthy individuals. The study included patients from the Internal Medicine Service of Ramiro Prialé – Essalud Hospital of Huancayo, Peru, with their respective controls who were apparently healthy individuals. Patients older than 20 years who were from Huancayo and other localities of the Mantaro Valley were studied from March 2016 to July 2016. The cases were persons who had one of the following risk factors: hypertension (HTN), diabetes mellitus (DM), obesity, or a history of stroke or coronary artery disease. The controls did not have any chronic medical condition as verified by their clinical chart, physical examination, and laboratory evaluation. Controls with dyslipidemia or nondiabetic dysglycemia were excluded.
Individuals with hypersensitivity to nitrates, pronounced hypotension, a history of recent (less than one year) myocardial infarction, marked anemia, or using sildenafil were excluded, since isosorbide use is contraindicated in such situations.
According to the International Brachial Artery Reactivity Task Force,12 a total of 20–30 individuals are needed in a crossover study in order to detect a significant increment of 1.5%–2% in FMD. A total of 61 patients, 28 cases and 33 controls, were included. The approval of the project was obtained from the Ethics and Research Committee of Essalud – Junín. The consent and the study were in accordance with the Declaration of Helsinki. All individuals provided written consent.
Clinical data were collected and physical examination performed before ED was assessed by FMD. Two physicians verified that the controls had no pathological conditions.
FMD technique
Endothelium-dependent and endothelium-independent FMD were evaluated according to current protocols.11,12,13 The patients had fasted and had not smoked or performed physical exercise at least 8 hours before the study. No exhaled carbon monoxide was measured to confirm if the subjects had not smoked because not one of them was a smoker for young females, evaluation was not done during their menstrual period.
FMD test was performed in the supine position, at a room temperature of 16°C and at noon. During the test, after 5 minutes of resting, the brachial artery was identified 5–10 cm above the elbow and measured by a Mindray DC-3 ultrasound machine with Mindray linear transducer of 7.5 Hz (Shenzhen, People’s Republic of China). After identifying the artery’s longitudinal axis, basal diameter was measured during diastole, which was determined manually through pulse oximetry plethysmography.
The sphygmomanometer cuff was inflated to 200 mmHg for a period of 5 minutes. Immediately, one minute after the cuff relaxation, the diameter of the brachial artery was measured. After a 10-minute rest, the same measurements were taken following the administration of 5 mg sublingual isosorbide dinitrate. The test was determined to be positive for ED if the dilation was less than 10% of the baseline diameter.9 Finally the intima–media complex in the right carotid artery was measured.
Statistical analysis
Data were processed with Microsoft Excel program for Windows 2013 and then analyzed with SPSS. Categorical variables were described as frequencies and percentages. For the bivariate analysis, χ2 test was employed, while continuous variables were evaluated with analysis of variance. A p-value <0.05 was considered statistically significant. Linear logistic regression was performed comparing cases and controls.
Results
A total of 61 patients, with an mean age of 60.6±16.5 years, were included; 68.9% (n=42) of them were female (Table 1). All individuals lived at Mantaro valley permanently for the last 5 years. Of them, 83.6% were born in Junín, Pasco, or Huacavelica, in cities above 3,200 meters above sea level, and 75.4% (n=46) lived in Huancayo, the capital of the department. All patients were of mestizo race, having a combination of Amerindian, Spaniard, Chinese, and African traits. Most of them (59%, n=36) were married and had obtained their high school degree (32.8%, n=20). Half of the subjects were housewives (49.2%, n=30).
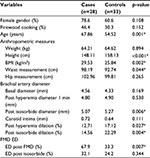  | Table 1 Comparison of patient characteristics Notes: *significant p-value. Abbreviations: BMI, body mass index; ED, endothelial dysfunction; FMD, flow-mediated dilation; SD, standard deviation. |
Overall, the medical history of the participants showed the following: a history of firewood cooking (37.7%), HTN (29.5%), obesity (27.1%) and type 2 DM (6.6%).
Evaluation of cases and controls
Cases and controls were compared (Table 1); cases were older with a mean age of 67.8 years versus 54.52 in controls. They had higher body mass index (29.53 versus 25.84 kg/m2), were shorter in height (148.1 versus 158.1 cm), and had more abdominal fat (waist circumference 98.19 versus 92.74 cm). The differences were statistically significant.
Cases also had worse responses to endothelium-dependent and endothelium-independent vasodilation: FMD post hyperemia showed an increase of 12.71% in cases and 17.12% in controls (p<0.05), while endothelium-independent vasodilation, evaluated according to the response to sublingual nitrate, displayed an increment of 22.29% in controls and only of 14.56% in cases (p<0.05).
ED was found in 67.9% (n=19) of cases and 33.3% (n=11) of controls (p<0.05).
Patients with diabetes or HTN had a greater thickness of the carotid artery intima media layer as compared to controls (1.092 versus 0.664 cm) (p=0.037). No significant difference was found between obese and controls.
Evaluation of patients with and without ED according to FMD results
The FMD test was positive in 30 patients (49.2%) who had a mean flow-mediated vasodilation of the brachial artery of 3.84±8.9%, while those with a negative test had a mean of 23.16±9.5%.
There was no difference between patients with positive and negative test regarding age (62.13±13.9 years versus 59.2±18.9 years), waist circumference (96.7 versus 93.83 cm), body mass index (27.8 versus 27.3 kg/m2), or presence of risk factors (57.9% versus 45.2%) (Table 2).
  | Table 2 Anthropometric variables in patients with ED and normal FMD results Abbreviations: BMI, body mass index; ED, endothelial dysfunction; FMD, flow-mediated dilation; SD, standard deviation. |
Thickness of the intima media was greater among patients with ED compared to those with normal results (0.9±0.2 versus 0.64±0.1 cm), although not statistically significant (p=0.147).
100% of diabetic patients had impaired endothelial function, whereas, in patients with HTN, this characteristic was found only in 55.6%, and it was present in 66.8% of obese individuals.
A positive test was associated with a greater basal diameter of the brachial artery (4.66±0.62 versus 4.23±0.59 cm) (p=0.02). Of those patients with ED, a total of 7 patients (23.3%) presented paradoxical response, developing posthyperemia vasoconstriction.
It is noteworthy that no patient without endothelium-dependent dysfunction had a positive nitrate test. A total of 56.7% (n=17) of those with endothelial-dependent dysfunction also had endothelium-independent dysfunction (p<0.01).
Discussion
ED has been implicated as a key event in the pathogenesis of atherosclerosis, coronary vasoconstriction, and myocardial ischemia. The demonstration that this condition may be reversible opens the possibility of slowing the progression of atherosclerosis, thereby reducing the risk of cardiovascular events.14
The normal endothelium produces the relaxing molecule NO as well as antioxidants, and the contracting products endothelin-1 and angiotensin.15 ED is present when there is decreased production or bioavailability of NO, a molecule responsible for the regulation of vasomotor and vascular tone.
The production of reactive oxygen species (ROS) induces an increase in vascular tone, activating the expression of cytokines, chemokines, and adhesion molecules. This change, known as endothelial activation, is a key mechanism in the development of atherosclerosis.2 Signaling mechanisms that are activated by ROS lead to the adhesion of leukocytes and platelets, leading to a localized inflammatory state, which, under normal conditions, is activated in response to an attack by microorganisms, but in this case represents a maladaptive response.16 The development of a persistent maladaptive response could be a consequence of the extension, duration, and combination of proinflammatory factors, which can lead to ED. Among these, we find risk factors such as hypercholesterolemia, hypertension, DM, and hypoxia; the latter may lead to increased production of mitochondrial ROS.2
FMD evaluates the brachial artery vasodilator response by measuring its diameter and flow using Doppler echocardiography technique with results obtained before and after transient ischemia.12,13,17 FMD is produced by the frictional pressure of the bloodstream that rushes abruptly into the vessel and is the main stimulus that triggers the release of NO by the endothelium. In the general population, the response is greater in young people and decreases progressively with age. At high altitude, the response has been evaluated only in Tibetan, Sherpas, lowlander Nepalese, Han, and Italians.3,4 Of these ethnic groups, the first two mentioned are genetically adapted to high altitude, while the remaining, as compared to Andean people, do not live in high altitude.
The proportion of patients with ED found in this study was higher in diabetics (100%) and obese individuals (68.8%) compared to other reports done on patients at sea level.18,19 The percentage of hypertensive patients with endothelial dysfunction (55.6%) was similar to that in other studies.9 In those without ED, the increase in diameter of the brachial artery was 23.16%, similar to the results of studies done at sea level, which reported rates of 19.1% and 21.9%.18,19
Insulin resistance, a condition that plays a role in the pathogenesis of obesity, diabetes, and HTN is known to decrease endothelial nitric oxygen synthase and increase endothelin-1 production.20 This abnormal physiological state, aggravated by a hypoxic environment, may lead to ED in these patients.
Special emphasis needs to be placed on the influence of age in ED. Previous studies have shown that ED increases with age.21,22 In our population, even though the age of the control group was lower than that of the case group, when comparing patients with and without ED, there was no statistically significant difference regarding age. This finding points to a more important contribution of risk factors like obesity, HTN, and diabetes to ED than age itself.
When performing FMD with isosorbide, we evaluated the response to extrinsic nitrite administration (endothelium-independent vasodilation). A total of 58.6% (n=19) of those with endothelium-dependent dysfunction failed to vasodilate after receiving isosorbide. These results indicate a great proportion of individuals with severe ED, involving both dependent and independent ED mechanisms. Moreover, 23.3% of patients with ED showed a paradoxical vasoconstrictor response. According to Nguyen et al,23 the presence of flow-mediated paradoxical vasoconstriction has 61.9% sensitivity and 65.6% specificity to identify asymptomatic coronary artery disease in diabetics.
Most patients had an adequate response to isosorbide dinitrate, even though the oral bioavailability of this drug fluctuates between 40% and 50%24 and, according to Straehl,25 it can be only 29%. Sublingual nitroglycerine usually acts within 1.9 minutes; isosorbide needs 3.4 minutes at least.24 For that reason, the protocol considered a 10-minute rest before procedure
On the other hand, those healthy individuals with no risk factors fared better than their counterparts in other high-altitude studies: FMD increased in 6.7% in Sherpas and 7.9% in Nepalese lowlanders,3 and 6.44% in Italians,4 while healthy Andeans in this study showed an increment of 15.42%. Sublingual nitrate increased FMD in 19.19%, compared to 14.5% in Sherpas and 16.6% in Nepalese.3 Increase in FMD in Andean patients with risk factors was similar to healthy Sherpas, at 13.99%.
Our study had some limitations. Both groups were not matched for gender, and the population with risk factors included few diabetic patients. FMD cutoff of 10% allowed us to compare our results with those of Latino-american origin, but not with other studies that do not use cutoffs3,4 or use other distinctive values.12
Conclusion
The rate of ED demonstrated by FMD was high among controls and patients with risk factors, especially in obese and diabetic patients. Notwithstanding, Andean individuals with no risk factors had higher FMD increments as compared to genetically adapted subjects and subjects who live at sea level in other countries.
Acknowledgments
The authors thank Dr. Juan Carlos de la Cruz Rocha for his support in the evaluation of patients and students Leticia Ribbeck, Betina Zárate-Rodríguez, and Fany Ugarte for their help in collecting data.
Disclosure
The authors report no conflicts of interest in this work.
References
Cines DB, Pollak ES, Buck CK, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. | ||
Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285–1295. | ||
Lewis NCS, Bain AR, Wildfong KW, Green DJ, Ainslie PN. Acute hypoxemia and vascular function in healthy humans. Exp Physiol. Epub 2017 Sep 13. | ||
Bruno RM, Cogo A, Ghiadoni L, et al. Cardiovascular function in healthy himalayan high-altitude dwellers. Atherosclerosis. 2014;236:47–53. | ||
Lewis NC, Bailey DM, duManoir GR, et al. Conduit artery structure and function in lowlanders and native highlanders: relationships with oxidative stress and role of sympathoexcitation. J Physiol. 2014;592:1009–1024. | ||
Wang B, Zhang YB, Zhang F, et al. On the origin of tibetans and their genetic basis in adapting high-altitude environments. PLoS One. 2011;6(2):e17002. | ||
Bhandari S, Zhang X, Cui C, et al. Sherpas share genetic variations with Tibetans for high-altitude adaptation. Mol Genet Genomic Med. 2017;5(1):76–84. Available from: http://doi.org/10.1002/mgg3.264. | ||
Rupert JL, Hochachka PW. Genetic approaches to understanding human adaptation to altitude in the Andes. J Exp Biol. 2001;204(Pt 18):3151–3160. | ||
Maggiorini M, Leon-Velarde F. High-altitude pulmonary hypertension: a pathophysiological entity to different diseases. Eur Respir J. 2003;22:1019–1025. | ||
Maruhashi T, Kajikawa M, Nakashima A, et al. Nitroglycerine-induced vasodilation in coronary and brachial arteries in patients with suspected coronary artery disease. Int J Cardiol. 2016;219:312–316. | ||
Meirelles Cde M, Leite SP, Montenegro CA, Gomes PS. Reliability of brachial artery flow-mediated dilatation measurement using ultrasound. Arq Bras Cardiol. 2007;89(3):160–167, 176–183. | ||
Corretti MC, Anderson TJ, Benjamin EJ, et al; International Brachial Artery Reactivity Task Force. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39(2):257–265. | ||
Thijssen DH, Black MA, Pyke KE, et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2011;300(1):H2–H12. | ||
Gibbons GH, Dzau VJ. Molecular therapies for vascular diseases. Science. 1996;272:689–693. | ||
Szmitko PE, Wang CH, Weisel RD, de Almeida JR, Anderson TJ, Verma S. New markers of inflammation and endothelial cell activation: Part I. Circulation. 2003;108(16):1917–1923. | ||
Nilsson J, Hansson GK. Autoimmunity in atherosclerosis: a protective response losing control? J Intern Med. 2008;263:464–478. | ||
Bruno RM, Bianchini E, Faita F, Taddei S, Ghiadoni L. Intima media thickness, pulse wave velocity, and flow mediated dilation. Cardiovasc Ultrasound. 2014;12:34. | ||
Vilariño JL, Cacharon JL, Suarez DH, et al. Evaluación de la función endotelial por ecodoppler. Influencia de la edad, sexo y factores de riesgo. Rev Argent Cardiol. 1998;66(5):523–532. | ||
Navarta DAC, Albarrazin KS, Trejo G, et al. Evaluación de la disfunción endotelial en pacientes ambulatorios mayores de 75 años atendidos en un hospital de la tercera edad. Insuf Card. 2013;8(3):119–124. | ||
Potenza MA, Marasciulo FL, Chieppa DM, et al. Insulin resistance in spontaneously hypertensive rats is associated with endothelial dysfunction characterized by imbalance between NO and ET-1 production. Am J Physiol Heart Circ Physiol. 2005;289:H813–H822. | ||
Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–476. | ||
Parker BA, Ridout SJ, Proctor DN. Age and flow-mediated dilation: a comparison of dilatory responsiveness in the brachial and popliteal arteries. Am J Physiol Heart Circ Physiol. 2006;291(6):H3043–H3049. | ||
Nguyen MT, Pham I, Valensi P, et al. Flow-mediated-paradoxical vasoconstriction is independently associated with asymptomatic myocardial ischemia and coronary artery disease in type 2 diabetic patients. Cardiovasc Diabetol. 2014;13:20. | ||
Isordil (Isosorbide dinitrate, sublingual Tablets): FDA Professional Drug Information. Available from: https://www.drugs.com/pro/isordil.html | ||
Straehl P, Galeazzi RL. Isosorbide dinitrate bioavailability, kinetics, and metabolism. Clin Pharmacol Ther. 1985;38(2):140–149. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
