Back to Journals » Veterinary Medicine: Research and Reports » Volume 10
Emphysematous cystitis: review of current literature, diagnosis and management challenges
Authors Fumeo M , Manfredi S , Volta A
Received 29 March 2019
Accepted for publication 5 July 2019
Published 30 July 2019 Volume 2019:10 Pages 77—83
DOI https://doi.org/10.2147/VMRR.S210463
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Young Lyoo
Video abstract presented by Martina Fumeo.
Views: 7761
Martina Fumeo, Sabrina Manfredi, Antonella Volta
Department of Veterinary Science, University of Parma, 43126 Parma, Italy
Abstract: Emphysematous cystitis (EC) is a rare disease in human as well as in veterinary medicine; in both it is defined as an uncommon form of complicated urinary tract infection (UTI), characterized by the presence of gas within the bladder wall and lumen. While female dogs are at increased risk of developing an UTI, patients with EC are not subject to gender or age predisposition and may present with variable clinical manifestations. Diabetes mellitus (DM) appears as the most common risk factor for the development of EC, but many other underlying conditions have been mentioned in literature and should be taken into consideration. In case of EC, Escherichia coli appears to be the most common pathogen isolated from urine cultures. A conclusive diagnosis of EC requires necessarily the recourse to imaging methods, such as abdominal radiography and ultrasonography. An early diagnosis and appropriate medical therapy, consisting in protracted antibiotic treatment associated with control over underlying diseases, could lead to avoid surgical intervention.
Keywords: emphysematous cystitis dog, dog, cat, gas, urinary, bladder, infection
Introduction
Emphysematous cystitis (EC) is a rare disease in human as well as in veterinary medicine; in both, it is defined as an uncommon form of complicated urinary tract infection (UTI), characterized by the presence of gas within the bladder wall and lumen.1–3
It was first portrayed as pneumaturia in 1671 in human medicine1 and finally defined as “cystitis emphysematosa” by Bailey in 1961, when a link between EC and pneumaturia was first identified.2,3 In fact, pneumaturia is described as the passage of gas or “air” in the urine, a condition that can also occur for iatrogenic causes, for example, through the use of instruments in the urogenital tract, or in case of fistulous connections between the bladder and colon or the vagina, while EC is an inflammatory disease of the bladder generally resulting from a gas-producing bacterial infection.4
EC has also been reported in dogs, cats and in a cow,5 and it was first reported in a diabetic dog in 1926.6 Since then, EC has been described both in diabetic and in nondiabetic dogs and cats.
The aim of this review is to offer a general and inclusive description in dogs, with an eye to human medicine.
Prevalence and impact
EC is a rare form of complicated UTI whose clinical diagnosis is based on evidence of gas accumulation within the bladder lumen and wall found with diagnostic imaging techniques. Radiographic or ultrasonographic examinations are rarely requested in dogs with bacteriuria or glycosuria, causing a probable underestimation of this disease, whose true prevalence is still unknown.7
EC is almost always associated with glucosuria and complicated urinary tract infection, but by far the most common occurrences both in humans and in animals are in patients with diabetes mellitus;4 in humans, almost 70% of the patients have diabetes mellitus.1
EC has been diagnosed in patients with primary renal glycosuria (Fanconi’s syndrome), urinary tract obstruction, chronic urinary tract infections, neurogenic bladder dysfunction, morphologic abnormalities and immunosuppression.4,8–11
The exact mechanism of pneumaturia is still unclear and not fully understood. However, in diabetic patients, as well as in case of primary renal glycosuria, it has been suggested that carbon may be produced through fermentation by the pathogens, whenever glucose concentrations are high. Since the infection also affects nondiabetics, it is thought that tissue proteins and urinary lactulose can also serve as substrates.10 On the other hand, the occurrence of gas formation only in some patients with a UTI could be explained by survival mechanisms of bacteria in patients with certain comorbidities associated with EC or by the presence of specific virulence factors in some uropathogenic bacteria.7 Impaired gas transport due to local inflammation or obstructive processes (which increase intracellular pressure while simultaneously decreasing circulation) may also increase gas accumulation within the tissues and the risk of pneumaturia.7,11 The release of bacterial endotoxins in complicated UTIs may contribute to the inflammatory process and may induce urinary tract paralysis and urinary stasis.2,11
Other potential risk factors identified in dogs include vesicoureteral reflux, bladder trigone diverticulum and long-term administration of steroids.5,7,12 With regard to prolonged glucocorticoid administration, impairment of host defense mechanisms is probably one the most important factor disposing the patient to recurrent complicated UTI,4 assuming that an immunosuppressive therapy (for example, with steroids but even with chemotherapeutic drugs) is a potential risk factor for developing recurrent UTI and EC.
Finally, a recent report described an emphysematous pyonephrosis occurring in a dog with congenital extrahepatic portosystemic shunt (PSS);13 although the report did not mention the occurrence of EC, nonetheless, PSS could be considered as a potential risk factor of the disease, as it may cause a urinary obstruction and UTI.
In humans, cases of EC are reported approximately twice as often in women as in men; in general, middle-aged, diabetic women appear to be at a significantly higher risk for disease.1 While female dogs and cats are at increased risk of developing an UTI, and several studies demonstrated an increased risk with increasing age, for EC no gender nor age predisposition has been reported in dogs or cats until now.8,14
As mentioned before, no studies were focused on the prevalence of the disease, as it may be difficult to evaluate due to its underestimation; even so, EC is most frequently associated with diabetic patients. Despite that, in two retrospective studies made on a quite numerous sample, diabetes mellitus was present, respectively, in 33.3% out of 27 dogs and in 10, 5% out of 36 dogs and 2 cats, while patients with neurologic diseases (26% and 18, 4%, respectively), impaired immune system (22, 2% and 26, 3%), different forms of complicated or recurrent UTI (65, 8%) and other underlying disease were more represented than it usually appears.7,15 This could suggest that not only further studies could be made about EC prevalence, but also that in dogs DM could not represent the primary risk factors, giving more importance to other predisposing pathologies.
Microbiological agents
Most of the UTIs in dogs and cats (approximately 75%) involve a single agent, with Escherichia coli being responsible for up to half of the infections in dogs and being also the most common pathogens in cats.14
In humans, the two major organisms isolated in urine cultures in cases of EC are Escherichia coli (60%) and Klebsiella pneumoniae (10–20%).1 Other microorganisms isolated in human EC cases include Klebsiella aerogenes (previously known as Enterobacter aerogenes), Proteus mirabilis, Aerobacter spp., Citrobacter spp., Staphylococcus aureus, Streptococcus spp., Nocardia spp., Clostridium perfringes and Clostridium welchii, some fungi such as Candida albicans, Candida tropicalis and even Aspergillus spp.1,4
In dogs and cats, E. coli is the most common urinary tract pathogen, with and without EC, as well as both in diabetic and in nondiabetic small animals.4,7 Other gas-producing bacteria previously reported in animals include Klebsiella spp., Proteus spp., Klebsiella aerogenes and Costridium perfringens,4 and mixed infections are not infrequent. Based on the cases reported until now, it could be assumed that the bacterial species isolated are often similar to those isolated from humans. On the other hand, although according to our knowledge fungi have not been reported as microbiological agents of EC in animals until now, they should be considered as potential pathogens.
Clinical signs and laboratory findings
Clinical presentation is variable. Both in humans and in dogs, EC may present similar to uncomplicated cystitis, which is characterized by nonspecific UTI signs such as hematuria, dysuria, stranguria or pollakiuria, abdominal pain, and urinary urgency and frequency.7 In some cases, pneumaturia is present and could represent a more specific clinical sign, but it is often not recognized or not noted; in humans, it is observed in 70% of patients with bladder catheterization.1 Fever has been reported as a common feature in humans, even without emphysematous pyelonephritis (EP), in contrast with the animal population, in which this clinical sign was unfrequently described.1,7
Patients may be asymptomatic or showing signs associated to their underlying diseases. It should be taken into consideration that EP and peritonitis have been reported as consequences of EC in dogs, cats, and humans,1,7,8,12,16 and in human medicine, a case of severe sepsis secondary to EC has been described.18 Early detection of EC is therefore of utter importance; therefore, greater attention is required on the presence of risk factors for EC.
Urinalysis and aerobic and anaerobic urine culture should be performed routinely and are recommended as a starting point of the diagnostic process if any form of UTI is suspected.14 Antibiogram should then be performed, in order to implement effective antibiotic therapy. In the reported cases of EC in veterinary medicine, cultures have been achieved from samples collected via cystocentesis, sterile catheterization or, in few cases, by mid-stream voiding.4,5,7–9,12–14,16 Cystocentesis is a simple but invasive form of paracentesis used to obtain sterile urine samples; it has been reported that in some cases, a few bubbles of air entrapped in the wall bladder can be detected after this procedure.17 Therefore, both cystocentesis and catheterization can be considered as procedures that could introduce air in the bladder lumen or wall and should be performed only after any diagnostic imaging evaluation.
Urinalysis usually reveals hematuria, pyuria and bacteriuria, and often glycosuria.4,5,7–9
Finally, evaluation of complete blood count (CBC) and serum biochemistry panels should be performed, although there are no specific data suggesting the presence of EC. CBC is advised to evaluate the presence of systemic or local inflammation,7 and together with serum biochemical profiles, it should be estimated to detect the eventual underlying condition, such as endocrine or renal diseases. In human medicine, it has been reported that 50% of patients with EC have bacteremia, advising blood culture as an additional laboratory test.1
Diagnostic imaging
Diagnostic imaging techniques are necessary for the diagnosis of EC, which is based on detection of an inflammatory disease of the bladder with gas accumulation in the lumen and/or in the wall of the urinary bladder by radiography, ultrasonography and/or CT in humans and dogs. EC is a cause of pneumaturia (defined as “air in the urine”). Gas in the urinary tract may also be iatrogenically introduced or may be secondary to a fistulous connection with the intestine or genital tract.12
Although diagnostic imaging is not recommended for uncomplicated UTI diagnosis, it may be considered as a useful tool to better characterize the lesions and eventually rule out EC especially in recurrent or complicated UTI, in particular in those cases where EC risk factors are present, and where the urine culture is positive.7
Iatrogenic causes such as procedures that could introduce air into the bladder (eg, catheterization, cystocentesis, cystoscopy, urological surgery) and anatomic conditions (such as fistulas between the vagina/bowel and urinary bladder) must first be ruled out.7
Plain conventional abdominal radiography is the most common imaging method for EC detection both in humans and in dogs.1,5,8,17,18 Plain radiography shows a radiolucent line of gas around the bladder wall, separated from the more dorsal rectal gas (Figure 1). The presence of air in the bladder wall is characterized by a cobblestoned or “beaded necklace” appearance, reflecting irregular thickening of the nondependent mucosal surface due to submucosal blebs.1 Three radiographic stages of EC have been described in human patients.20 In stage one, a clear 1-mm zone may be seen around the contrast medium and free gas is not present in the bladder lumen. In stage two, the bladder wall is irregular and thickened because of increased intramural gas, and there is still no free intraluminal gas. In stage three, free gas in the bladder lumen is evident radiographically. The lateral ligaments of the bladder may also become emphysematous, as it has been described in one dog.21 However, a radiographic classification has not been adopted yet in dogs. Gas accumulation in the urinary tract can be difficult to differentiate from air in overlying loops of bowel by plain radiography, an explanation to its low sensitivity reported in the human literature (33%).12,18 Moreover, in veterinary medicine, if the patient is emaciated, radiography may not provide adequate morphologic information on the kidneys, making the detection of an emphysematous upper UTI difficult to achieve.12
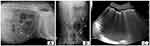 |
Figure 1 Radiographic (A, B) and ultrasonographic (C) appearance of an emphysematous cystitis by E. coli in a female 9-year-old Labrador retriever with a history of diabetes mellitus. |
For these reasons, some authors have suggested the superiority of ultrasonography to radiography for the detection of emphysematous changes particularly at an early stage and when only a small amount of gas is present.8,12,17
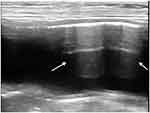
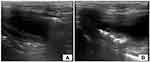
Ultrasonographic findings of mural EC include a hyperechoic stripe with reverberation artifact in the superficial bladder wall (Figure 2), while accumulation of gas immediately deep to the superficial urinary bladder mucosa with characteristic reverberation/ring down artifact is seen with luminal EC. Scanning the patient in both recumbent and standing positions (Figure 3) can help to differentiate movable, gravity-dependent calculi and hematomas from adherent mass lesions or to differentiate free gas in the bladder lumen from intramural gas.8,19
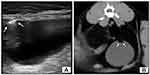
In humans, CT is the recommended imaging modality of choice for diagnosis, monitoring and prognosis of EC and emphysematous UTIs in general, having a reported sensitivity and specificity as high as 100%.1,2,10,11,16 CT can better define the extent and severity of disease and detect mild cases as well as rule out other causes of urinary gas such as vesicocolic fistulas.8,22 Although the use of CT for diagnosis of EC or emphysematous pyelonephritis has not been described thoroughly in the veterinary literature,8 a CT scan of the abdomen should be considered as a useful tool to reveal the presence of gas bubbles in the renal parenchyma and in the upper urinary tract in dogs as in humans (Figure 4). Even so, the risks associated with general anesthesia or sedation in debilitated patients should be carefully evaluated.12
Finally, cystoscopy could be evaluated as a different imaging modality for detection of EC; in human medicine, this examination is indicated as useful in revealing the presence of bladder outlet obstruction,1 but some authors report that it is not essential for diagnosis.18
Management and outcome
Antibiotic administration is the medical therapy of choice for EC, opportunely adjusted to the results of urine and/or blood cultures. In humans, medical therapy consists of antibiotics, bladder drainage by catheterization and treatment of predisposing conditions.1 Establishing glycemic control in diabetic patients is of utter importance, as many of the reported cases in literature involve poorly controlled diabetes.
Ideally, EC treatment should be based on bactericidal and lipid soluble drugs, which should reach high concentrations both in urine and in tissue, as infection may extend beyond the urothelium.7 Trimethoprim-sulfamethoxazole is recommended as a first-line drug for the treatment of UTIs by the International Society for Companion Animal Infectious Diseases. Dogs with mixed infections that include Enterococcus spp. or Streptococcus spp. may require the addition of a penicillin, since a certain resistance to TMS has been shown by these organisms.7 In the context of increasing antimicrobial resistance worldwide, E. coli is one of the microorganisms to be monitored because of its frequent acquisition of antibiotic resistance mechanisms, including toward those antibiotics used as first-line treatment such as TMS or β-lactamics. Therefore, urine culture and antibiogram should be performed also during treatment, preferably 7 days after starting antibiotic therapy and 1 week after completing it.14
The duration of the medical treatment is unclear. Generally, treatment of complicated UTI is for 4 weeks.14
In complicated ECs, with an ascending infection of the urinary tract, surgical therapy may be needed; the severity of the disease determines the surgical method, for example, surgical debridement, partial cystectomy, total cystectomy or even nephrectomy in combined EC/EP cases.
The prognosis for canine patients with EC is generally favorable.4,5,19
In humans, the overall death rate among patients with EC has been reported to be approximately 7%.1 Nonetheless, if not treated promptly and adequately, EC can affect the upper UT and develop in EP, a disease with a poorer prognosis both in humans and in dogs, requiring more aggressive treatment and even surgical intervention in some cases.8,12,13
A good prognosis could be achieved only through an early diagnosis of EC, followed by a prompt medical management.
Conclusions
EC is a rare form of UTI characterized by the presence of gas within the bladder wall and/or lumen. It affects primarily patients with severe or uncontrolled DM, although it has been described in nondiabetic patients and it recognizes different potential risk factors. Urine culture and antibiogram should be performed in order to set up an appropriate antibiotic therapy, which could reduce the need for surgery, together with an early diagnosis. The prevalence of EC could be underestimated as diagnostic imaging is necessary for the detection of this condition, and it has been suggested that ultrasonography may be a more sensitive technique for detection of gas within the bladder at an early stage. No significant clinical features strongly suggestive of EC have been reported to date; hence, it could be assumed that every patient presenting UTI signs, even mildly, and with a history of diabetes or other risk factors, should be evaluated radiographically and ultrasonographically, to detect or exclude the presence of EC.
Acknowledgments
The authors declare that no financial support was received for this study.
Author contributions
Substantial contributions to conception and design, data acquisition, or data analysis and interpretation: Fumeo M, Manfredi S, Volta A. Drafting the article or critically revising it for important intellectual content: Fumeo M, Manfredi S, Volta A. Final approval of the version to be published: Fumeo M, Manfredi S, Volta A. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of the work are appropriately investigated and resolved: Fumeo M, Manfredi S, Volta A.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Amano M, Shimizu T. Emphysematous cystitis: a review of the literature. Inter Med. 2014;53(2):79–82. doi:10.2169/internalmedicine.53.1121
2. Thomas AA, Lane BR, Thomas AZ, Remer EM, Campbell SC, Shoskes DA. Emphysematous cystitis: a review of 135 cases. BJU Int. 2007;100(1):17–20. doi:10.1111/j.1464-410X.2007.06930.x
3. Bailey H. Cystitis emphysematosa; 19 cases with intraluminal and interstitial collections of gas. Am J Roentgenol Radium Ther Nucl Med. 1961;86:850–862.
4. Aizenberg I, Aroch I. Emphysematous cystitis due to Escherichia coli associated with prolonged chemotherapy in a non-diabetic dog. J Vet Med Ser B. 2003;50(8):396–398. doi:10.1046/j.1439-0450.2003.00692.x
5. Lobetti RGL, Goldin JP. Emphysematous cystitis and bladder trigone diverticulum in a dog. J Small Anim Pract. 1998;39(3):144–147.
6. Heuper W. Cystitis emphysematosa. Am J Pathol. 1926;2:159–165.
7. Merkel LK, Lulich J, Polzin D, Ober C, Westropp J, Sykes J. Clinicopathologic and microbiologic findings associated with emphysematous cystitis in 27 dogs. J Am Anim Hosp Assoc. 2017;553(6):313–320. doi:10.5326/JAAHA-MS-6722
8. Moon R, Biller DS, Smee NM. Emphysematous cystitis and pyelonephritis in a nondiabetic dog and a diabetic cat. J Am Anim Hosp Assoc. 2014;50(2):124–129. doi:10.5326/JAAHA-MS-5972
9. Davies NL, Williams JH. Emphysematous cystitis in a non-diabetic cat. J S Afr Vet Assoc. 1993;64(4):162–164.
10. Grupper M, Kravtsov A, Potasman I. Emphysematous cystitis: illustrative case report and review of the literature. Medicine. 2007;86(1):47–53. doi:10.1097/MD.0b013e3180307c3a
11. Eken A, Alma E. Emphysematous cystitis: the role of CT imaging and appropriate treatment. Can Urol Assoc J. 2013;7(11–12):E754. doi:10.5489/cuaj.472
12. Fabbi M, Manfredi S, Bianchi E, Gnudi G, Miduri F, Volta A. Emphysematous pyelitis and cystitis associated with vesicoureteral reflux in a diabetic dog. Can Vet J. 2016;57(4):382.
13. Lim J, Yoon Y, Jung D, Yeon S, Lee H. Emphysematous pyonephrosis associated with extrahepatic portosystemic shunt in a dog. J Vet Med Sci. 2016;78(4):697–700. doi:10.1292/jvms.15-0181
14. Byron JK. Urinary tract infection. Vet Cl Small Anim Pract. 2019;49(2):211–221.
15. Lippi I, Mannucci T, Santa DD, Barella G, Oranges M, Citi S. Emphysematous cystitis: retrospective evaluation of predisposing factors and ultrasound features in 36 dogs and 2 cats. Can Vet J La Rev Veterinaire Canadienne. 2019;60(5):514–518.
16. Gould EN, Cohen TA, Trivedi SR, Kim JY. Emphysematous pyelonephritis in a domestic shorthair cat. J Feline Med Surg. 2016;18(4):357–363. doi:10.1177/1098612X15600481
17. Manfredi S, Carvalho C, Fonti P, et al. Complications of ultrasound-guided cystocentesis in companion animals: 21 cases (2005-2016). Turk J Vet Anim Sci. 2018;42(5):459–466. doi:10.3906/vet-1802-11
18. Gargouri MM, Abid K, Kallel Y, Rhouma SB, Chelif M, Nouira Y. Severe sepsis secondary to emphysematous cystitis. Afr J Urol. 2015;21(1):41–43. doi:10.1016/j.afju.2014.11.001
19. Petite A, Busoni V, Heinen MP, Billen F, Snaps F. Radiographic and ultrasonographic findings of emphysematous cystitis in four nondiabetic female dogs. Vet Radiol Ultrasound. 2006;47(1):90–93. doi:10.1111/j.1740-8261.2005.00112.x
20. Ney C, Friedenberg RM. Inflammation of the bladder. In: Ney C, Friedenberg RM, editors. Radiographic Atlas of the Genitourinary System.
21. Root CR, Scott RC. Emphysematous cystitis and other radiographic manifestations of diabetes mellitus in dogs and cats. J Am Vet Med Assoc. 1971;158:721–728.
22. Cruse AM, Vaden SL, Mathews KG, Hill TL, Robertson ID. Use of computed tomography (CT) scanning and colorectal new methylene blue infusion in evaluation of an English Bulldog with a rectourethral fistula. J Vet Inter Med. 2009;23(4):931–934. doi:10.1111/j.1939-1676.2009.0320.x
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.