Back to Journals » Cancer Management and Research » Volume 13
Encouraging Pathological Complete Response Rate from Neoadjuvant Chemotherapy with Albumin-Bound Paclitaxel Plus Cisplatin and Capecitabine for Locally Advanced Esophageal Squamous Carcinoma: Preliminary Outcome of a Retrospective Study
Authors Zhang W, Li Y, Xue L , Qu D, Jiang Z , Wang Z, Yang Z, Zhou A
Received 22 December 2020
Accepted for publication 11 February 2021
Published 2 March 2021 Volume 2021:13 Pages 2163—2170
DOI https://doi.org/10.2147/CMAR.S298360
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Yong Teng
Wen Zhang,1,* Yong Li,2,* Liyan Xue,3 Dong Qu,4 Zhichao Jiang,1 Zhen Wang,2 Zhaoyang Yang,3 Aiping Zhou1
1Department of Medical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, People’s Republic of China; 2Department of Thoracic Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, People’s Republic of China; 3Department of Pathology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, People’s Republic of China; 4Department of Diagnostic Radiology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Aiping Zhou Email [email protected]
Objective: To evaluate the efficacy and safety of neoadjuvant chemotherapy with albumin-bound paclitaxel plus cisplatin and capecitabine for locally advanced esophageal squamous cell carcinoma (ESCC).
Methods: The data of thirty-one patients with locally advanced ESCC (cT1-2N+M0, cT3-4aNanyM0) received preoperative chemotherapy with albumin-bound paclitaxel plus cisplatin and capecitabine (referred as APCC regimen) were retrospectively analysed. The primary endpoint was pathological complete response (pCR) rate.
Results: The median number of chemotherapy cycles with APCC regimen every 3 weeks were 4 (range: 1– 6), which was completed by 23 patients. The clinical efficacy of 30 patients was evaluated and all showed reduction of tumours in varying degrees. Five patients received radiotherapy following chemotherapy. Four patients could not receive surgery due to COVID-19 pandemic. Of the 24 patients who underwent surgery, 3 received radiotherapy following chemotherapy, the resection rate of R0 was 95.8%, 9 cases (37.5%) showed pCR and 16 cases (66.7%) showed major pathological response (MPR). Postoperative pathology of 15 cases (62.5%) were stage I (ypT0-2N0M0). Of the 21 patients who underwent surgery after neoadjuvant chemotherapy alone, 8 (38.1%) had pCR and 15 (71.4%) had MPR. The most common grade 3/4 adverse events of chemotherapy included neutropenia (35.5%) and leukopenia (9.7%). Grade 2 postoperative complications occurred in 3 (12.5%) patients.
Conclusion: The preliminary results of this study suggest that preoperative chemotherapy with the triplet regimen of albumin-bound paclitaxel, cisplatin and capecitabine for patients with locally advanced ESCC revealed significant tumour downstage and encouraging pCR rate, with well-tolerable toxicities. The role of this regimen warrants further investigation.
Keywords: albumin-bound paclitaxel, cisplatin, capecitabine, esophageal squamous cell carcinoma, neoadjuvant chemotherapy, pathological complete response
Introduction
Esophageal cancer (EC) ranks seventh (572,000 new cases) in incidence and sixth (509,000 deaths) in mortality among all the malignant tumour globally in 2018.1 Adenocarcinoma is the most common subtype of EC in Western population,2 however, squamous cell carcinoma accounts for over 90% of the Chinese population.3 Esophageal squamous cell carcinoma (ESCC) remains a major concern in China, where the number of new and death cases reached 307,000 and 283,000 respectively, accounting for 53.7% and 55.7% of the total global new and death cases respectively in 2018.1 After surgery alone, the prognosis for patients with locally advanced EC remains poor, with a 5-year survival rate of only 25%.4 Combined modality therapy has been shown to significantly increase survival in esophageal cancer patients with locally advanced disease compared to resection alone.5
Neoadjuvant chemoradiotherapy (nCRT) has been considered as the standard care for locally advanced EC. Various studies have reported that nCRT along with surgery can significantly reduce tumour size and the recurrence rate while improving the R0 resection rate and overall survival.5–8 The pathological complete response (pCR) rate of nCRT was 13% to 22% for esophageal adenocarcinoma, and reached approximately 40% for squamous cell carcinoma.9–12 However, in some studies, concurrent chemoradiotherapy not only achieved good results, but also increased adverse reactions. As reported by Yang et al, the incidences of postoperative complications such as arrhythmia (13% vs 4.0%; P =0.001) as well as pretreatment mortality (2.2% vs 0.4%; P =0.212) were higher in the nCRT group over surgery alone.12 Similar findings were observed in the FFCD9901 study, where nCRT did not improve R0 resection rate or 3-year survival, but enhanced the postoperative mortality (11.1% vs 3.4%, P = 0.049) in patients with stage I or II EC compared with surgery alone.13
Recently, neoadjuvant chemotherapy (nCT) alone has also been shown to be effective in terms of improving resection rate of R0 and overall survival for locally advanced EC. For example, administration of 2 cycles of cisplatin with 5-FU, a previously most commonly used regimen, prior to surgery improved resection rate of R0 (60% vs 54%, P<0.0001) and 5-year survival rate (23% vs 17%, P = 0.03) compared with surgery alone in OEO2 study.14 However, in most studies, nCT with cisplatin and 5-FU, revealed an unsatisfactory pCR rate of only 1.7% to 9% in both esophageal adenocarcinoma and ESCC, which limits the application of nCT alone.11,14,15 So far, the best nCT regimen has not been well established. Thus, investigation of potent nCT regimen is of major clinical concern for locally advanced EC. In advanced ESCC, cisplatin plus paclitaxel seemed to increase objective response rate (42.5% vs 38.4%, P =0. 948) and improve progression-free survival (PFS) (7.85 months vs 6.53 months, P=0.02).16 Furthermore, a recent study demonstrated that preoperative regimen of cisplatin, 5-FU and paclitaxel improved the pCR rate by 24.1%, R0 resection rate was 82.5%, and the perioperative mortality rate was only 1.9%.17 As a solvent-free form of paclitaxel, albumin-bound paclitaxel is widely used in the treatment of advanced gastric cancer and pancreatic cancer due to advantages such as enhanced solubility, high affinity of binding to tumour tissue and low incidence of allergic reaction. Since 2018, we adopted a triplet regimen with albumin-bound paclitaxel, cisplatin and capecitabine (referred as APCC regimen) as neoadjuvant chemotherapy for locally advanced ESCC. Here, we reported the preliminary results.
Patients and Methods
Study Design
This was a retrospective study, in which the data of all eligible patients with locally advanced ESCC from April 2018 to October 2020 were collected retrospectively. The patients were included based on the following criteria: (1) histologically confirmed locally advanced ESCC; (2) clinical TNM staging of cT1-2N+M0, cT3-4aNanyM0 according to American Joint Committee on Cancer (AJCC 8th Edition); (3) an Eastern Cooperative Oncology Group (ECOG) score of 0–1; (4) those who received at least 1 cycle of neoadjuvant chemotherapy. The patients were excluded if they showed the presence of any one of the following: (1) non-squamous cell carcinoma on pathological examination; (2) cervical esophageal cancer; (3) previous chemotherapy or radiotherapy for esophageal lesions; (4) distant metastatic (M1) diseases; (5) other malignant tumours in the past 5 years, except for cervical carcinoma in situ, cutaneous squamous or basal cell carcinoma treated for radical purposes.
The study protocol was reviewed and approved by the Ethics Committee of Cancer Hospital Chinese Academy of Medical Sciences (approval No: 20/381-2577). The study met the requirements of the Declaration of Helsinki formulated by the World Medical Association. Personal data during the study will be protected in accordance with the law.
Chemotherapeutic Regimen
All the patients were treated with APCC regimen as neoadjuvant chemotherapy. The choice of APCC regimen was determined by the clinical experience of physicians. APCC regimen consisted of albumin-bound paclitaxel administered at dose of 125 mg/m2, day 1 and day 8; cisplatin at 60 mg/m2 dose at day 1, or divided into 2 days; and capecitabine 1750 mg/m2, oral twice a day, from day 1 to day14; every 21 days. During the period of chemotherapy, the efficacy was evaluated by enhanced computed tomography (CT) of the neck, chest and abdomen every 2 cycles. A maximum of 4 cycles of chemotherapy was planned in patients with clinical tumor remission. Radical esophagectomy was performed within 4 to 6 weeks after cessation of last dose of chemotherapy.
Efficacy and Toxicity Evaluation
The primary endpoint of the study was pCR rate. The secondary endpoints were major pathological response (MPR) rate, radical resection rate (R0 resection rate) and safety. Here, pCR was defined as no residual tumour cells found in the surgical specimens of primary esophageal lesions and drainage lymph nodes. Cases with residual high-grade dysplasia/carcinoma in situ without invasive carcinoma is also included in pCR. MPR was defined as tumour regression induced by neoadjuvant chemotherapy, when ≤10% of residual tumour tissue in resected primary tissue. The clinical and pathologic stages are expressed according to the American Joint Committee on Cancer (AJCC 8th Edition) TNM staging.18 In addition, the toxicities were graded according to US Department of Health and Human Services Common Terminology Criteria for Adverse Events (CTCAE) version 5.0,19 while Clavien-Dindo classification system was used to assess postoperative complications.20
Results
Baseline Characteristics
A total of 31 patients with locally advanced ESCC were included in the study. The baseline characteristics of the patients are summarized in Table 1. The median age of patients was 57 years (range: 25–75 years) and included 25 males and 6 females. Twenty-nine patients (93.5%) belonged to T3 and T4a, and further 21 patients (67.7%) were lymph node positive.
 |
Table 1 Patient Baseline Demographics and Clinic Pathological Characteristics (N = 31) |
Treatment Compliance
Among the 31 patients, 29 patients (93.5%) were treated with APCC regimen every 3 weeks, while the other 2 patients (aged over 70 years, determined by the physician) were adjusted to bi-weekly APCC regimen (albumin-bound paclitaxel 125 mg/m2, d1; cisplatin 40 mg/m2, d1 and capecitabine 1750 mg/m2, d1-10; 14 days as a cycle) (Figure 1). Most patients completed four cycles of neoadjuvant chemotherapy as planned, but some patients did not receive four cycles of chemotherapy as planned due to personal or epidemic reasons. The median number of preoperative treatment cycles was 4 (ranging from 1 to 6 cycles) in patients who received APCC regimen, every 3 weeks. Twenty-three (79.3%) of the 29 patients with APCC regimen, every 3 weeks, completed 4 cycles of chemotherapy, whereas 3 patients (10.3%) completed 2 cycles of chemotherapy, 2 patients (6.8%) completed 6 cycles of chemotherapy, and 1 patient (3.6%) completed one cycle of chemotherapy (discontinued treatment due to personal reasons) who was considered unevaluable for efficacy. Two patients with bi-weekly APCC regimen completed 3 and 6 cycles of chemotherapy, respectively. Five patients received local radiotherapy post completion of neoadjuvant chemotherapy, 3 of whom underwent surgical resection after radiotherapy. The causes of combined radiotherapy were as follows: in order to preserve the larynx in 1 case with upper thoracic esophageal carcinoma near neck, surgical intolerance in 1 case (73 years old), advanced baseline tumour staging in 1 case (cT4aN2), and close relationship between tumour and large vessels in 2 cases. Four patients failed to receive the expected surgery after 4 cycles of chemotherapy due to the COVID-19 outbreak. Ultimately, 24 patients underwent surgical resection. Of the 24 patients, 3 patients received additional preoperative radiotherapy, while 21 patients received preoperative chemotherapy alone.
 |
Figure 1 Patient flow diagram of this study. |
Efficacy Outcomes
The clinical efficacy outcomes were evaluated among 30 patients. The size of the tumour was reduced to varying degrees in all the patients post-treatment with APCC regimen. The R0 resection rate among 24 patients was 95.8% (23/24). One patient was detected with primary esophageal lesion invading the descending aorta during the surgery; hence, the tumour was not resected and the patient refused to receive radiation and continued chemotherapy post-surgery. Of the 24 patients who underwent surgery, 9 (37.5%) achieved pCR and 16 (66.7%) achieved MPR. Further, 15 (62.5%) patients were reduced to stage I (ypT0-2N0M0), 18 (75%) were N0, and 5 patients had positive lymph nodes, 4 were N1 stage and only 1 was N2 stage after surgery. Of the 21 patients who underwent surgery with neoadjuvant chemotherapy alone, 8 (38.1%) had pCR and 15 (71.4%) had achieved MPR (Table 2).
 |
Table 2 Efficacy Outcomes Post-Surgery with Neoadjuvant Chemotherapy Regimen |
Safety Outcomes
The commonly observed adverse events related to chemotherapy were leukopenia and neutropenia, as well as gastrointestinal adverse reactions, mostly of grade 1 and 2. The most common grade 3 or more adverse events were neutropenia (35.5%) and leukopenia (9.7%). A total of 3 patients (9.7%) were adjusted for drug dose due to adverse reactions, including 2 patients with grade 4 neutropenia and 1 patient with grade 2 hand foot syndrome. Significant complications occurred in 3 patients within 1 month after surgery, including thoracic infection (pleural effusion), pneumonia and mediastinal infection after drainage tube removal in each one patient, all of which were grade 2. The above postoperative complications were improved after symptomatic treatment, and the postoperative hospital stay was 15, 17, and 24 days, respectively. No treatment-related deaths occurred during neoadjuvant chemotherapy and within 30 days after surgery (Table 3). Of the 23 patients who underwent radical surgical resection, 4 patients continued the original regimen of chemotherapy until a total of 6 cycles after surgery and 1 patient was given postoperative radiotherapy considering the postoperative stage as ypT3N2.
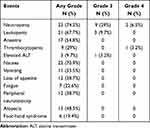 |
Table 3 Adverse Events Due to Neoadjuvant Chemotherapy (N = 31) |
As of November 15, 2020, with a median follow-up time of 12.4 months (range: 10.1–31.2), 3 patients died (2 patients did not receive surgery, 1 patient received surgery). Among the 23 patients who underwent R0 resection, the median follow-up time after surgery was 8.3 months (range: 1.1–26.1). One patient developed lung metastasis 7.9 months after surgery, and his overall survival time was 24 months. Due to the breakdown of COVID 19, one patient received surgery 6 months after the end of 4 cycles of preoperative chemotherapy. The postoperative pathological stage of this patient was ypTisN1. However here, the follow-up time was only 1.1 months.
Discussion
In this study, neoadjuvant chemotherapy with the triplet regimen of albumin-bound paclitaxel with cisplatin and capecitabine (APCC regimen) for locally advanced resectable ESCC showed high anti-tumor activities and significant effect on tumour downstaging. None of the evaluable patients (n=30) had disease progression after chemotherapy. Though six patients did not receive surgery after chemotherapy for various reasons and this might have confounded our findings of the actual results, a pCR rate of 38.1% and a MPR rate of 71.4% in the 21 patients who received neoadjuvant chemotherapy alone were quite encouraging. This pCR rate seemed to be higher than that of previous neoadjuvant chemotherapy with cisplatin and fluorouracil.11,14,15 Further, in all 24 patients received surgery, the R0 resection rate was 95.8%, the pCR rate was 37.5%, the MPR rate was 66.7%, and 62.5% of the patients’ postoperative pathological stage was stage I (ypT0-2N0). As with the pCR rate in the previously reported studies nCRT were believed to often has a significant advantage over nCT alone, especially for squamous cell carcinoma. The randomized-controlled CROSS study reported a pCR rate of 49% in the squamous cell carcinoma subgroup.9 NEOCRTEC5010 study use of vinorelbine and cisplatin with concurrent radiotherapy (DT 40Gy) resulted in a pCR rate of 43.2% in patients with locally advanced ESCC.12 However, whether the higher pCR rate of nCRT over nCT could transfer to the survival benefit need further investigation. In the Phase 2 randomized trial NeoRes I, a higher pCR rate of 28% from cisplatin and fluorouracil based nCRT did not produce significant OS improvement over cisplatin and fluorouracil neoadjuvant chemotherapy alone.21 There are two ongoing randomized Phase III trials to compare the efficacy of platinum and paclitaxel concurrent radiotherapy and neochemotherapy alone for locally advanced ESCC.22,23 Our study findings suggest that APCC regimen seems to be able to further narrow the gap in pCR rate between nCT and nCRT. Recently, immune checkpoint combined with chemotherapy has been tried for neoadjuvant treatment of locally advanced esophageal cancer, but the results are still preliminary. Two prospective small sample studies showed that 2 cycles of anti-PD-1 monoclonal antibody combined with nab-paclitaxel plus S-1 or paclitaxel plus carboplatin regimen as nCT achieved pathological complete response rates of 16.67% and 45.4%, respectively.24,25
79.3% of patients in this study completed 4 cycles of preoperative chemotherapy with APCC regimen, which was longer than the duration of chemotherapy reported in other relevant studies with EC.14,15 At present, there is no clear information with regards to the optimal number of cycles of perioperative chemotherapy for locally advanced EC. In the previous studies of nCRT and nCT, usually 2 to 3 cycles of chemotherapy were used. Findings by Zhao et al17 suggested that prolonging the number of chemotherapy cycles during perioperative period could further reduce the recurrence rate and improve the overall survival. The study compared the efficacy of 4 and 2 cycles of paclitaxel, cisplatin and 5-FU during the perioperative period. It was observed that treatment with 4 cycles of chemotherapy resulted in better 5-year disease-free survival (DFS) (31% vs. 17%, HR 0.62; 95% CI, 0.49–0.73; P<0.001) and OS (38% vs 22%, HR 0.79; 95% CI 0.59–0.95; P<0.001).
In this study, 2 to 4 cycles of preoperative neoadjuvant triplet chemotherapy with APCC regimen were well tolerated. Most of the common adverse reactions were grade 1 or 2 neutropenia and gastrointestinal adverse events with the most common grade 3 or 4 adverse reactions being neutropenia (35.5%). However, 3 (12.5%) patients had chest, lung or mediastinal infection after surgery, which need more attention to in future research.
There are some limitations on this study that warrant mention. Since this was a retrospective study with a limited sample size and short follow-up time, the tumour recurrence rate and overall survival was not reached yet. In addition, as mentioned earlier, the inability of 6 (20%) patients to undergo surgery due to the age, personal will and COVID-19 epidemic might affect the accurate interpretation of pCR rate resulted from this regimen.
Conclusion
The preliminary results of this study suggest that the triplet regimen of albumin-bound paclitaxel, cisplatin and capecitabine as preoperative neoadjuvant chemotherapy for patients with locally advanced ESCC revealed significant tumour downstaging and encouraging pCR rate, with favourable safety profile. The role of this triplet regimen warrants further investigation in the neoadjuvant treatment for ESCC and a prospective Phase II study (NCT04390958) has already been in progress.
Ethics Approval
The study protocol was reviewed and approved by the Ethics Committee of Cancer Hospital Chinese Academy of Medical Sciences (approval No: 20/381-2577). This study is a retrospective non-intervention study, which does not interfere with the routine diagnosis and treatment, does not affect any medical rights and interests of patients, and does not increase the risk of patients. The results of the study will be published in the form of statistical analysis without any identifiable patient information. Most of the patients in this study have completed the treatment and cannot obtain their informed consent objectively. Based on the above reasons. We have applied to the Ethics Committee for exemption from the informed consent of all patients in this study and have been approved.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research received no external funding.
Disclosure
The authors declare no conflict of interest.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Arnold M, Soerjomataram I, Ferlay J, Forman D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64(3):381–387. doi:10.1136/gutjnl-2014-308124
3. Liang H, Fan J-H, Qiao Y-L. Epidemiology, etiology, and prevention of esophageal squamous cell carcinoma in China. Cancer Biol Med. 2017;14(1):33–41. doi:10.20892/j.issn.2095-3941.2016.0093
4. Herskovic A, Russell W, Liptay M, et al. Esophageal carcinoma advances in treatment results for locally advanced disease: review. Ann Oncol. 2012;23(5):1095–1103. doi:10.1093/annonc/mdr433
5. Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12(7):681–692. doi:10.1016/S1470-2045(11)70142-5
6. van Hagen P, Hulshof MC, Van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–2084. doi:10.1056/NEJMoa1112088
7. Gebski V, Burmeister B, Smithers BM, et al. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol. 2007;8(3):226–234. doi:10.1016/S1470-2045(07)70039-6
8. Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26(7):1086–1092. doi:10.1200/JCO.2007.12.9593
9. Shapiro J, van Lanschot JJB, Hulshof MCCM. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16(9):1090–1098. doi:10.1016/S1470-2045(15)00040-6
10. Burmeister BH, Thomas JM, Burmeister EA. Is concurrent radiation therapy required in patients receiving preoperative chemotherapy for adenocarcinoma of the oesophagus? A randomised phase II trial. Eur J Cancer. 2011;47(3):354–360. doi:10.1016/j.ejca.2010.09.009
11. Klevebro F, Alexandersson von Döbeln G, Wang N. A randomized clinical trial of neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the oesophagus or gastro-oesophageal junction. Ann Oncol. 2016;27(4):660–667. doi:10.1093/annonc/mdw010
12. Yang H, Liu H, Chen Y, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a phase III multicenter, randomized, open-label clinical trial. J Clin Oncol. 2018;36(27):2796–2803. doi:10.1200/JCO.2018.79.1483
13. Mariette C, Dahan L, Mornex F, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol. 2014;32(23):2416–2422. doi:10.1200/JCO.2013.53.6532
14. Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27(30):5062–5067. doi:10.1200/JCO.2009.22.2083
15. Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. 2012;19(1):68–74. doi:10.1245/s10434-011-2049-9
16. Liu Y, Ren Z, Yuan L, et al. Paclitaxel plus cisplatin vs. 5-fluorouracil plus cisplatin as first-line treatment for patients with advanced squamous cell esophageal cancer. Am J Cancer Res. 2016;6(10):2345–2350.
17. Zhao Y, Dai Z, Min W, et al. Perioperative versus preoperative chemotherapy with surgery in patients with resectable squamous cell carcinoma of esophagus: a phase III randomized trial. J Thorac Oncol. 2015;10(9):1349–1356. doi:10.1097/JTO.0000000000000612
18. Rice TW, Ishwaran H, Ferguson MK, Blackstone EH, Goldstraw P. Cancer of the Esophagus and Esophagogastric junction: an eighth edition staging primer. J Thorac Oncol. 2017;12(1):36–42. doi:10.1016/j.jtho.2016.10.016
19. Common Terminology Criteria for Adverse Events (CTCAE). Protocol development. CTEP [Internet]. [cited November 26, 2020]. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm.
20. Clavien PA, Sanabria JR, Strasberg SM. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery. 1992;111(5):518–526.
21. von Döbeln GA, Klevebro F, Jacobsen AB. Neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the esophagus or gastroesophageal junction: long-term results of a randomized clinical trial. Dis Esophagus. 2019;32(2). doi:10.1093/dote/doy078
22. Sun HB, Xing WQ, Liu XB. Neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for locally advanced oesophageal squamous cell carcinoma: a single-centre, open-label, randomized, controlled, clinical trial (HCHTOG1903). BMC Cancer. 2020;20(1):303. doi:10.1186/s12885-020-06824-2
23. Tang H, Tan L, Shen Y, et al. CMISG1701: a multicenter prospective randomized phase III clinical trial comparing neoadjuvant chemoradiotherapy to neoadjuvant chemotherapy followed by minimally invasive esophagectomy in patients with locally advanced resectable esophageal squamous cell carcinoma (cT3-4aN0-1M0) (NCT03001596). BMC Cancer. 2017;17:450. doi:10.1186/s12885-017-3446-7
24. Jun L, Zhigang L, Yang Y, et al. A prospective phase II clinical trial exploring neoadjuvant immunotherapy combined with chemotherapy in resectable thoracic esophageal squamous cell cancer(TESCC) wth multi-station lymph node metastases (NICE study): preliminary results. Ann Oncol. 2020;31(S6):S1292. doi:10.1016/j.annonc.2020.10.148
25. Zhang G, Hu Y, Yang B, et al. A single-centre, prospective, open-label, single-arm trial of toripalimab with nab-paclitaxel and S-1 as a neoadjuvant therapy for esophageal squamous cell carcinoma(ESCC). Ann Oncol. 2020;31(S4):S722. doi:10.1016/j.annonc.2020.08.1178
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
