Back to Journals » Journal of Inflammation Research » Volume 13
Encephalitozoon cuniculi Genotype II Concentrates in Inflammation Foci
Authors Brdíčková K, Sak B, Holubová N , Květoňová D, Hlásková L, Kicia M, Kopacz, Kváč M
Received 17 July 2020
Accepted for publication 24 August 2020
Published 25 September 2020 Volume 2020:13 Pages 583—593
DOI https://doi.org/10.2147/JIR.S271628
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Klára Brdíčková,1,2 Bohumil Sak,3 Nikola Holubová,3,4 Dana Květoňová,3 Lenka Hlásková,3 Marta Kicia,5 Żaneta Kopacz,5 Martin Kváč3,4
1Department of Clinical Microbiology, Bulovka Hospital, Prague, Czech Republic; 2Faculty of Science, University of South Bohemia in České Budějovice, České Budějovice, Czech Republic; 3Institute of Parasitology, Biology Centre, Czech Academy of Science, České Budějovice, Czech Republic; 4Faculty of Agriculture, University of South Bohemia in České Budějovice, České Budějovice, Czech Republic; 5Department of Biology and Medical Parasitology, Wroclaw Medical University, Wroclaw, Poland
Correspondence: Bohumil Sak
Institute of Parasitology, Branišovská 31, České Budějovice 37005, Czech Republic
Tel +420387775421
Fax +420385310388
Email [email protected]
Background: Microsporidia of the genus Encephalitozoon are generally connected with severe infections with lethal outcome in immunodeficient hosts. In immunocompetent hosts, microsporidiosis typically establishes a balanced host–parasite relationship that produces minimal clinically overt disease. Although the alimentary tract represents one of the main primary target tissues, the mechanisms of reaching other tissues during systemic microsporidian infections remain unclear.
Methods: In the present study, we tested the relation between inflammation induction in immunocompetent and immunodeficient mice and the presence of spores of E. cuniculi genotype II in selected organs and in fecal specimens by using molecular and histology methods.
Results: We reported the positive connection between inflammation induction and the significant increase of E. cuniculi genotype II occurrence in inflammation foci in both immunocompetent BALB/c and immunodeficient severe combined immunodeficient (SCID) mice in the acute phase of infection and the re-activation of latent microsporidial infection following inflammation induction in immunocompetent mice.
Conclusion: The results imply possible involvement of immune cells serving as vehicles transporting E. cuniculi genotype II purposefully across the whole host body towards inflammation. With increasing number of records of infections, it is necessary to reconsider microsporidia as agents responsible for various pathologies. The elucidation of possible connection with pro-inflammatory immune responses represents an important challenge with consequences for human health and development of therapeutic strategies.
Keywords: Encephalitozoon cuniculi, inflammation, targeted migration
Introduction
Microsporidia are obligatory intracellular eukaryotic parasites belonging to the phylum Microsporidia within the kingdom Opisthokonta infecting a broad range of animals including protists, invertebrates, and vertebrates. They have been studied for more than 150 years mainly for their ability to cause extensive damage to the host organism, mainly in husbandly important farmed animals such as silkworms, bees, and fish.1,2 In mammals, infections typically establish a balanced host–parasite relationship that produce minimal clinically overt disease in immunocompetent natural hosts.3–8 A shift in the balance toward immune deficiency or hyperimmune responses, however, produces overt clinical signs of disease. In the past, human microsporidial infections were most commonly associated with immunodeficiency, so they were often diagnosed in AIDS patients, organ recipients, and children.3 Of the ~1500 described microsporidia species, 17 species are known to infect humans, of which Encephalitozoon spp. and Enterocytozoon bieneusi are reported from most of the symptomatic patients.8,9 The first microsporidian successfully isolated for long-term culture, Encephalitozoon cuniculi, belongs to the best-studied microsporidian species with four different, not strictly host-specific genotypes/strains (I=“rabbit”, II=“mouse”, III=“dog”, IV=“human”) determined according to the number of short repeats in the ribosomal internal transcribed spacer (ITS) region.10,11 Encephalitozoon species infect several cell types in mammalian hosts, including epithelial and endothelial cells, fibroblasts, macrophages, and astrocytes.12 In addition to gastrointestinal tract involvement, patients with encephalitis, ocular infection, sinusitis, myositis, and disseminated infection are described in the literature.13–17
With the establishment of modern diagnostic methods, microsporidia have been detected in immunocompetent individuals, such as travelers, diabetics, pregnant women, and the elderly.9,–18–24 Immune suppression caused by chemotherapy, immunomodulating drugs, and solid organ or bone marrow transplantation, has been demonstrated to be an important risk factor for the development of microsporidiosis.25 Microsporidiosis is not limited, however, to immunocompromised states, and infections with these organisms occur in immunocompetent individuals as well.26,27 Furthermore, asymptomatic microsporidia infections have been recognized in the case of diseases with an ambiguous etiology, such as encephalitis or periprosthetic osteolysis.8,28,29 Microsporidia are often overlooked due to the problematic diagnosis, resulting in the increased possibility of hidden infections causing huge damage and various non-specific pathologies, often without application of effective treatment.30
The present study was designed to answer the question raised based on literary data29 and factual lack of active motility of microsporidia, if the microsporidian E. cuniculi could be secondarily transferred to the site of experimentally induced inflammation in cellular vehicles attracted to the inflammation focus.
Materials and Methods
Ethics Statement
All experimental procedures were performed in accordance with the law of Czech Republic (Act No 246/1992 Coll., on the protection of animals against cruelty). The study design was approved by ethical committees at the Biology Centre of the Czech Academy of Sciences, the State Veterinary Administration, and the Central Commission for Animal Welfare under protocols no. 100/2016 and 35/2020, respectively.
Mice
Eight-week-old severe combined immunodeficient (SCID) and immunocompetent (BALB/c) mice originally obtained from Charles River (Sulzfeld, Germany) were bred under sterile condition and supplied with a sterilized diet and sterilized water ad libitum.
Parasites
The spores of E. cuniculi genotype II originally isolated from a dexamethasone-treated laboratory mouse31 were cultivated in vitro for the purpose of the experiment according to Kotková et al.32
Experimental Protocol
All mice were per orally infected with the dose of 107 E. cuniculi genotype II spores in 0.2 mL of deionized water using intragastric gavage. The inflammation in experimental SCID and BALB/c was induced by the usage of 50 μL of Freund’s Incomplete Adjuvant (Sigma-Aldrich, St. Louis, MO, USA) inoculated in either acute (7 or 28 days post-infection (DPI) in BALB/c mice, respectively; seven DPI in SCID mice) or chronic phase (56 or 77 DPI in BALB/c mice, respectively) of infection32 to the hind limb muscles (Table 1). Mice of both strains injected intramuscularly with 50 μL of sterile phosphate buffered solution (PBS) at the same intervals were used as negative controls. Three mice from each strain were separately examined for microsporidia shedding in feces on a daily basis and fecal samples were stored at −20°C for further molecular analyses. The animal’s health, mortality, and morbidity were recorded at 12-hour intervals.
 |
Table 1 Design of Experiments |
Three mice from each group were euthanized by cervical dislocation every seventh DPI and sterile samples for molecular detection and histology were prepared from limb muscles and liver by the usage of a different pair of sterile dissection tools (Table 1).
DNA Isolation
Total DNA from samples was extracted as described by Sak et al33 following bead homogenization. Extracted DNA was stored at −20°C.
PCR Amplification
Nested PCR protocols34,35 were used for amplification of a partial sequence of 16S rRNA. Encephalitozoon cuniculi genotype I DNA and ultrapure water were used as positive and negative controls, respectively.
qRT-PCR
The protocols described by Wolk et al36 and Dai et al37 were used for amplification of 16S rRNA gene of E. cuniculi and β-actin as a housekeeping gene, respectively. Each run included unspiked specimens and diluent blanks as negative controls. Positive results were determined based on mathematical algorithms included with the LightCycler system (Roche, Praha, the Czech Republic). Results were determined to be positive when the fluorescence signal crossed the baseline at ≤43 cycles. The total amount of spores in 1 g of individual fecal or tissue samples was calculated based on the standard curve derived from serial dilutions of spores in water and fecal samples of known weight or recalculated based on the number of β-actin copies in the tissue sample, respectively.38,39
Histology
Tissues samples were processed by the usual paraffin method following fixation in 10% buffered formalin. The sections were stained with Brown and Brenn Gram stain.40
Statistical Analysis
Differences in microsporidia presence in inflammatory foci compared to noninduced site were analyzed by student T-tests and the conformity of the variances of the tested groups was verified by an F-test at a confidence level of α=0.05 and lower. The differences in the spore shedding frequencies within individual groups were analyzed by non-parametric Mann–Whitney U-tests. All computations were made using Statistica 6.0 software (StatSoft CR, Praha, Czech Republic).
Results
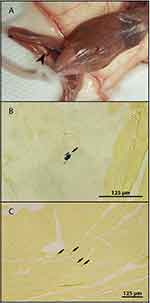
The application of Freund’s Incomplete Adjuvant induced localized inflammatory immune response in all animals (Figure 1A). There was no difference observed in the induction of lesions between infected and non-infected groups. On the other hand, neither macroscopic nor microscopic changes were observed in PBS inoculated mice.
Induction of Inflammation in BALB/c Mice in Acute Phase of Infection
In immunocompetent BALB/c mice, E. cuniculi genotype II was detected already prior to induction of inflammation in all screened tissue samples at least in one animal per group (Figure 2A). An induction of inflammation in the acute phase of infection 7. DPI in immunocompetent BALB/c mice led to the disappearance of microsporidia from non-induced leg and increase of their concentration towards induced leg in all experimental mice (P<0.05) from 14 day post-infection (DPI) to 35 DPI reaching up to 7.8×102 spores per 1 g of tissue reported 1 week post-inflammation induction (Figure 2A). On the other hand, no difference was observed between the occurrence frequencies and spore burden of microsporidia in legs in the control group (Figure 2A).
After secondary induction of inflammation, 28 DPI in the left leg E. cuniculi genotype II shifted to the new site of inflammation, especially 42 DPI reaching 4.8×104 spores per gram of tissue compared to the right leg, where 3.0×102 were detected. Also the frequency of occurrence of E. cuniculi genotype II in a particular leg was higher in induced legs, as the microsporidia were detected at least in two of three examined animals per interval, compared to non-induced ones showing the presence of E. cuniculi genotype II mostly in one animal per interval. The occurrence of E. cuniculi genotype II was also confirmed histologically at 42 DPI in the left induced leg (Figure 1B). Surprisingly, induction of inflammation led to a prolonged occurrence of E. cuniculi genotype II in all screened tissues of the experimental group (P<0.05) compared to the control group, where gradual disappearance of E. cuniculi genotype II from tested tissues was observed starting from 35 DPI, indicating a positive correlation between the presence of inflammation and the persistence of detectable infection (Figure 2A).
Encephalitozoon cuniculi genotype II was detected in feces in both groups of BALB/c mice in the acute phase of infection from the 2 DPI (Figure 3A). Induction of inflammation by Freund’s adjuvant led to discontinued excretion of spores in the feces for 15 days after primary induction and irregular spores secretion after secondary induction of inflammation, whereas in the control group specific E. cuniculi DNA in the feces was observed significantly more frequently during whole experiment without any relevant interruption.
Induction of Inflammation in BALB/c Mice in Chronic Phase of Infection
In the control immunocompetent BALB/c mice inoculated with PBS in the chronic phase of infection, microsporidia were not detected in selected organs and tissues throughout the whole experimental period (Figure 2B). However, after the inflammation induction in the chronic phase of infection of 56 DPI in BALB/c mice (Figure 2B), the infection was reactivated in all experimental mice and microsporidia were detected in both legs regardless of the site of inflammation induction. In addition, E. cuniculi genotype II reappeared also in the liver, even though the maximum spore burden was 10-times lower than in the case of induction of inflammation in the acute phase of infection (Figure 2B).
A similar effect was observed after secondary induction of inflammation in the chronic phase of infection of 77 DPI with no specific migration of E. cuniculi genotype II to the inflammatory focus, but activation of latent infection in experimental BALB/c mice, showing a significant increase in the spore burden in the induced group compared to control mice (P<0.05) (Figure 2B).
Similar to the experiment performed in the acute phase of the infection, a significant decrease in fecal spore excretion was observed in the group with induced inflammation (Figure 3B). Although the presence of specific E. cuniculi genotype II DNA in feces was demonstrated irregularly in both groups of immunocompetent mice until the 55 DPI, E. cuniculi genotype II was no longer excreted in feces after induction of inflammation in the experimental group.
Induction of Inflammation in SCID Mice
In immunodeficient SCID mice, E. cuniculi genotype II was detected in all screened tissue already prior to induction of inflammation (Figure 2C). After induction of inflammation of 7 DPI, in the experimental group a significant (P<0.05) increase in the number of spores in the induced right hind leg of 21 DPI reaching 7.8×105 spores per gram of tissue was observed compared to the left leg containing 3.5×105 spores per gram of tissue. In addition, there was an overall increase in the number of spores in both limbs and liver of induced animals compared to the control mice, especially 21 DPI. At the end of the experiment, 28 DPI, however, the number of spores in both legs was similar and comparable to control animals, where the spore burden was approximately the same for both limbs throughout the whole experiment. The presence of spores of microsporidia in the site of inflammation in the right leg was also observed histologically (Figure 1C).
In most SCID mice, E. cuniculi specific DNA in feces were recorded starting from 2 DPI (Figure 3C). Spores were excreted in the feces throughout the infection until the end of the experiment, regardless of the induction of inflammation.
In contrast to BALB/c mice, clinical signs of microsporidiosis, including bristly hair, hunched back, and cachexia, were observed in SCID mice, especially at the end of the study period. However, diarrhea as one of the main symptoms of microsporidiosis caused by E. cuniculi was not observed in SCID mice either.
Discussion
Microsporidia of the genus Encephalitozoon are typically described as chronic, slow acting pathogens and, thus, less virulent than other pathogen groups. However, they are able to multiply successfully in an appropriate host to an enormous amount without any obvious signs of infection in immunocompetent hosts, revealing that high infectivity, high pathogenicity, or both do not necessarily predict the level at which a microsporidian pathogen impacts the host population.33,39,41 The problematics are complicated by the fact that microsporidia are often overlooked and underdiagnosed, resulting in the increased possibility of hidden infections causing huge damage and various non-specific pathologies prior getting detected and successfully treated.28
Environmentally resistant life cycle stages of microsporidia, spores, are excreted in feces, urine, or sputum causing infection by ingesting contaminated food or water and less often by inhaling infectious spores.42,43 Horizontally transmitted microsporidia of the genus Encephalitozoon access host tissues by germinating in the gut lumen where infections are typically initiated in gut epithelial cells, and sometimes muscle cells. However, the target tissues – those in which microsporidia develop to the infectious stage, represent a species-specific interaction with its host. The alimentary tract is a common target tissue for the production of infective spores, and infection can be restricted to one portion of the gut or include several tissue types. The intensity of infection, including the amount and frequency of E. cuniculi spores secreted, depends on the age and health status of the infected individual as well as depends on the species and genotype of the infectious agent.42 The results of this work correspond to the data published by Sak et al38,39 and Kotková et al,32 ie, immunocompetent BALB/c mice shed E. cuniculi spores at a significantly lower frequency than immunodeficient SCID mice. The sporadic presence of E. cuniculi spores in the feces of an infected individual has also been demonstrated in other studies.26,27,29,32,41 A positive correlation between the frequency of excretion and the intensity of infection was observed in BALB/c and SCID mice; while BALB/c mice excreted intermittently with an infection intensity of 3.2×106, SCID mice excreted almost daily with an intensity of 1.2×108 spores per gram of feces.32,38,39 Similarly, a child without clinical signs of the disease has been shown to excrete 1.2×105 spores per gram of stool,44 whereas an immunodeficient individual can excrete up to 4.4×108 per gram of diarrhea.45
Systemic E. cuniculi infections acquired by ingesting spores are also initiated in the gut epithelial cells followed by the invasion of other tissues. The rapid spreading of Encephalitozoon has been reported in murine models, where systemic infection was reported within several days.32,39,41 However, spores of E. cuniculi are not motile, short distance dispersal in the host is limited to a unique mechanism of invasion of host cells that involves a highly specialized structure, the 10–50 μm long polar tube, which is responsible for the delivery of this organism to the host cell. Nevertheless, Encephalitozoon organisms may replicate to produce mature spores in a variety of immune cells including resident and trafficking macrophages and other phagocytic cells such as neutrophils, monocytes, dendritic cells, and eosinophils which may contribute to the spread of E. cuniculi throughout the host organism,46,47 suggesting that induction of chemokines for inducing innate immune inflammation may also promote recruitment of host cells for continued infection and dissemination48–50 On the other hand, there may be a situation in which microsporidia occurring in the area of induced inflammation are eliminated due to the increased occurrence of IFNγ-, LPS-, and TNFα-activated macrophages10,–50–54 by the means of cytotoxic cytokines, cationic proteins, lipid mediators, metalloproteinases, and components of the oxygen burst.10,51,55
Encephalitozoon cuniculi was recognized as a neglected etiological agent for more common, sometimes life-threatening diseases, such as encephalitis and meningitis, in otherwise healthy individuals28 or contribute to aseptic periprosthetic osteolysis after primary hip arthroplasty, leading to implant loosening and urgent arthroplasty revision.29 However, the fact that microsporidia occurred in the affected area before the onset of inflammatory processes, or whether they enter the affected area secondarily, either through macrophages or other cells involved in the development of inflammation serving as vehicles remained unanswered. Our results confirmed the targeted migration of E. cuniculi genotype II to the site of inflammation. Although E. cuniculi may be common in muscle, as confirmed by Sak et al56 in a recent study demonstrating the occurrence of E. cuniculi genotype II in pig muscle, reaching 60 to 250 spores per gram of meat tissue, in this work we showed that the induction of inflammation led to a significant increase of E. cuniculi genotype II at the induced site in immunocompetent BALB/c mice. Compared to the control group injected with sterile PBS, the occurrence of microsporidia in the limbs was random and, from 42 DPI, microsporidia in selected organs and tissues were not detected at all, suggesting that during our experiment they may have been eliminated by the host immune system or microsporidia remain in the body in undetectable amounts. Also induction of inflammation in the experimental group of SCID mice led to a significant increase of spore burden at the site of inflammation, while in the control group, the amount of spores per gram of tissue in both limbs was approximately identical. The high concentration of spores in muscle tissues at the end of the experiment could be attributed to the systemic spread of E. cuniculi in the terminal phase infection, as already described by Kotková et al.32
So far, the information available from experimental and natural infections suggests that E. cuniculi is able to spread in a host rapidly and survive in asymptomatic carriers for a long time period, but in some cases the E. cuniculi could be activated and trigger diseases with ambiguous etiology depending on the location. The results obtained in Kicia et al43 pointed to a significant association between the occurrence of E. cuniculi and respiratory symptoms in patients who have undergone kidney transplantation. However, the question of if microsporidia occurred in the respiratory tract in these individuals prior to immunosuppressive treatment, as Kotková et al32 and Sak et al39 described the common presence of Encephalitozoon species in the lungs of immunocompetent individuals, remains unanswered. Our experiments suggested that E. cuniculi genotype II could be activated from latent phase not even by chemotherapy, but also with inflammation occurrence. As induction of inflammation in the chronic phase of infection in immunocompetent BALB/c mice did not directly affect targeted migration of E. cuniculi genotype II to the inflammatory focus, but it led to reactivation of latent infection and E. cuniculi spores were repeatedly detected in both limbs and liver. These results correspond to the results of the study by Kotková et al,32 who described the reactivation of latent E. cuniculi genotype II infection in immunocompetent mice after immunosuppression, as well as the ability of this microsporidian to spread to various tissues during chronic infection, including muscles, and to persist at these sites. However, the mechanism of reactivation of E. cuniculi by inflammation remains unclear. It can be assumed that local induction of chronic inflammation could lead to a reduction in the capacity of the immune system, which previously kept the amount of microsporidia below the detection limit of the methods used (qRT PCR). Similarly, Bannoura et al57 described the reactivation of a latent Toxoplasma gondii infection in a 4-year-old child after application of steroid hormones against encephalomyelitis.
Unexpectedly, whereas the presence of E. cuniculi genotype II in the feces of control groups of immunocompetent BALB/c mice in both acute and chronic phase of infection was detected intermittently thorough the whole experimental period, in experimental groups the excretion of E. cuniculi in feces was reduced for several days or disappeared, respectively, after inflammation induction. This phenomenon can be explained by concurrent increase of occurrence of E. cuniculi genotype II in foci of inflammation and disappearance of the parasite from the gastrointestinal tract towards organs outside the gastrointestinal tract as previously reported in albendazole treated mice.30,39,58
Conclusions
Based on our results we can conclude that the induction of inflammation had caused increased migration of E. cuniculi genotype II to inflammatory foci as the frequency of spores was not random but shifted in favor of the site of induced inflammation compared to control groups, where spores were accidental or spores or their specific DNA was not detected at all. It can be assumed that E. cuniculi genotype II could be secondarily transported to the site of inflammation with the contribution of immune cells participating in its formation. E. cuniculi are also able to survive for a long time in immunocompetent hosts without any clinical signs, under certain circumstances the infection may be reactivated after inflammation induction. However, the mechanisms of such migration as well as the vehicle cells and mechanism of infection reactivation remain speculative and need to be specified in detail.
The elucidation of possible connection with pro-inflammatory immune responses, addressing the tissue specificity and the mechanisms of transfer to target tissues represent an important challenge with potential consequences for human health and brings novel insight into microsporidia problematic and developing reliable preventive and therapeutic strategies.
Funding
The study was supported by grants from the Grant Agency of the Czech Republic (17-12871S and 20-10706S).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Balbiani G. Sur les microsporidies ou psorospermies des articules. C R Acad Sci. 1882;95:1168–1171.
2. Lom J, Dyková I. Microsporidia (phylum microspora spraque, 1977). In: Protozoan Parasites of Fishes Developments in Aquaculture and Fisheries Science. Vol. 26. Amsterdam: Elsevier; 1992.
3. Didier ES, Didier PJ, Snowden KF, Shadduck JA. Microsporidiosis in mammals. Microbes Infect. 2000;2(6):709–720. doi:10.1016/S1286-4579(00)00354-3
4. Desoubeaux G, Pantin A, Peschke R, Joachim A, Cray C. Application of western blot analysis for the diagnosis of Encephalitozoon cuniculi infection in rabbits: example of a quantitative approach.. Parasitol Res. 2017;116(2):743–750. doi:10.1007/s00436-016-5343-4
5. Desoubeaux G, Piqueras MC, Pantin A, et al. Application of mass spectrometry to elucidate the pathophysiology of Encephalitozoon cuniculi infection in rabbits. PLoS One. 2017;12(7):e0177961. doi:10.1371/journal.pone.0177961
6. Desoubeaux G, Peschke R, Le-Bert C, et al. Seroprevalence survey for microsporidia in common bottlenose dolphin (Tursiops truncatus): example of a quantitative approach based on immunoblotting. J Wildl Dis. 2018;54(4):870–873. doi:10.7589/2017-11-287
7. Shadduck JA, Orenstein JM. Comparative pathology of microsporidiosis. Arch Pathol Lab Med. 1993;117(12):1215–1219.
8. Vávra J, Lukeš J. Microsporidia and ‘the art of living together’. Adv Parasitol. 2013;82:253–319.
9. Didier ES, Khan IA. The immunology of microsporidiosis in mammals. In: Weiss LM, Becnel JJ, editors. Microsporidia Pathogens of Opportunity. Chichester UK: John Wiley & Sons, Inc.; 2014.
10. Didier ES, Vossbrinck CR, Baker MD, Rogers LB, Bertucci DC, Shadduck JA. Identification and characterization of three Encephalitozoon cuniculi strains. Parasitology. 1995;111(4):411–421. doi:10.1017/S0031182000065914
11. Talabani H, Sarfati C, Pillebout E, van Gool T, Derouin F, Menotti J. Disseminated infection with a new genovar of Encephalitozoon cuniculi in a renal transplant recipient. J Clin Microbiol. 2010;48(7):2651–2653. doi:10.1128/JCM.02539-09
12. Didier ES, Stovall ME, Green LC, Brindley PJ, Sestak K, Didier PJ. Epidemiology of microsporidiosis: sources and modes of transmission. Vet Parasitol. 2004;126(1–2):145–166. doi:10.1016/j.vetpar.2004.09.006
13. Weber R, Bryan RT. Microsporidial infections in immunodeficient and immunocompetent patients. Clin Infect Dis. 1994;19(3):517–521. doi:10.1093/clinids/19.3.517
14. Weber R, Bryan RT, Schwartz DA, Owen RL. Human microsporidial infections. Clin Microbiol Rev. 1994;7(4):426–461. doi:10.1128/CMR.7.4.426
15. Weiss LM. And now microsporidiosis. Ann Intern Med. 1995;123(12):954–956. doi:10.7326/0003-4819-123-12-199512150-00012
16. Weiss LM, Keohane EM. Microsporidia at the turn of the millenium: raleigh 1999. J Eukaryot Microbiol. 1999;46(5):3S–5S. doi:10.1111/j.1550-7408.1999.tb06055.x
17. Wittner M, Weiss L. The Microsporidia and Microsporidiosis. Washington DC: ASM Press; 1995.
18. Del Aguila C, Rueda C, de la Camara C, Fenoy S. Seroprevalence of anti-Encephalitozoon antibodies in Spanish immunocompetent subject. J Microbiol. 2001;48:75–78.
19. Enriquez FJ, Taren D, Cruz-Lopez A, Muramoto M, Palting JD, Cruz P. Prevalence of intestinal encephalitozoonnosis in Mexico. Clin Infect Dis. 1998;26(5):1227–1229. doi:10.1086/520278
20. Lopez-Velez R, Turrientes MC, Garron C, et al. Microsporidiosis in travelers with diarrhea from the tropics. J Travel Med. 1999;6(4):223–237. doi:10.1111/j.1708-8305.1999.tb00522.x
21. Lores B, Lopez-Miragaya I, Arias C, Fenoy S, Torres J, Del Aguila C. Intestinal microsporidiosis due to Enterocytozoon bieneusi in elderly human immunodeficiency virus–negative patients from Vigo, Spain. Clin Infect Dis. 2002;4(7):918–921. doi:10.1086/339205
22. Müller A, Bialek R, Kamper A, Fatkenheuer G, Salzberger B, Franzen C. Detection of microsporidia in travelers with diarrhea. J Clin Microbiol. 2001;39(4):1630–1632. doi:10.1128/JCM.39.4.1630-1632.2001
23. van Gool T, Vetter JCM, Weinmayr B, van Dam A, Derouin F, Dankert J. High seroprevalence of Encephalitozoon species in immunocompetent subjects. J Infect Dis. 1997;175(4):1020–1024. doi:10.1086/513963
24. Morio F, Poirier P, Le Govic Y, et al. Assessment of the first commercial multiplex PCR kit (ParaGENIE crypto-micro real-time PCR) for the detection of Cryptosporidium spp., Enterocytozoon bieneusi, and Encephalitozoon intestinalis from fecal samples. Diagn Microbiol Infect Dis. 2019;95(1):34–37. doi:10.1016/j.diagmicrobio.2019.04.004
25. Desoubeaux G, Caumont C, Passot C, et al. Two cases of opportunistic parasite infections in patients receiving alemtuzumab. J Clin Pathol. 2012;65(1):92–95. doi:10.1136/jclinpath-2011-200403
26. Sak B, Brady D, Pelikánová M, Květoňová D, Rost M, Kostka M. Unapparent microsporidial infection among immunocompetent humans in the Czech Republic. J Clin Microbiol. 2011;49(3):1064–1070. doi:10.1128/JCM.01147-10
27. Sak B, Kváč M, Kučerová Z, Květoňová D, Saková K. Latent microsporidial infection in immunocompetent individuals – a longitudinal study. PLoS Negl Trop Dis. 2011;5(5):e1162. doi:10.1371/journal.pntd.0001162
28. Ditrich O, Chrdle A, Sak B, et al. Encephalitozoon cuniculi genotype I as a causative agent of brain abscess in an immunocompetent patient. J Clin Microbiol. 2011;49(7):2769–2771. doi:10.1128/JCM.00620-11
29. Kicia M, Weselowska M, Kopacz Z, et al. Disseminated infection of Encephalitozoon cuniculi associated with osteolysis of hip periprosthetic tissue. Clin Infect Dis. 2018;67(8):1228–1234. doi:10.1093/cid/ciy256
30. Lallo MA, da Costa LF, de Castro JM. Effect of three drugs against Encephalitozoon cuniculi infection in immunosuppressed mice. Antimicrob Agents Chemother. 2013;57(7):3067–3071. doi:10.1128/AAC.00157-13
31. Koudela B, Lom J, Vítovec J, Kučerová Z, Ditrich O, Trávníček J. In vivo efficacy of albendazole against Encephalitozoon cuniculi in SCID mice. J Eukaryot Microbiol. 1994;41:49–50.
32. Kotková M, Sak B, Květoňová D, Kváč M. Latent microsporidiosis caused by Encephalitozoon cuniculi in immunocompetent hosts: a murine model demonstrating the ineffectiveness of the immune system and treatment with albendazole. PLoS One. 2013;8(4):e60941. doi:10.1371/journal.pone.0060941
33. Sak B, Brdíčková K, Holubová N, Květoňová D, Hlásková L, Kváč M. Encephalitozoon cuniculi genotype III evinces a resistance to albendazole treatment in both immunodeficient and immunocompetent mice. Antimicrob Agents Chemother. 2020;64(5):e00058–20. doi:10.1128/AAC.00058-20
34. De Bosscuere H, Wang Z, Orlandi PA. First diagnosis of Encephalitozoon intestinalis and E. hellem in a European brown hare (Lepus europaeus) with kidney lesions. Zoonoses Public Health. 2007;54(3–4):131–134. doi:10.1111/j.1863-2378.2007.01034.x
35. Katzwinkel-Wladarsch S, Lieb M, Helse W, Löscher T, Rinder H. Direct amplification and species determination of microsporidian DNA from stool specimens. Trop Med Int Health. 1996;1(3):373–378. doi:10.1046/j.1365-3156.1996.d01-51.x
36. Wolk DM, Schneider SK, Wengenack NL, Sloan LM, Rosenblatt JE. Real-time PCR method for detection of Encephalitozoon intestinalis from stool specimens. J Clin Microbiol. 2002;40(11):3922–3928. doi:10.1128/JCM.40.11.3922-3928.2002
37. Dai J, Wang P, Adusumilli S, et al. Antibodies against a tick protein, Salp15, protect mice from the Lyme disease agent. Cell Host Microbe. 2009;6(5):482–492. doi:10.1016/j.chom.2009.10.006
38. Sak B, Jandová A, Doležal K, et al. Effects of selected Indonesian plant extracts on E. cuniculi infection in vivo. Exp Parasitol. 2017;181:94–101. doi:10.1016/j.exppara.2017.07.014
39. Sak B, Kotková M, Hlásková L, Kváč M. Limited effect of adaptive immune response to control encephalitozoonosis. Parasite Immunol. 2017;39(12):e12496. doi:10.1111/pim.12496
40. Brown JH, Brenn L. A method for the differential gram-positive and gram-negative bacteria in tissue sections. Bull Johns Hopkins Hosp. 1931;48:69.
41. Kotková M, Sak B, Kváč M. Differences in the intensity of infection caused by Encephalitozoon cuniculi genotype II and III – comparison using quantitative real-time PCR. Exp Parasitol. 2018;192:93–97. doi:10.1016/j.exppara.2018.07.019
42. Didier ES, Weiss LM. Microsporidiosis: not just in AIDS patients. Curr Opin Infect Dis. 2011;24(5):490–495. doi:10.1097/QCO.0b013e32834aa152
43. Kicia M, Szydłowict M, Cebulski K, et al. Symptomatic respiratory Encephalitozoon cuniculi infection in renal transplant recipients. Int J Infect Dis. 2019;79:21–25. doi:10.1016/j.ijid.2018.10.016
44. Mungthin M, Subrungruang I, Naaglor T, Aimpun P, Areekul W, Leelayoova S. Spore shedding pattern of Enterocytozoon bieneusi in asymptomatic children. J Med Microbiol. 2005;54(5):473–476. doi:10.1099/jmm.0.45832-0
45. Goodgame R, Stager C, Marcantel B, Alcocer E, Segura AM. Intensity of infection in AIDS-related intestinal microsporidiosis. J Infect Dis. 1999;180(3):929–932. doi:10.1086/314914
46. Couzinet S, Cejas E, Schittny J, Deplazes P, Weber R, Zimmerli S. Phagocytic uptake of Encephalitozoon cuniculi by nonprofessional phagocytes. Infect Immun. 2000;68(12):6939–6945. doi:10.1128/IAI.68.12.6939-6945.2000
47. Nassonova E, Tokarev YS, Trammer T, Entzeroth R, Sokolova J. Phagocytosis of Nosema grylli (microsporidia, nosematidae) spores in vivo and in vitro. J Eukaryot Microbiol. 2001;48:83–84. doi:10.1111/j.1550-7408.2001.tb00462.x
48. Fischer J, Tran D, Juneau R, Hale-Donze H. Kinetics of Encephalitozoon spp. infection of human macrophages. J Parasitol. 2008;94(1):169–175. doi:10.1645/GE-1303.1
49. Niederkorn JY, Shadduck JA. Role of antibody and complement in the control of Encephalitozoon cuniculi infections by rabbit macrophages. Infect Immun. 1980;27(3):995–1002. doi:10.1128/IAI.27.3.995-1002.1980
50. Weidner E. Interactions between Encephalitozoon cuniculi and macrophages. Parasitophorous vacuole growth and the absence of lysosomal fusion. Z Parasitenkd. 1975;47(1):1–9. doi:10.1007/BF00418060
51. Didier ES, Bowers LC, Martin AD, Kuroda MJ, Khan IA, Didier PJ. Reactive nitrogen and oxygen species, and iron sequestration contribute to macrophage-mediated control of Encephalitozoon cuniculi (phylum microsporidia) infection in vitro and in vivo. Microbes Infect. 2010;12(14–15):1244–1251. doi:10.1016/j.micinf.2010.09.010
52. Didier ES, Shadduck JA. IFN-gamma and LPS induce murine macrophages to kill Encephalitozoon cuniculi in vitro. J Eukaryot Microbiol. 1994;41:34S.
53. Schmidt EC, Shadduck JA. Mechanisms of resistance to the intracellular protozoan Encephalitozoon cuniculi in mice. J Immunol. 1984;133(5):2712–2719.
54. Jelínek J, Salát J, Sak B, Kopecký J. Effect of interferon gamma and specific polyclonal antibody on the infection of murine peritoneal macrophages and murine macrophage cell line PMJ2-R with Encephalitozoon cuniculi. Folia Parasitol. 2007;54(3):172–176. doi:10.14411/fp.2007.024
55. Didier ES, Varner PW, Didier PJ, et al. Experimental microsporidiosis in immunocompetent and immunodeficient mice and monkeys. Folia Parasitol. 1994;41(1):1–11.
56. Sak B, Vecková T, Brdíčková K, et al. Experimental Encephalitozoon cuniculi infection acquired from fermented meat products. Foodborne Pathog Dis. 2019;16(6):394–398. doi:10.1089/fpd.2018.2569
57. Bannoura S, El Hajj R, Khalifeh I, El Hajj H. Acute disseminated encephalomyelitis and reactivation of cerebral toxoplasmosis in a child: case report. IDCases. 2018;13:e00434. doi:10.1016/j.idcr.2018.e00434
58. Kotková M, Sak B, Hlásková L, Kváč M. The course of infection caused by Encephalitozoon cuniculi genotype III in immunocompetent and immunodeficient mice. Exp Parasitol. 2017;182:16–21. doi:10.1016/j.exppara.2017.09.022
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.