Back to Journals » International Medical Case Reports Journal » Volume 12
Endoscopic resection of an intraventricular cavernoma: a case report
Authors Fehrenbach MK, Kuzman P, Quaeschling U, Meixensberger J, Nestler U
Received 8 May 2019
Accepted for publication 9 July 2019
Published 6 August 2019 Volume 2019:12 Pages 249—252
DOI https://doi.org/10.2147/IMCRJ.S214917
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ronald Prineas
MK Fehrenbach,1 P Kuzman,2 U Quaeschling,3 J Meixensberger,1 U Nestler1
1Department of Neurosurgery, University Clinic of Leipzig, Leipzig 04103, Germany; 2Department of Neuropathology, University Clinic of Leipzig, Leipzig 04103, Germany; 3Department of Neuroradiology, University Clinic of Leipzig, Leipzig 04103, Germany
Abstract: Cerebral cavernous malformations occur in 0.5% of the population. They consist of thin-walled vessels and can be found as congenital or sporadic lesions. Most of them are asymptomatic, however, due to their anatomical features blood leakage into the surrounding tissue can cause severe neurological symptoms. Although risk of bleeding is low, symptomatic lesions should be treated, with microsurgical resection being the therapy of choice for surgically accessible cavernomas. Intraventricular cavernous malformations are a rare subtype, and due to their anatomical localization, they are eligible for endoscopic surgery. However, there are only a few reports on endoscopic resection of intraventricular cavernomas to be found in the literature. We report the case of a 48-year-old woman who suffers from multiple cerebral cavernous malformations. Since the first diagnosis, several of these cavernomas had been removed in open microsurgical interventions. Most recently, a new lesion arose intraventricularly, adjacent to the ependymal wall of the right lateral ventricle. In follow-up, cranial MR imaging microbleeding and an increasing size were detected. Eventually, the lesion was endoscopically removed. Presurgery the patient suffered from right-sided sensibility loss and gait disturbances as a consequence of prior surgeries. Postsurgery, no new neurological symptoms could be found. We here present MR images and intraoperative pictures as well as a short video of the resection itself. In our opinion, endoscopic resection of intraventricular cavernomas should be considered in selected cases.
Keywords: cerebral cavernous malformation, endoscopic resection
Introduction
According to the latest International Society for the Study of Vascular Anomalies, classification cerebral cavernous malformations, also referred to as cavernomas, cavernous hemangiomas or cavernous angiomas are defined as simple vascular malformations in the subgroup of venous malformations.1 They are present in approximately 0.5% of the population while most of them remain asymptomatic. Cavernomas found in the central nervous system may cause headaches, seizures, stroke, hydrocephalus or focal neurological symptoms. Cavernomas are usually small (2 mm to few centimeters) and are composed of thin-walled, dilated capillaries that lack elastic fibers and smooth muscles.2 Most lesions are considered to be congenital; however, they can also form sporadically or de novo after brain surgery or radiotherapy.3 Three gene mutations associated with the formation of cavernomas have been reported (CCM1, CCM2 and CCM3).4
While asymptomatic cavernomas are usually observed, symptomatic lesions should be removed by microsurgical resection, if the lesions are surgically accessible.5 Symptomatic lesions which are not surgically accessible may be treated with stereotactic radiosurgery. Despite the fact that radiation decreases the risk of bleeding, radiation-induced permanent neurological defects are considerably higher in this patient group.6 More recent studies showed better long-term neurological outcome in patients after radiosurgery, compared to the natural course with cumulative morbidity due to repeated hemorrhages.7,8 Of note, recent reports suggest a positive effect on recurrent bleeding and development of new lesions for propranolol.9
Intraventricular cavernous malformations are a rare subgroup. They constitute 2.5–10.8% of all cerebral cavernomas.10 Intraventricular cavernomas reportedly have a higher tendency for bleeding and re-bleeding, which may result in the development of an obstructive hydrocephalus. Due to their anatomical position, intraventricular cavernomas are suitable for endoscopic surgery, though the risk of intraoperative bleeding has to be considered, eventually obstructing the endoscopic view and necessitating a switch to open microscopic neurosurgery.
Case report
We present the case of a 48-year-old woman with a known cerebral cavernomatosis combined with epilepsy. It is known that her mother was diagnosed with cerebral cavernous malformation as an incidental finding in a cranial MR imaging, yet our patient refused genetic testing. She suffers from multiple cerebral cavernous malformations (more than 15) and underwent microsurgical removal of symptomatic lesions four times in the course of the last 18 years. Three of these were located in the left hemisphere, the fourth one had provoked a left-sided cerebellar hemorrhage 12 years ago. A hysterectomy for hypermenorrhagia was performed 4 years ago. The patient receives levothyroxine substitution and lamotrigine. Although advised otherwise, she continues to smoke cigarettes.
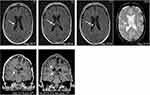
In follow-up cranial MR imaging, a cavernous malformation located in the right lateral ventricle had occurred about two years ago and demonstrated not only an increasing size but also microbleeding (Figure 1, upper row). The proposition was made to remove this lesion endoscopically via a frontal burr hole guided by neuronavigation to spare the patient a fifth trepanation.
The patient remained reluctant and, only after a further 6-month MRI control depicting a still increasing size of the intraventricular cavernoma, finally consented to the neurosurgical intervention. The operation and postoperative period were uneventful. Histologic examination showed an atypical dilatated blood vessel with signs of recurrent hemorrhage, in the given clinical context compatible with parts of a cavernous hemangioma. Postoperative MR imaging demonstrated a complete resection (Figure 1, lower row). The patient could be discharged 4 days after surgery. Neurologic examination revealed right-sided sensibility loss and gait disturbances as sequelae of the former four neurosurgical interventions. The symptoms remained unchanged after the endoscopy, without the occurrence of further deficits or an epileptic seizure. Postsurgery wound pain relieved quickly. Noteworthily, neither the de novo occurrence of the cavernous malformation nor the microbleeding caused any new neurological symptoms.
Total operation time was 2 hrs 18 mins including the positioning of the patient in supine position, setting up the neuronavigation and the endoscope. The time needed for resection (skin to skin) of the lesion was 1 hr 15 mins. For surgical access, Kocher’s point was chosen. A small, approx. 3-cm-long incision was made and a single burr hole with a diameter of 1 cm was placed. The dura was opened with a small incision and the endoscope was carefully advanced toward the lesion following the trajectory of the neuronavigation. Video 1 gives an impression of the endoscopic view, displaying the use of electrocautery to coagulate the attachment of the cavernoma to the lateral thalamic wall and the use of the forceps to remove the lesion in one piece by seizing the thinned and coagulated stalk.
Written informed consent has been provided by the patient to have the case details and any accompanying images published. No institutional approval was required to publish this case report.
Discussion
In our limited experience with endoscopic resection of cavernous malformation, we can report that this operation method represents a good alternative to open surgery in selected cases. The surgical access is considerably smaller, causing less trauma to the surrounding brain tissue and the total operation time is notably shorter. There is no craniotomy needed, a simple burr hole is sufficient. We can, therefore, confirm the findings of Gianetti et al, Palandri et al and Prat et al, who had reported successful endoscopic resection of intraventricular cavernous malformations.11–13
However, it must be noted that intraoperative complications, especially bleeding, are more difficult to control, as not only the freedom of movement is very limited in endoscopical procedures but also the view can more easily be blocked. Patients should therefore carefully be selected considering the position and the size of the cavernoma, and adequate preoperative planning including possible switching to an open access has to be performed.
The endoscopic approach is limited to intraventricular cavernous malformations, as intraparenchymal lesions are not accessible with this technique. Although technical advantages have been made in recent years, endoscopic neurosurgical approaches still require preformed cavities as the ventricular system or large cysts found in complex hydrocephalus.14 In our opinion, endoscopic resection should at least be considered as a surgical approach for intraventricular cavernous malformations within the lateral ventricles, the interventricular foramina and to some extent the third ventricle. It should be noted that large lesions may be difficult to handle as they more easily block the view of the endoscope.
Acknowledgment
We acknowledge support from the German Research Foundation (DFG) and Universität Leipzig within the program of Open Access Publishing.
Disclosure
The authors report no conflicts of interest in this work.
References
1. ISSVA: Classification of Vascular Anomalies. ©2018 International Society for the Study of Vascular Anomalies “issvaorg/classification”; 2018.
2. Raychaudhuri R, Batjer HH, Awad IA. Intracranial cavernous angioma: a practical review of clinical and biological aspects. Surg Neurol. 2005;63:319–328. discussion 328. doi:10.1016/j.surneu.2004.05.032
3. Fritschi JA, Reulen HJ, Spetzler RF, Zabramski JM. Cavernous malformations of the brain stem. A review of 139 cases. Acta Neurochir (Wien). 1994;130:35–46.
4. Mouchtouris N, Chalouhi N, Chitale A, et al. Management of cerebral cavernous malformations: from diagnosis to treatment. ScientificWorldJournal. 2015;2015:808314. doi:10.1155/2015/808314
5. Fontanella M, Bacigaluppi S. Treatment of cerebral cavernous malformations: where do we stand? J Neurosurg Sci. 2015;59:199–200.
6. Porter RW, Detwiler PW, Han PP, Spetzler RF. Stereotactic radiosurgery for cavernous malformations: kjellberg’s experience with proton beam therapy in 98 cases at the harvard cyclotron. Neurosurgery. 1999;44:424–425. doi:10.1097/00006123-199902000-00125
7. Nagy G, Burkitt W, Stokes SS, et al. Contemporary radiosurgery of cerebral cavernous malformations: part 1. Treatment outcome for critically located hemorrhagic lesions. J Neurosurg. 2018;1:1–9.
8. Nagy G, Stokes SS, Eross LG, et al. Contemporary radiosurgery of cerebral cavernous malformations: part 2. Treatment outcome for hemispheric lesions. J Neurosurg. 2018;1:1–9.
9. Reinhard M, Schuchardt F, Meckel S, et al. Propranolol stops progressive multiple cerebral cavernoma in an adult patient. J Neurol Sci. 2016;367:15–17. doi:10.1016/j.jns.2016.04.053
10. Kivelev J, Niemela M, Kivisaari R, Hernesniemi J. Intraventricular cerebral cavernomas: a series of 12 patients and review of the literature. J Neurosurg. 2010;112:140–149. doi:10.3171/2009.3.JNS081693
11. Giannetti AV. Purely neuroendoscopic resection of an intraventricular cavernous angioma: case report. J Neurol Surg A Cent Eur Neurosurg. 2013;74:47–50. doi:10.1055/s-0032-1325632
12. Palandri G, Sorenson T, Zucchelli M, Acciarri N, Mantovani P, Sturiale C. Endoscopic resection of hemorrhaged third ventricle cavernous malformation: 2-dimensional operative video. Oper Neurosurg (Hagerstown). 2018;16(2):E51–E51.
13. Prat R, Galeano I. Endoscopic resection of cavernoma of foramen of monro in a patient with familial multiple cavernomatosis. Clin Neurol Neurosurg. 2008;110:834–837. doi:10.1016/j.clineuro.2008.05.011
14. Stolzenburg JU, Do M, Pfeiffer H, Konig F, Aedtner B, Dorschner W. The endoscopic extraperitoneal radical prostatectomy (EERPE): technique and initial experience. World J Urol. 2002;20:48–55.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.