Back to Journals » Journal of Pain Research » Volume 9
Endogenous inhibition of pain and spinal nociception in women with premenstrual dysphoric disorder
Authors Palit S, Bartley E, Kuhn B, Kerr K, DelVentura J, Terry E, Rhudy J
Received 25 September 2015
Accepted for publication 14 December 2015
Published 11 February 2016 Volume 2016:9 Pages 57—66
DOI https://doi.org/10.2147/JPR.S97109
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael Schatman
Shreela Palit,1 Emily J Bartley,2 Bethany L Kuhn,1 Kara L Kerr,1 Jennifer L DelVentura,1 Ellen L Terry,1 Jamie L Rhudy1
1Department of Psychology, University of Tulsa, Tulsa, OK, USA; 2Department of Community Dentistry and Behavioral Science, Pain Research and Intervention Center of Excellence, University of Florida, Gainesville, FL, USA
Purpose: Premenstrual dysphoric disorder (PMDD) is characterized by severe affective and physical symptoms, such as increased pain, during the late-luteal phase of the menstrual cycle. The mechanisms underlying hyperalgesia in women with PMDD have yet to be identified, and supraspinal pain modulation has yet to be examined in this population. The present study assessed endogenous pain inhibitory processing by examining conditioned pain modulation (CPM, a painful conditioning stimulus inhibiting pain evoked by a test stimulus at a distal body site) of pain and the nociceptive flexion reflex (NFR, a spinally-mediated withdrawal reflex) during the mid-follicular, ovulatory, and late-luteal phases of the menstrual cycle.
Methods: Participants were regularly-cycling women (14 without PMDD; 14 with PMDD). CPM was assessed by delivering electrocutaneous test stimuli to the sural nerve before, during, and after a painful conditioning ischemia task. Participants rated their pain to electrocutaneous stimuli, and NFR magnitudes were measured. A linear mixed model analysis was used to assess the influence of group and menstrual phase on CPM.
Results: Compared with controls, women with PMDD experienced greater pain during the late-luteal phase and enhanced spinal nociception during the ovulation phase, both of which were independent of CPM. Both groups showed CPM inhibition of pain that did not differ by menstrual phase. Only women with PMDD evidenced CPM inhibition of NFR.
Conclusion: Endogenous modulation of pain and spinal nociception is not disrupted in women with PMDD. Additionally, greater NFR magnitudes during ovulation in PMDD may be due to tonically-engaged descending mechanisms that facilitate spinal nociception, leading to enhanced pain during the premenstrual phase.
Keywords: premenstrual dysphoric disorder, menstrual cycle, pain, conditioned pain modulation, nociception
Introduction
An estimated 3%–8% of women suffer from premenstrual dysphoric disorder (PMDD), a cyclical syndrome associated with severe affective (eg, depression, irritability, emotional lability), physical (eg, musculoskeletal pain, weight gain, headaches), and behavioral (eg, lethargy, appetite change) symptoms that result in significant functional impairment during the late-luteal phase of the menstrual cycle. Considerable burden is associated with PMDD. Its impact on physical and emotional quality of life is similar to that associated with other chronic medical conditions (ie, osteoarthritis, rheumatoid arthritis, hypertension, type 2 diabetes), and may even surpass the impact of chronic back pain.1 Additionally, interpersonal functioning is often compromised and substantial work impairment is prevalent in the days preceding menses onset.2,3
Evidence suggests that among other somatic symptoms, women with PMDD experience heightened premenstrual pain, including headaches4 and musculoskeletal pain.5 This increase in pain has been found to lead to significant distress, impaired functioning, and disturbed quality of life in women with PMDD.5,6 Although pain is a significant symptom in PMDD,6 only five published studies have used experimental pain to assess pain processing in PMDD.7–11 Kuczmierczyk et al7 did not find any differences in pressure pain threshold or tolerance in healthy women vs those with severe premenstrual symptoms across the intermenstrual (days 7–22) and premenstrual (days 24–28) phases of the menstrual cycle; however, the premenstrual symptoms group reported higher overall pain ratings to pressure stimuli when compared with the healthy group. Fillingim et al8 extended this work to assess thermal and ischemia pain thresholds and tolerances in women with and without PMDD. Although they did not find any group differences in thermal pain outcomes, the PMDD group exhibited lower ischemia pain thresholds and tolerances, as well as higher ischemia pain ratings. However, it is important to note that these authors only tested participants during the luteal phase. Straneva et al9 assessed ischemia pain during the follicular (days 4–9) and luteal (8–12 days post-luteinizing hormone [LH] surge) phases and found that, compared to healthy women, the PMDD group had lower pain thresholds and tolerances across phases. More recently, Klatzkin et al10 assessed ischemia pain threshold/tolerance and cold pressor pain, but only during the luteal (5–12 days post-LH surge) phase in PMDD and healthy women. No significant differences were found across groups in regard to pain sensitivity, but PMDD women with a history of major depressive disorder reported greater unpleasantness in response to cold pressor pain. Bartley et al11 examined pain sensitivity in PMDD across the mid-follicular (days 5–8), ovulatory, and late-luteal phases (9–11 days post-LH surge) and found that, when compared to healthy women, those with PMDD reported higher sensory and affective pain ratings of electrocutaneous stimuli, as well as lower ischemic pain thresholds. Although these studies suggest potential differences in pain processing in PMDD, it is unclear what mechanisms underlie these deficits.
Abnormalities in the ability to regulate pain and nociception may increase risk for clinical pain;12,13 therefore, this could account for enhanced pain symptomatology and sensitivity in PMDD. One factor that may account for enhanced clinical pain and pain sensitivity in PMDD is through abnormalities in endogenous pain inhibitory processes. Conditioned pain modulation (CPM) is a method that experimentally assesses endogenous pain inhibition. CPM involves the application of a tonic painful stimulus (conditioning stimulus) to inhibit pain evoked by a phasic, test stimulus at a distal body site. CPM has been studied extensively in both healthy and clinical populations, but only three known studies have assessed CPM across the menstrual cycle, and all of the studies were of healthy women.14–16 Their findings suggest that CPM inhibition of pain occurs across all menstrual cycle phases, but may be greater during ovulation compared to follicular and luteal phases.14,15 Our laboratory was the first to show that CPM inhibition of the nociceptive flexion reflex (NFR, a spinally-mediated withdrawal reflex used as a physiological correlate of spinal nociception) does not vary across mid-follicular, ovulatory, and late-luteal phases of the menstrual cycle, suggesting that descending brain-to-spinal cord modulation of spinal nociception may not differ across these menstrual phases in healthy women.16 However, there have been no investigations assessing CPM in PMDD across the menstrual cycle. Such an investigation could identify the mechanisms that contribute to pain in PMDD (eg, failure of endogenous inhibition).
The purpose of the present study was to determine whether endogenous inhibition of pain and NFR varies across the mid-follicular, ovulatory, and late-luteal phases of the menstrual cycle in women with PMDD. Results were compared to a matched group of healthy women without PMDD. At each menstrual phase, pain ratings and NFRs in response to electrocutaneous stimuli were measured before, during, and after a painful ischemia task. In healthy women, pain and NFR should be inhibited during ischemia relative to pre-ischemia. In contrast, women with PMDD experience enhanced pain which may result from variation in endogenous inhibition, and we therefore hypothesized that women with PMDD would report greater pain and show deficits in CPM inhibition of pain and NFR. Although aspects of this study have been published elsewhere,11,17 these data are novel.
Methods
Participants
Healthy women with PMDD and matched healthy controls (HC) without PMDD were recruited from the community via fliers, newspaper/radio advertisements, and online postings. All participants provided verbal and written informed consent. Participants were excluded for: <18 years of age; menopausal or postmenopausal; irregular menstrual cycle; use of hormone preparations in the last 6 months; pregnant or trying to get pregnant; pregnant or breastfeeding in the last 6 months; history of tubal ligation; polycystic ovarian syndrome; endometriosis; body mass index >35; history of serious cardiovascular, neuroendocrine, or neurological disorders; hypertension; history of chronic pain; current opioid, antidepressant, or anxiolytic medication use; recent psychological trauma as defined by Diagnostic and Statistical Manual of Mental Disorders, 4th Edition Text Revision (DSM-IV-TR);18 or current psychiatric condition (other than PMDD) as determined by a structured clinical interview (Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition).19 Participants received an honorarium of up to US$375 for participation in the study. Finally, 14 women with PMDD and 14 matched HC women were recruited and contributed to data analysis. These sample sizes were determined to be adequate based on our prior studies of group differences in modulation of pain and NFR that used similar sample sizes,20,21 but also because the current study design was three times more statistically powerful than these previous studies given that CPM was measured during three different menstrual phases.
Procedure
This study and all procedures were fully approved by the University of Tulsa Institutional Review Board. Potentially eligible participants attended an initial laboratory session during which an experimenter provided a thorough overview of the experiment, obtained informed consent, and conducted an assessment of inclusion/exclusion criteria. If deemed eligible to participate, participants were given instructions on menstrual cycle monitoring and then randomly assigned to one of six testing orders: 1) mid-follicular – ovulatory – late-luteal; 2) mid-follicular – late-luteal – ovulatory; 3) ovulatory – mid-follicular – late-luteal; 4) ovulatory – late-luteal – mid-follicular; 5) late-luteal – mid-follicular – ovulatory; 6) late-luteal – ovulatory – mid-follicular. Participants then tracked their menstrual phases for three cycles. Cycle 1 was used to establish cycle length and ovulation timing. Experimental testing occurred during cycles 2 and 3 (with the exception of participants assigned to a luteal/ovulatory/follicular testing order, for whom the first pain testing session occurred during cycle 1).
Upon arrival at the laboratory for the first experimental testing session, a complete overview of the procedures was provided, followed by a review of informed consent and health status. Participants were then instructed on the use of the Numerical Rating Scale (NRS) to rate pain. Next, sensors were applied to participants for physiological recording. The procedures in the testing session proceeded in the following order: NFR threshold testing, electrocutaneous pain threshold assessment, emotional controls of nociception (ECON), and CPM. ECON data are reported elsewhere.17 At the end of the session, participants were reminded to continue tracking their menstrual phases until all three cycles (and all three testing sessions) were completed.
Menstrual cycle monitoring and phase determination
The Prospective Record of the Impact and Severity of Menstrual Symptoms (PRISM)22 calendar was used to record daily symptoms. The PRISM calendar contains affective (eg, depression), behavioral (eg, insomnia), and physical (eg, breast tenderness) symptoms that are rated daily for severity (absent, mild, moderate, severe). The PRISM calendar also includes measures of lifestyle impact to assess functional impairment (eg, neglected housework, time off from work). Participants completed the calendars daily for three menstrual cycles and were asked to mail in calendars on a weekly basis to discourage retrospective reporting. The mid-follicular phase was defined as days 5–8 following menses onset and the late-luteal phase was defined as days 1–6 prior to menses (approximately 9–11 days following the LH surge that triggers ovulation). Verification of ovulation was obtained from an LH surge assessed from home-administered urine test kits, (ie, Clearblue© Easy). Positive LH tests were dated and retained for verification of results by an experimenter. Participants attended ovulatory phase testing sessions within 24–48 hours of receiving a positive ovulation test result. In addition, phases were verified via salivary hormone levels (ie, estradiol and progesterone).
Determination of PMDD diagnosis
PMDD diagnosis was determined prospectively from PRISM calendars. The DSM-IV-TR18 defines the diagnostic criteria for PMDD as having ≥5 of the following eleven symptoms present during the late-luteal phase: 1) depressed mood, hopelessness, or self-deprecating thoughts; 2) anxiety, tension; 3) affective lability; 4) persistent and marked anger or irritability or increased interpersonal conflicts; 5) decreased interest in usual activities; 6) difficulty concentrating; 7) lethargy, easy fatigability, or marked lack of energy; 8) change in appetite, overeating, or specific food cravings; 9) hypersomnia or insomnia; 10) overwhelmed or feeling out of control; or 11) physical symptoms. Symptoms must significantly interfere with work, school, or usual social activities and relationships with others, not be due to another disorder, and at least one symptom must be mood related. Further, PMDD must be prospectively confirmed from two consecutive symptomatic cycles. All participants with PMDD met the aforementioned criteria. However, due to the brief nature of the study requirements (ie, completion of only three cycle calendars), participants were said to have PMDD as long as they met criteria for at least two of the three monitored cycles even if they were nonconsecutive. Control women did not meet criteria for PMDD and experienced only mild affective symptoms during the late-luteal phase (premenstrual) days, and moderate physical symptoms on fewer than 3 premenstrual days.9
Apparatus, electrode application, and signal acquisition
All testing procedures were completed in an electrically-shielded and sound-attenuated experiment room. Experimenters monitored participants from an adjacent room via a 17″ flat panel monitor connected to a video camera with a microphone. All data acquisition, as well as stimuli and questionnaire presentation, was controlled by a personal computer equipped with dual monitors and analog to digital converter board, as well as LabVIEW software (National Instruments, Austin, TX, USA). One video output from the computer was used to present questionnaires to the participant, while a second video output was displayed on a second computer for monitoring of physiological signals and experimental timing. Electrocutaneous stimuli were delivered to the left ankle over the retromalleolar pathway of the sural nerve using a Digitimer stimulator (DS7A; Digitimer, Hertfordshire, England) and a Nicolet bipolar stimulating electrode (019-401400; Nicolet, Madison, WI, USA). Stimulation timing was controlled by computer (maximum stimulation intensity =50 mA). A Lafayette Instrument Hand Dynamometer (models 78010 and 78011; Lafayette, IN, USA) and Prestige Medical blood pressure cuff (Northridge, CA, USA) were used to evoke forearm ischemia.
To apply electromyographic (EMG) and stimulating electrodes, the skin was first cleaned with alcohol and exfoliated using Nuprep gel (Weaver and Company, Aurora, CO, USA) until impedances below 5 kΩ were achieved. Electrodes were then filled with conductive gel (EC60; Grass Technologies, West Warwick, RI, USA) before being applied to the skin. For NFR recording, two electrodes were placed over the ipsilateral biceps femoris muscle of the left leg 10 cm superior to the popliteal fossa, and a common reference electrode was placed over the lateral epicondyle of the femur. All physiological signals were recorded using a Grass Technologies amplifier. Biceps femoris EMG was sampled at 1,000 Hz, amplified (×10,000), bandpass filtered (10–300 Hz), and rectified.
Pain outcomes
Ratings of electrocutaneous stimuli
Following each electric stimulus, participants rated their experience using a computer-presented NRS. The scale ranged from 0 to 100 with the following labels: 0 (no sensation), 1 (just noticeable), 25 (uncomfortable), 50 (painful), 75 (very painful), and 100 (maximum tolerable). Participants responded by using a computer mouse to move an indicator along the scale to make their ratings.
NFR threshold and magnitude assessment
The NFR is a spinally-mediated withdrawal reflex primarily elicited by Aδ pain fiber activation following noxious stimulation. Electric stimuli delivered during CPM testing were set at either 120% NFR threshold or 120% pain threshold (whichever value was greater). NFR and pain thresholds were therefore assessed prior to CPM testing. To measure NFR threshold, three ascending–descending staircases of electric stimulations were delivered to the sural nerve. Trains of five 1 ms rectangular wave pulses at 250 Hz (ie, 3 ms interpulse interval) were delivered with a varying inter-train interval of 8–12 seconds to reduce stimulus predictability. The first train started at 0 mA (current) and was increased in steps of 2 mA until a reflex was observed. The stimulus intensity was then decreased in 1 mA steps until a reflex was no longer detected. This ascending–descending staircase process was repeated two more times, but with the use of 1 mA steps. NFR threshold was defined as the average stimulation intensity (in mA) of the last two peaks and troughs of the ascending–descending staircase procedure.
During CPM testing, NFR magnitude was used as a physiological measure of spinal nociception because the size of the reflex correlates with subjective pain intensity, as well as activity of nociceptive dorsal horn neurons.23,24 The NFR was defined as a mean biceps femoris EMG response in the 90–150 ms post-stimulus interval that exceeded mean EMG activity during the 60 ms pre-stimulus baseline interval by 1.4 standard deviations (SDs).25,26 NFR magnitude was converted to Cohen’s d units (d= [mean EMG of 90–150 ms post-stimulation interval – mean EMG of 60 ms pre-stimulation interval]/average SD of EMG from pre- and post-stimulation intervals). This method was chosen because it has previously been shown to produce a stronger correlation with pain report (ie, external validity coefficient) than other NFR scoring methods (eg, mean EMG).27,28
Electrocutaneous pain threshold assessment
Pain threshold was assessed using three ascending– descending staircases of electric stimuli. Stimulus parameters and interstimulus intervals were the same as during NFR testing. Participants rated their pain intensity on the NRS immediately following each stimulus. Stimulation intensity of the first ascending–descending staircase started at 0 mA and increased in 4 mA steps until the participant reached pain threshold (rating ≥50). The stimulation intensity was then decreased in 2 mA steps until a stimulus was rated ≤25. The second and third ascending–descending staircases continued with 2 mA steps. Pain threshold was defined as the average stimulation intensity (mA) of the four stimuli immediately above and immediately below a rating of 50 on the last two ascending–descending staircases.
CPM of pain and NFR
CPM involves the application of a tonic, painful, conditioning stimulus to dampen pain evoked at a distal (heterotopic) body site by a painful test stimulus. For the present study, forearm ischemia was used as the conditioning stimulus and electrocutaneous stimulation of the ankle was used as the test stimulus. Ischemia pain was evoked by having participants conduct hand exercises using a dynamometer at 50% of their maximal effort at a rate of one compression per second for 2 minutes. The arm was then elevated for 15 seconds for exsanguination. After this, blood flow was occluded to the forearm by placing a blood pressure cuff around the biceps of the arm and inflating it to 220 mm/Hg for 2 minutes. For the test stimulus, suprathreshold electric stimuli were delivered to the sural nerve at the left ankle with a 15–25-second variable interstimulus interval. CPM was assessed by delivering four painful electrocutaneous stimulations during the 2 minutes prior to ischemia (baseline), four during the 2 minutes of ischemia, and four during the 2 minutes after (post) the ischemia procedure (12 stimulations in total). The NRS was administered following each stimulus to assess pain. Biceps femoris EMG was recorded throughout the procedure to assess NFR magnitudes. Following the CPM procedure, participants used the NRS to rate the overall pain in response to the blood pressure cuff on their arm (ie, forearm ischemia pain).
Statistical analyses
For all analyses, alpha level was set at P<0.05 (two-tailed). To analyze group differences in background variables (eg, age, education, body mass index), independent samples Student’s t-tests were conducted and Levene’s test was performed to test the assumption of homogeneity of variance. If this assumption was violated, degrees of freedom were adjusted. Chi-square analyses were conducted to analyze group differences in categorical variables (eg, ethnicity, marital status, and employment).
Given that testing involved multiple data points, the SPSS MIXED procedure (ie, a linear mixed model analysis) was used to maximize statistical power in analyses of ischemia ratings, CPM of pain, and CPM of NFR. Data were arranged in long form (ie, each participant contributed up to 36 rows of data; 12 CPM stimuli per menstrual cycle phase ×3 phases). In these mixed models, maximum likelihood estimation was used and the repeated measures covariance was modeled by an autoregressive (AR1) structure. Diagnosis (PMDD vs HC), menstrual phase (mid-follicular, ovulatory, and late-luteal), and CPM phase (baseline, ischemia, post-ischemia) were entered as independent variables. It is important to note that for there to be group differences in CPM of pain or NFR, a significant interaction must contain the CPM phase independent variable. To control for habituation of NFR and sensitization of pain ratings within each CPM phase, the order of the four stimulations within each phase was entered as a continuous variable called “stimulation number”. This provided more power in the models to detect CPM effects by removing variance due to habituation/sensitization that would normally be attributed to the error terms of the models.
Results
Participant characteristics
Means, SDs, and Student’s t-test results for group characteristics are reported in Table 1. In general, participants were employed, single, non-Hispanic, and had approximately 15 years of education. There were no group differences in any of the demographic or clinical variables.
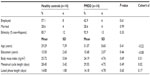  | Table 1 Participant characteristics by group |
Salivary hormone verification
Results of the salivary hormone tests to verify menstrual phase are reported in detail elsewhere.11 In sum, estradiol and progesterone levels indicated that experimental sessions were timed appropriately and occurred during the intended menstrual cycle phases.
Ischemia pain ratings
There was a main effect of menstrual phase on ischemia pain ratings following the CPM procedure (F[2, 49.23]=3.35, P=0.04). This effect was qualified by a significant group × menstrual phase interaction (F[2, 49.23]=5.34, P=0.01), indicating that there were menstrual cycle phase-related differences in ischemia ratings in the PMDD group (simple effect of menstrual phase; F[2, 49.25]=8.50, P=0.001), but not in HC (simple effect of menstrual phase; F[2, 49.15]=0.13, P=0.88). Ischemia pain ratings were greatest in the PMDD group during the late-luteal phase, compared to the mid-follicular and ovulation phases (P-values<0.05), but mid-follicular pain ratings were not different from ovulation (P=0.35, see Figure 1).
Modulation of pain
As shown in Figure 2A, there was a significant main effect of CPM phase (F[2, 757.28]=5.20, P=0.01), but there was neither a significant group × CPM phase interaction (F[2, 790.13]=0.25, P=0.78) nor a group × menstrual phase × CPM phase interaction (F[4, 592.54]=1.87, P=0.11). Both HC and women with PMDD demonstrated pain inhibition during the ischemia and post-ischemia phases, relative to baseline (P-values<0.05), but there was no difference between ischemia and post-ischemia (P=0.52) (Figure 2A). As shown in Figure 2B, the group × menstrual phase interaction approached significance (F[2, 179.14]=2.41, P=0.09). Contrasts for the simple effect of menstrual phase found that the PMDD group rated the electrocutaneous stimulations as more painful during the late-luteal phase compared to the ovulation phase (P=0.02), and there was a trend towards significance compared to the mid-follicular phase (P=0.07). However, there was no difference between the mid-follicular and ovulation phase (P=0.75). HC did not evidence any differences in pain ratings across the menstrual cycle (P-values>0.20). Overall, these data indicate that CPM modulation did not vary by group or menstrual phase. However, there were menstrual phase differences in pain sensitivity (mean pain ratings at each phase) across each group, with the PMDD group rating the electrocutaneous stimulations as more painful during the late-luteal phase.
Modulation of NFR magnitude
As shown in Figure 3A, there was a significant main effect of CPM phase [F(2, 598.38)=3.03, P<0.05] that was qualified by a significant group × CPM phase interaction [F(2, 604.78)=4.22, P=0.02]. The PMDD group exhibited lower NFR magnitude during the ischemia phase vs the pre-ischemia baseline (P=0.001) (Figure 3A); however, HC did not show NFR modulation during the CPM procedure (P-values>0.16). There was no significant group × menstrual phase × CPM phase interaction [F(4, 490.53)=0.79, P=0.53]. The main effect of menstrual phase was not significant (P=0.19); however, there was a significant interaction of group × menstrual phase [F(2, 234.45)=4.31, P=0.01]. As shown in Figure 3B, the PMDD group evidenced an increase in NFR magnitude during the ovulation phase compared to the mid-follicular (P=0.03) and the late-luteal phases (P=0.01). There was no significant difference in NFR magnitude during the mid-follicular phase compared to the late-luteal phase (P=0.64). In contrast, HC did not show any differences in NFR magnitude across menstrual phases (P-values>0.09). This indicates that PMDD showed modulation of NFR magnitude during CPM, as well as differences in NFR across menstrual cycle phases, while HC did not.
Discussion
Nociception is modulated by facilitatory and inhibitory mechanisms that regulate the degree of pain experienced. Abnormalities in the functioning and balance of this system are thought to confer risk for the development and persistence of chronic pain.12,20,21,29 Several studies support PMDD-related deficits in pain processing, as heightened sensitivity to both clinical and experimental pain has been observed in this population. While enhanced pain facilitation may contribute to hyperalgesia in PMDD, it is also plausible that under-activation of descending inhibition may lead to augmented pain. Therefore, the current study was designed to examine this issue by assessing endogenous inhibition of pain perception and spinal nociception across the mid-follicular, ovulatory, and late-luteal phases of the menstrual cycle in women with PMDD compared to healthy women. Three notable findings emerged: 1) the PMDD group exhibited greater ischemia and electrocutaneous pain during the late-luteal phase; 2) CPM inhibition of pain and NFR was intact in the PMDD group; and 3) spinal nociception was tonically heightened during ovulation in PMDD.
Overall, women with PMDD experienced enhanced sensitivity to ischemia pain during the late-luteal (premenstrual) phase, while a non-significant trend (P=0.09) towards greater electrocutaneous pain intensity was also observed. This is consistent with heightened premenstrual pain symptomatology commonly observed in women with PMDD, and is supported by studies reporting increased sensitivity to experimental pain during the symptomatic phase.9 For instance, Straneva et al9 measured pain threshold and tolerance in response to forearm ischemia in a group of 27 women with PMDD (and 27 HC) across the follicular and luteal phases of the menstrual cycle. Although women with PMDD exhibited a phase-independent hyperalgesia in ischemia pain thresholds and tolerances, ratings of pain intensity and unpleasantness were generally higher during the luteal phase. Further, our laboratory found that enhanced pain in women with PMDD is demonstrated on retrospective measures of pain report, rather than immediate evaluation of pain (eg, pain threshold),11 an effect also observed by Klatzkin et al.10 As we have previously noted,11 this could suggest that PMDD symptomatology influences memory for pain above and beyond immediate behavioral measures of pain (threshold/tolerance).9–11
Interestingly, there was no evidence of group-related deficits in CPM inhibition of pain ratings, which suggests intact endogenous modulation of pain in both PMDD women and HC. Further, these outcomes did not differ across the menstrual cycle. Although CPM has not been previously examined in PMDD, there have been two studies, to our knowledge, that have investigated CPM in healthy individuals across the follicular, ovulatory, and luteal phases. Tousignant-Laflamme and Marchand14 found that cold pressor inhibition of heat pain was greatest during ovulation compared to early-follicular (days 1–3) and mid-luteal (days 19–23) phases. Similarly, using the cold pressor as a conditioning stimulus, Rezaii et al15 observed greater inhibition of pressure pain during the ovulatory phase, when compared with the early-follicular (days 3–5) and mid-luteal phases (days 7–8 post-ovulation). Although it is unclear why we did not observe phase-related differences in our HC group in CPM modulation, it is important to note that variation in menstrual timing and pain stimuli may have accounted for the divergence across studies. Regardless, current findings suggest that group differences in CPM inhibition of pain do not likely contribute to enhanced premenstrual pain observed in PMDD, at least not during the menstrual phases assessed in our study.
Notably, while CPM inhibition of NFR occurred in PMDD women, this effect was absent in HC. Although an unexpected outcome, it is plausible that group differences in the salience of the conditioning stimuli (ischemia) may have impacted the current study findings. Specifically, ischemia pain produces a deep, prolonged aching sensation that is believed to resemble some forms of clinical pain (musculoskeletal pain). Hence, this particular pain modality may closely emulate clinical pain commonly experienced in PMDD, and thus be more clinically relevant to this group.30,31 Therefore, when presented with a challenge that approximates their clinical experience, women with PMDD may engage their physiological pain modulatory processes, an effect which may have led to enhanced inhibition of spinal nociception in this group. However, this is speculative and warrants further investigation. Another interesting finding was that NFR varied across the menstrual phases, with NFR magnitude being greater during ovulation in PMDD. Overall, this suggests that descending brain-to-spinal cord mechanisms that heighten spinal nociception were tonically engaged during ovulation. While the exact nature of this outcome is unclear, it is possible that this is a mechanism that initiates and/or influences PMDD symptom expression and pain symptomatology during the premenstrual phase.
Although physiological differences may play a role in significant pain-related symptomatology in PMDD, there is not enough evidence at this time to identify whether centrally-mediated pain regulatory mechanisms account for variability of pain experienced in this group. It is possible that rather than brain-to-spinal cord processes, enhanced pain in PMDD may be better accounted for by neuroendocrine variability (eg, beta-endorphins). Individual differences in sex hormones (eg, testosterone, estradiol) may also contribute to pain facilitation in PMDD, whether through independent effects or in conjunction with other processes (eg, differential endogenous opioid system receptor activation).17 Additionally, Tassorelli et al32 speculated that desensitization of opiate receptors during ovulation could be a potential mechanism by which pain is enhanced during the luteal phase in this group. Further exploration is warranted to determine whether these factors contribute to heightened pain in women with PMDD, especially during the luteal phase.
Study limitations
The present study had a number of strengths, including a within-subjects design; assessment of hormone levels; verification of menstrual phases, ovulation, and cycle regularity; and counterbalance of menstrual phase testing order. Further, this is the first study to examine CPM in PMDD, and the second to investigate CPM of NFR across the menstrual cycle. However, a few limitations are worth noting. First, our sample sizes were small which reduced statistical power, making it difficult to achieve statistical significance on some outcomes. However, our sample sizes are comparable to previous studies,7–10 which highlights the difficulty of obtaining a large sample of women with PMDD. Second, the external validity of these results may be compromised, as this PMDD sample may not be representative of other women with PMDD. The level of participant involvement and conscientiousness required for completing this study (tracking symptoms over 3 months, attending experimental sessions, and testing during the late-luteal phase) lends itself to the idea that this sample may be resilient or less symptomatic than the general PMDD population. Third, the study used acute electrocutaneous stimulation as the test stimuli during CPM, which may have limited ecological validity in terms of the quality of pain symptomology experienced in PMDD. However, in order to study modulation of spinal nociception (ie, NFR), it was necessary to use electrocutaneous stimuli because only electrocutaneous or laser stimuli can evoke this reflex. Finally, methodological differences in experimental pain assessment or the definition of menstrual cycle phases may contribute to inconsistent findings among studies. Thus, replication using the same modalities across similar windows of time is recommended.
Conclusion
In summary, this study suggests that women with PMDD do not differ from HC in CPM of pain, and that they do not have disrupted CPM of spinal nociception. However, women with PMDD do evidence greater ischemic and electrocutaneous pain sensitivity during the late-luteal phase and enhanced spinal nociception during ovulation. Overall, this suggests that PMDD-related symptomatology may not be related to a disruption of the circuits that mediate CPM-related inhibition. Future research is necessary to determine whether other mechanisms differentially impact PMDD and ultimately contribute to enhanced pain symptoms in this population.
Acknowledgments
This work was funded by a grant (HR09-080) from the Oklahoma Center for the Advancement of Science and Technology (OCAST) awarded to Jamie L Rhudy. The authors would like to thank Satin Martin, Lauren Guderian, Abby Greenhaw, and Yvette Guereca for their assistance with data collection.
Disclosure
The authors report no conflicts of interest in this work.
References
Yang M, Wallenstein G, Hagan M, Guo A, Chang J, Kornstein S. Burden of premenstrual dysphoric disorder on health-related quality of life. J Womens Health (Larchmt). 2008;17(1):113–121. | |
Halbreich U, Borenstein J, Pearlstein T, Kahn LS. The prevalence, impairment, impact, and burden of premenstrual dysphoric disorder (PMS/PMDD). Psychoneuroendocrinology. 2003;28 Suppl 3:1–23. | |
Freeman EW. Effects of antidepressants on quality of life in women with premenstrual dysphoric disorder. Pharmacoeconomics. 2005;23(5):433–444. | |
Keenan PA, Lindamer LA. Non-migraine headache across the menstrual cycle in women with and without premenstrual syndrome. Cephalalgia. 1992;12(6):356–359. | |
Di Giulio G, Reissing ED. Premenstrual dysphoric disorder: prevalence, diagnostic considerations, and controversies. J Psychosom Obstet Gynaecol. 2006;27(4):201–210. | |
Steiner M, Born L. Advances in the diagnosis and treatment of premenstrual dysphoria. CNS Drugs. 2000;13(4):287–304. | |
Kuczmierczyk AR, Adams HE, Calhoun KS, et al. Pain responsivity in women with premenstrual syndrome across the menstrual cycle. Percept Mot Skills. 1986;63(2 Pt 1):387–393. | |
Fillingim RB, Girdler SS, Booker DK, Light KC, Harris MB, Maixner W. Pain sensitivity in women with premenstrual dysphoric disorder: a preliminary report. Journal of Women’s Health. 1995;4(4):367–374. | |
Straneva PA, Maixner W, Light KC, Pedersen CA, Costello NL, Girdler SS. Menstrual cycle, beta-endorphins, and pain sensitivity in premenstrual dysphoric disorder. Health Psychol. 2002;21(4):358–367. | |
Klatzkin RR, Lindgren ME, Forneris CA, Girdler SS. Histories of major depression and premenstrual dysphoric disorder: Evidence for phenotypic differences. Biol Psychol. 2010;84(2):235–247. | |
Bartley EJ, Palit S, Kuhn BL, et al. Nociceptive processing in women with premenstrual dysphoric disorder (PMDD): the role of menstrual phase and sex hormones. Clin J Pain. 2015;31(4):304–314. | |
Naugle KM, Riley JL 3rd. Self-reported physical activity predicts pain inhibitory and facilitatory function. Med Sci Sports Exerc. 2014;46(3):622–629. | |
Edwards RR. Individual differences in endogenous pain modulation as a risk factor for chronic pain. Neurology. 2005;65(3):437–443. | |
Tousignant-Laflamme Y, Marchand S. Excitatory and inhibitory pain mechanisms during the menstrual cycle in healthy women. Pain. 2009;146(1–2):47–55. | |
Rezaii T, Hirschberg AL, Carlström K, Ernberg M. The influence of menstrual phases on pain modulation in healthy women. J Pain. 2012;13(7):646–655. | |
Bartley EJ, Rhudy JL. Endogenous inhibition of the nociceptive flexion reflex (NFR) and pain ratings during the menstrual cycle in healthy women. Ann Behav Med. 2012;43(3):343–351. | |
Rhudy JL, Bartley EJ, Palit S, et al. Affective disturbance associated with premenstrual dysphoric disorder does not disrupt emotional modulation of pain and spinal nociception. Pain. 2014;155(10):2144–2152. | |
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th Edition Text Revision. Arlington, VA: American Psychiatric Association; 2000. | |
First MB, Spitzer RL, Gibbons M, Williams JB. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. (SCID-I/NP) New York: Biometrics Research, New York State Psychiatric Institute; 2002. | |
DelVentura JL, Terry EL, Bartley EJ, Rhudy JL. Emotional modulation of pain and spinal nociception in persons with severe insomnia symptoms. Ann Behav Med. 2014;47(3):303–315. | |
Terry EL, DelVentura JL, Bartley EJ, Vincent AL, Rhudy JL. Emotional modulation of pain and spinal nociception in persons with major depressive disorder (MDD). Pain. 2013;154(12):2759–2768. | |
Reid RL. Premenstrual syndrome. In: Leventhal JM, Hoffman JJ, Keith LG, Taylor PJ, editors. Current Problems in Obstetrics, Gynecology, and Fertility. Chicago: Year Book Medical Publishers; 1985:345–353. | |
Sandrini G, Serrao M, Rossi P, Romaniello A, Cruccu G, Willer JC. The lower limb flexion reflex in humans. Prog Neurobiol. 2005;77(6):353–395. | |
Rhudy JL, Williams AE, McCabe KM, Nguyen MA, Rambo P. Affective modulation of nociception at spinal and supraspinal levels. Psychophysiology. 2005;42(5):579–587. | |
France CR, Rhudy JL, McGlone S. Using normalized EMG to define the nociceptive flexion reflex (NFR) threshold: further evaluation of standardized scoring criteria. Pain. 2009;145(1–2):211–218. | |
Rhudy JL, France CR. Defining the nociceptive flexion reflex (NFR) threshold in human participants: a comparison of different scoring criteria Pain. 2007;128(3):244–253. | |
Rhudy JL, Green BA, Arnau RC, France CR. Taxometric analysis of biceps femoris EMG following electrocutaneous stimulation over the sural nerve: Determining the latent structure of the nociceptive flexion reflex (NFR). Int J Psychophysiol. 2008;69(1):18–26. | |
Rhudy JL, France CR, Bartley EJ, McCabe KM, Williams AE. Psychophysiological responses to pain: further validation of the nociceptive flexion reflex (NFR) as a measure of nociception using multilevel modeling. Psychophysiology. 2009;46(5):939–948. | |
Rhudy JL, DelVentura JL, Terry EL, et al. Emotional modulation of pain and spinal nociception in fibromyalgia. Pain. 2013;154(7):1045–1056. | |
Fillingim RB, Maixner W. The influence of resting blood pressure and gender on pain responses. Psychosom Med. 1996;58(4):326–332. | |
Fillingim RB, Maixner W, Girdler SS, et al. Ischemic but not thermal pain sensitivity varies across the menstrual cycle. Psychosom Med. 1997;59(5):512–520. | |
Tassorelli C, Sandrini G, Cecchini AP, Nappi RE, Sances G, Martignoni E. Changes in nociceptive flexion reflex threshold across the menstrual cycle in healthy women. Psychosom Med. 2002;64(4):621–626. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



