Back to Journals » OncoTargets and Therapy » Volume 10
Emodin suppresses proliferation, migration and invasion in ovarian cancer cells by down regulating ILK in vitro and in vivo
Authors Lu JJ, Xu Y, Zhao Z, Ke XN, Wei X, Kang J, Zong X, Mao HL, Liu PS
Received 29 March 2017
Accepted for publication 24 May 2017
Published 19 July 2017 Volume 2017:10 Pages 3579—3589
DOI https://doi.org/10.2147/OTT.S138217
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ingrid Espinoza
This paper has been retracted.
Jingjing Lu,1,2,* Ying Xu,1,* Zhe Zhao,1 Xiaoning Ke,2 Xuan Wei,1 Jia Kang,1 Xuan Zong,1 Hongluan Mao,1 Peishu Liu1
1Department of Obstetrics and Gynecology, Qilu Hospital of Shandong University, Shandong, 2Department of Obstetrics and Gynecology, Handan Central Hospital, Handan, People’s Republic of China
*These authors contributed equally to this work
Objective: Although our previous studies have confirmed that 1, 3, 8-trihydroxy-6-methylanthraquinone (emodin) inhibits migration and invasion in epithelial ovarian cancer (EOC) cells, the underlying molecular mechanism remains unknown. Here, the aim was to investigate the effects of emodin on EOC cells and to study further the mechanism underlying this process, both in vitro and in vivo.
Materials and methods: Cell proliferation was evaluated by the methylthiazolyl tetrazolium assay. Cell migration and invasion abilities were tested using the transwell assay. The expression of integrin-linked kinase (ILK) and epithelial–mesenchymal transition (EMT)-associated factors were measured with western blotting.
Results: Exogenous ILK enhanced the proliferation, migration and invasion properties of A2780 and SK-OV-3 cells. After treatment with emodin, the survival rate of cells was gradually reduced, including those of SK-OV-3/pLVX-ILK and A2780/pLVX-ILK cells, with increasing emodin concentrations. The migration and invasion abilities of A2780 and SK-OV-3 cells were effectively increased by the transfection of pLVX-ILK, which could be abrogated by following this with 48 hours of emodin treatment. Treatment with emodin significantly downregulated the expression of ILK and EMT-related proteins. So, emodin suppressed proliferation, migration and invasion in ovarian cancer cells by downregulating ILK in vitro. SK-OV-3/pLVX-Con and SK-OV-3/pLVX-ILK cells were used to generate xenografts in nude mice. Tumors grew more rapidly in the SK-OV-3/pLVX-ILK group compared with the control group, and this could be significantly inhibited by emodin. Also, the expression of E-cadherin was downregulated, while the expression of Slug, MMP-9 and Vimentin were upregulated in the SK-OV-3/pLVX-ILK group, and this could be reversed by following treatment with emodin. Emodin did not demonstrate target toxicity on hepatocytes, nephrocytes and cardiomyocytes.
Conclusion: Emodin suppresses proliferation, migration and invasion in ovarian cancer by targeting ILK.
Keywords: emodin, ILK, epithelial ovarian cancer, epithelial–mesenchymal transition, Slug, xenografts in nude mice
Introduction
Epithelial ovarian cancer (EOC) is the most lethal disease among female genital malignant tumors.1 The lack of effective early detective markers and pathognomonic clinical symptoms means that there is currently no effective technique for the routine screening of the population.2 As a consequence, 80% of ovarian cancers are diagnosed at the advanced stages with widespread metastases.3 The prevalence of metastasis arises from the poor prognosis of EOC and leads to high mortality rates. Hence, clarifying the molecular mechanisms underlying metastases is the approach with the greatest potential for improving the outcome for EOC patients.
Integrin-linked kinase (ILK) is a 59 kDa serine/threonine protein kinase, which interacts with the cytoplasmic domain of integrin β1 and β3.4,5 ILK is viewed as a core regulator of the phosphoinositide 3-kinase signaling pathway. Once activated, ILK directly activates kinase B/Akt (PKB/Akt) and restrains glycogen synthase kinase 3β (GSK3β) to regulate the extracellular matrix. ILK has been implicated in multiple cellular processes including cell survival, cell cycle progression, inflammation, tumorigenesis and epithelial–mesenchymal transition (EMT).6,7
EMT is a vital process during embryogenesis, tissue remodeling, wound healing and tumor metastasis, where cells lose their epithelial phenotypes, such as E-cadherin, and acquire mesenchymal traits, such as Vimentin.8–10 Overexpression of ILK has been found to occur in prostate cancer,11 bladder cancer,12 colorectal cancer13 and other cancers,14,15 and this promotes cancer cell invasion and migration by regulating EMT-related genes. The overexpression of ILK in rat epithelial cells has been reported to induce cell growth in culture and tumorigenesis.16 Downregulation of ILK inhibits EMT and metastasis in bladder cancer cells.17 The same phenomenon has been found in EOC.18,19 ILK expression is positively correlated with ovarian tumor grade, and ILK modulates the metastatic behavior of ovarian cancer cells.20,21
1, 3, 8-Trihydroxy-6-methylanthraquinone (emodin) has been isolated from the rhizomes of rhubarb, aloes and other plants known to possess anti-bacterial, anti-inflammatory, immunosuppressive and anti-cancer effects.22 Emodin induces apoptosis in human lung adenocarcinoma cells.23 Recent studies have demonstrated that emodin inhibited migration and invasion through the downregulation of MMP-2 and MMP-9 in human breast cancer cells.24 In addition, emodin was shown to repress cell migration and invasion by regulating EMT-related genes in colorectal cancer, head and neck squamous cell carcinoma and ovarian cancer.25–27 Furthermore, our previous studies have demonstrated that emodin inhibits the EMT of EOC cells via the ILK/GSK3β/Slug signaling pathway in vitro.28
The present study was aimed at furthering understanding the role of ILK in EOC cells, and determining whether emodin could inhibit invasion and migration in EOC cells by inhibiting EMT by targeting ILK in vitro and in vivo.
Materials and methods
Cell lines and culture conditions
The human EOC cell lines A2780 and SK-OV-3 cells were purchased from Cell Bank (Chinese Academy of Sciences, Shanghai, People’s Republic of China). A2780 cells were maintained in RPMI-1640 medium (GE Healthcare Life Sciences, HyClone Laboratories, Logan, UT, USA), supplemented with 10% (v/v) fetal bovine serum (FBS; Gibco Life Technologies, Mount Waverley, VIC, Australia). SK-OV-3 cells were cultured in McCoy’s 5A medium (M&C Gene Technology, Beijing, People’s Republic of China) with 10% FBS (v/v). Cells were kept in an incubator at 37°C containing air/CO2 (95:5, v/v).
Antibodies and chemicals
Emodin and poloxamer 188 were purchased from Sigma Aldrich (St Louis, MO, USA). Dimethyl sulfoxide (DMSO) and propanediol were obtained from Solarbio (Beijing, People’s Republic of China). Emodin was diluted in DMSO in vitro, and the final concentration of DMSO was below 0.1% (v/v). For peritoneal injection, emodin was dissolved in a solution, which was mixed with propanediol/normal saline/poloxamer 188 (1:6:6, v/v). The primary antibodies against E-cadherin (3195S), Vimentin (5741S), Slug (9585S), ILK (3826S) and GAPDH (2118S) were purchased from Cell Signaling Technology (CST, MA, USA). The primary antibody MMP-9(ab76003) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Construction of cells with ILK overexpression
ILK was exogenously expressed in A2780 and SK-OV-3 cells by transfecting them with ILK overexpression lentiviral vector (pLVX-C1-ILK). The lentiviral-containing ILK (pLVX-ILK) or control vector (pLVX-Con) was purchased from the GenePharma Company (Shanghai, People’s Republic of China). The A2780 and SK-OV-3 cells were seeded into 24-well plates with appropriate medium with 10% FBS (v/v), treated for 6 hours and when the cell density was approximately 30%, the cells were transfected with lentiviral, according to the manufacturer’s protocol. After 48 hours of transfection, cells were selected with puromycin for 7–10 days. Then, stable, transfected cells were obtained: A2780/pLVX-ILK, A2780/pLVX-Con, SK-OV-3/pLVX-Con and SK-OV-3/pLVX-ILK.
Methylthiazolyl tetrazolium
Cell viability was assessed using the methylthiazolyl tetrazolium (MTT) assay. To detect the ability of cells to proliferate, cells were seeded into seven 96-well, flat-bottomed plates at a density of 1,000 cells/well and incubated overnight. Then, every day at the same time point, 10 μL MTT (5 g/L) solution (Sigma Aldrich) was added to each well and then incubated for an additional 4 hours before being treated with DMSO (100 μL/well). Absorbance at 490 nm using a plate reader was used as a measure of the ability of cells to proliferate (Bio-Rad, Hercules, CA, USA). To determine the effects of emodin on cell viability, cells (3,000 cells/well) were seeded into a 96-well plate and incubated overnight. Thereafter, cells were treated with increasing concentrations of emodin (0–80 μM) for 48 hours.
Transwell migration and invasion assays
Transwell migration and invasion assays were performed with 24-well Boyden chambers (Corning Costar, Cambridge, MA, USA). Cells were re-suspended in 100 μL serum-free medium (3×104 SK-OV-3 cells or 6×104 A2780 cells) and then seeded onto the top of the chamber in 100 μL serum-free medium. The lower chamber was filled with 750 μL medium containing 10% (v/v) FBS. After incubation for 24 hours, the cells that had migrated, on the lower surface of the filter, were fixed with methanol solution for 5 minutes and 3.7% (v/v) paraformaldehyde for 5 minutes and then stained with Giemsa stain. Images (200×) of stained cells were captured with an Olympus IX51 inverted microscope (Olympus Corporation, Tokyo, Japan). Cells were counted in five visual fields per chamber. For the invasion assay, Boyden chambers were pre-coated with 100 μL Matrigel (1:9 dilution in serum free medium; BD Biosciences, San Jose, CA, USA) prior to experimentation. Following this, similar steps were performed as in the migration assay.
Western blot analysis
Total cellular protein and tumor tissue protein were extracted by adding RIPA lysis buffer (Beyotime, Jiangsu, People’s Republic of China) containing 1% (v/v) phenylmethanesulfonyl fluoride (Beyotime) on ice. The proteins were separated in 10% (w/v) tris-glycine gel and transferred electrophoretically to a pure nitrocellulose blotting membrane. Primary antibodies (anti-ILK, anti-E-cadherin, anti-Vimentin, anti-Slug, anti-MMP-9 and anti-GAPDH, 1:1,000) were incubated with membranes overnight at 4°C. The following day, membranes were washed and incubated with the respective horseradish peroxidase (HRP)-conjugated second antibodies (1:3,000; Millipore, Billerica, MA, USA) for 1 hour at 37°C. Antibody-positive bands were detected with Image Quant LAS 4000. ImageJ software was used for densitometric analysis.
Xenografts in nude mice
Four-week-old female BALB/C nude mice were purchased from the HFK Bioscience Company. The treatment protocol followed the guidelines of “Laboratory Animal – Requirements of Environment and Housing Facilities”. Our research was approved by Experimental Animal Ethics Committee of Qilu Hospital of Shandong University (approval number: DWLL-2015-004). Mice were randomized into two groups, and 200 μL re-suspended serum-free PBS with either 1.0×107 viable SK-OV-3/pLVX-Con or SK-OV-3/pLVX-ILK cells was injected into the right armpit of mice, according to their group allocation. The volume (V = [length × width2]/2) of subcutaneous tumors was measured every other day. When tumor volume reached 100 mm3, each group of mice was randomized in two groups (n=6) to receive different treatments. One group was treated with an intra-peritoneal injection, every day for 21 days, of 50 mg/kg/d of emodin. The other mice were injected with the drug vehicle. On the 21st day after drug injection, blood was sampled from the eyes of the experimental mice to measure liver, renal and cardiac functions. Finally, tumors were removed, weighed and divided into two parts, one for immunohistochemical (IHC) staining and the other for immunoblotting assays.
Immunohistochemistry
Tumor xenografts were stored in 3.7% (w/v) paraformaldehyde. Paraformaldehyde-fixed, paraffin-embedded tissues were cut to produce sections of 4 mm thickness for IHC staining. Tissue sections were probed with anti-ILK (1:200), anti-E-cadherin (1:100), anti-Vimentin (1:200), anti-MMP-9 (1:200) and anti-Slug (1:200) for determining the expression of these proteins in every tissue. Antibody binding was amplified using HRP-conjugated second antibodies for 30 minutes, and the IHC staining was visualized using diaminobenzidine (DAB). Cell nuclei were lightly stained with hematoxylin (Solarbio). Micrographs (400×) were produced using an Olympus IX51 inverted microscope (Olympus Corporation). Positive DAB staining was used to assess tissue sections. The intensity of the protein expression was scored blindly as 0, 1, 2 and 3, which corresponded to negative, weak, moderate, and strong immunoreactivity, respectively. Sections were reviewed by two observers independently in order to verify the IHC scores.
Statistical analysis
GraphPad Prism Version 5.01 was used for all statistical analyses. Data are shown as means ± SD of three independent experiments. The student’s t-test was used to analyze the differences between any two groups. One-way analysis of variance was used to assess the means among groups. P<0.05 was considered to be statistically significant in all analyses.
Results
Exogenous ILK enhanced the proliferation, migration and invasion properties of EOC cells
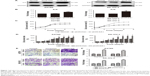
Western blotting was conducted in order to test ILK in A2780, A2780/pLVX-ILK, A2780/pLVX-Con, SK-OV-3, SK-OV-3/pLVX-Con and SK-OV-3/pLVX-ILK expression. The expression of ILK was significantly upregulated by the transfection of pLVX-ILK in A2780 and SK-OV-3 cells (Figure 1A and B). Then, the effect of exogenous expression of ILK on the proliferation abilities of human EOC cells was determined and it was found that A2780/pLVX-ILK and SK-OV-3/pLVX-ILK showed much stronger proliferation abilities (Figure 1C and D). Simultaneously, the migration and invasion abilities of human EOC cells were enhanced by the transfection of pLVX-ILK (Figure 1E and F).
Emodin suppressed the proliferation, migration and invasion of EOC cells
To examine the effect of emodin on the proliferation of A2780, A2780/pLVX-ILK, A2780/pLVX-Con, SK-OV-3, SK-OV-3/pLVX-Con and SK-OV-3/pLVX-ILK, we treated the cells with a range of doses of emodin (0–80 μM) for 48 hours. We found that emodin suppressed the proliferation of A2780, A2780/pLVX-ILK and A2780/pLVX-Con in a dose-dependent manner (Figure 2A; IC50s were 34.02 μM, 37.05 μM and 42.59 μM for A2780, A2780/pLVX-Con and A2780/pLVX-ILK, respectively). Similar results were found for SK-OV-3 cells (IC50s were 37.31 μM, 36.84 μM and 43.31 μM for SK-OV-3, SK-OV-3/pLVX-Con and SK-OV-3/pLVX-ILK cells, respectively; Figure 2B). The effect of emodin on the migration and invasion capacity of cells was measured with Transwell assays. The migration and invasion abilities of A2780 and SK-OV-3 cell lines were increased by the transfection of pLVX-ILK, and this effect could be abrogated by following the treatment with emodin (20 μM) for 48 hours (Figure 2C and D). The expression of EMT-related molecules was detected by western blotting analysis to examine whether emodin decreased migration and invasion abilities by inhibiting the EMT of SK-OV-3/pLVX-ILK and A2780/pLVX-ILK. We tested the expression of E-cadherin, Vimentin, MMP-9 and Slug. After transfection of pLVX-ILK, the expression of E-cadherin had decreased, while the levels of Vimentin, MMP-9 and Slug had increased in A2780 and SK-OV-3 cells, which could be counteracted by emodin (Figure 2E and F). Therefore, emodin arrested the migration and invasion abilities of EOC cells by inhibiting EMT through targeting ILK.
Emodin repressed the proliferation of human tumor cells in vivo
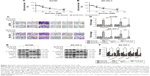
On the basis of the in vitro findings, we moved on to determine the effects of ILK and the role of emodin in vivo. After transfection of the empty vector or ILK into SK-OV-3 cells, we injected SK-OV-3/pLVX-Con and SK-OV-3/pLVX-ILK cells hypodermically into the armpits of female nude mice. We observed that tumors grew more rapidly in the SK-OV-3/pLVX-ILK group compared with the control group (SK-OV-3/pLVX-Con), and growth could be inhibited significantly by emodin (Figure 3A and B). As expected, the nodules were heavier in the SK-OV-3/pLVX-ILK group, when compared to the control group (Figure 3C and D). At the same time, the effects of overexpression of ILK on EOC cells were counteracted by emodin (Figure 3C and D). The effects of ILK on EOC cells were confirmed by the results in vivo. Simultaneously, the ability of EOC cancer cells to proliferate was decreased by emodin. So, emodin inhibited the proliferation of EOC cancer cells by targeting ILK in vivo.
Emodin inhibited the EMT of tumor cells in vivo
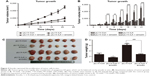
The tumors removed from nude mice were stained immunohistochemically and subjected to western blot assays. It was found that the expression of E-cadherin was downregulated, while the expression of Slug, MMP-9 and Vimentin were upregulated in the SK-OV-3/pLVX-ILK group. Contrary results were obtained from emodin-treated groups (Figure 4A and B). In addition, IHC staining showed that the levels of Slug, MMP-9 and Vimentin were increased, while the level of E-cadherin was decreased after exogenous expression of ILK. This could be reversed by emodin (Figure 4C–F). Our results demonstrate that ILK was associated significantly with EMT of EOC cells in vivo and that the impact of exogenous ILK could be abrogated by emodin.
The cardiac, liver and renal toxicities of emodin were mild in vivo
The concentrations of serum alanine aminotransferase, aspartate aminotransferase, urea nitrogen, creatinine and creatine kinase were measured by extracting eyeball blood. Hematologic toxicity test results showed little or no effects on the functional indices of liver, kidney and heart (Figure 5A–C). Thus, emodin was not toxic at the applied concentrations.
Discussion
ILK is a factor that is known to be crucial in regulating the extracellular matrix and cell–cell interactions by phosphorylating its downstream target kinases, B/Akt (PKB/Akt) and GSK3β.7 ILK has been found to promote the metastatic behavior of ovarian cancer cells.20,29 ILK has been reported to facilitate cancer cell migration and invasion by regulating EMT processes.15,30,31 Consistently, the knockdown of ILK inhibited tumor invasion and metastasis by inhibiting the EMT process in bladder cancer and in oral squamous cell carcinoma.17,32 In the present study, we found that the overexpression of ILK of A2780 and SK-OV-3 cells had a greater potential for proliferation, migration and invasion. An in vivo mouse model of exogenous expression of ILK was successfully established, and using this, it was demonstrated that the overexpression of ILK enhanced tumor growth and metastasis in vivo. This means that ILK may play an important role in metastasis in ovarian cancer and it could be a novel marker for the targeting of molecular therapies for advanced ovarian cancer.
Emodin exerts diverse biological activities, including inhibition of proliferation, migration and invasion in many different kinds of cells.23,24,33 EMT plays an essential role in tumor metastasis, where cells downregulate their epithelial markers, such as E-cadherin, and upregulate mesenchymal traits, such as Vimentin.34 Emodin inhibited EMT in head and neck squamous cell carcinoma cells.25 In the present study, it was found that emodin could inhibit the proliferation, migration and invasion abilities of EOC cells by inhibiting EMT, which is in accord with previous studies. The inhibitive effect of emodin on EMT is, therefore, known, but the underlying molecular mechanism still requires elucidation. Recent studies have implied that the effect of emodin on ILK is mediated via the MEK/ERK signaling pathway.35 Emodin was first reported to regulate the expression of ILK in tubular epithelial to myofibroblast transdifferentiation.36 It was subsequently shown to ameliorate high-glucose-induced podocyte EMT through ILK both in vitro and in vivo.37 In addition, emodin was reported to inhibit migration and invasion by promoting mesenchymal–epithelial transition by targeting ILK in human endometrial stromal cells.38 Further, in a previous study, carried out in our own laboratory, it was speculated that emodin inhibits EMT via the ILK/GSK3β/Slug signaling pathway in vitro.28 In our current study, it was also found that emodin decreased the expression of ILK, resulting in a repression of the abilities of EOC cells to migrate and invade through restraining EMT. Further, the effect of the overexpression of ILK could be reduced by emodin treatment in vitro. In terms of xenografts in nude mice, the tumors grew more quickly in the SK-OV-3/pLVX-ILK group compared to the control group (SK-OV-3/pLVX-Con), and this could be decelerated significantly by emodin. The expression of E-cadherin was downregulated, while the expression of Slug, MMP-9 and Vimentin were upregulated in the tumors of the mice in the SK-OV-3/pLVX-ILK group, which was crosscurrent in the tumors from the emodin-treated group of mice. Hematologic toxicity test results indicated that emodin (50 mg/kg/d) was not toxic and did not alter liver, kidney and cardiac function indices, but a change in stool characteristics induced by emodin was observed. Taking these new data into account, emodin could be considered to have potential clinical value due to its actions against ovarian cancer in the future.
In summary, exogenous expression of ILK enhanced the proliferation, migration and invasion properties of A2780 and SK-OV-3 cells, and this could be abrogated by emodin in vitro. A xenograft tumor model was used to show that emodin targeted the ILK signaling pathway, thereby inhibiting EOC cell growth and EMT-associated protein expression in vivo. In our experiment, fact that no metastatic lesions were observed we attributed to the short-term nature of the observations and the low level of the technology used. The underlying mechanisms by which emodin targets ILK and by which ILK accelerates EMT are still unclear. Additionally, in vivo studies are preferable for the construction of a xenograft tumor model with knockdown ILK and for investigating the therapeutic effects of emodin on ovarian cancer.
Conclusion
In this study it has been demonstrated that ILK has a role in EOC cells, and a molecular basis for further studies has been provided. It has been shown that ILK could enhance the proliferation, migration and invasion abilities of EOC cells, and these effects could be neutralized by emodin in vitro and in vivo. Furthermore, emodin inhibited the migration and invasion abilities of EOC cells by inhibiting the EMT of EOC cells through the targeting of ILK. The findings presented here suggest a novel therapeutic approach to the treatment of ovarian cancer and highlights the possible value of using ILK as a prognostic marker in advanced ovarian cancer.
Acknowledgment
This study was funded by grants from Science and Technology Development Project of Shandong Province (2008GG2NS02017).
Disclosure
The authors report no conflicts of interest in this work.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. | ||
Morales-Vásquez F, Pedernera E, Reynaga-Obregón J, et al. High levels of pretreatment CA125 are associated to improved survival in high grade serous ovarian carcinoma. J Ovarian Res. 2016;9(1):41. | ||
Lim HJ, Ledger W. Targeted therapy in ovarian cancer. Womens Health (Lond). 2016;12(3):363–378. | ||
Wu C, Dedhar S. Integrin-linked kinase (ILK) and its interactors: a new paradigm for the coupling of extracellular matrix to actin cytoskeleton and signaling complexes. J Cell Biol. 2001;155(4):505–510. | ||
Dedhar S, Williams B, Hannigan G. Integrin-linked kinase (ILK): a regulator of integrin and growth-factor signalling. Trends Cell Biol. 1999;9(8):319–323. | ||
Dedhar S. Cell-substrate interactions and signaling through ILK. Curr Opin Cell Biol. 2000;12(2):250–256. | ||
Persad S, Dedhar S. The role of integrin-linked kinase (ILK) in cancer progression. Cancer Metastasis Rev. 2003;22(4):375–384. | ||
Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172(7):973–981. | ||
Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28(1–2):15–33. | ||
Grassi ML, Palma CS, Thomé CH, Lanfredi GP, Poersch A, Faça VM. Proteomic analysis of ovarian cancer cells during epithelial-mesenchymal transition (EMT) induced by epidermal growth factor (EGF) reveals mechanisms of cell cycle control. J Proteomics. 2017;151:2–11. | ||
Becker-Santos DD, Guo Y, Ghaffari M, et al. Integrin-linked kinase as a target for ERG-mediated invasive properties in prostate cancer models. Carcinogenesis. 2012;33(12):2558–2567. | ||
Matsui Y, Assi K, Ogawa O, et al. The importance of integrin-linked kinase in the regulation of bladder cancer invasion. Int J Cancer. 2012;130(3):521–531. | ||
Yan Z, Yin H, Wang R, et al. Overexpression of integrin-linked kinase (ILK) promotes migration and invasion of colorectal cancer cells by inducing epithelial-mesenchymal transition via NF-κB signaling. Acta Histochem. 2014;116(3):527–533. | ||
Wang SC, Makino K, Xia W, et al. DOC-2/hDab-2 inhibits ILK activity and induces anoikis in breast cancer cells through an Akt-independent pathway. Oncogene. 2001;20(47):6960–6964. | ||
Chen D, Zhang Y, Zhang X, et al. Overexpression of integrin-linked kinase correlates with malignant phenotype in non-small cell lung cancer and promotes lung cancer cell invasion and migration via regulating epithelial-mesenchymal transition (EMT)-related genes. Acta Histochem. 2013;115(2):128–136. | ||
Radeva G, Petrocelli T, Behrend E, et al. Overexpression of the integrin-linked kinase promotes anchorage-independent cell cycle progression. J Biol Chem. 1997;272(21):13937–13944. | ||
Zhu J, Pan X, Zhang Z, Gao J, Zhang L, Chen J. Downregulation of integrin-linked kinase inhibits epithelial-to-mesenchymal transition and metastasis in bladder cancer cells. Cell Signal. 2012;24(6):1323–1332. | ||
Cruet-Hennequart S, Maubant S, Luis J, Gauduchon P, Staedel C, Dedhar S. Alpha(v) integrins regulate cell proliferation through integrin-linked kinase (ILK) in ovarian cancer cells. Oncogene. 2003;22(11):1688–1702. | ||
Li Q, Li C, Zhang YY, et al. Silencing of integrin-linked kinase suppresses in vivo tumorigenesis of human ovarian carcinoma cells. Mol Med Rep. 2013;7(3):1050–1054. | ||
Bruney L, Liu Y, Grisoli A, Ravosa MJ, Stack MS. Integrin-linked kinase activity modulates the pro-metastatic behavior of ovarian cancer cells. Oncotarget. 2016;7(16):21968–21981. | ||
Ahmed N, Riley C, Oliva K, Stutt E, Rice GE, Quinn MA. Integrin-linked kinase expression increases with ovarian tumour grade and is sustained by peritoneal tumour fluid. J Pathol. 2003;201(2):229–237. | ||
Huang Q, Lu G, Shen HM, Chung MC, Ong CN. Anti-cancer properties of anthraquinones from rhubarb. Med Res Rev. 2007;27(5):609–630. | ||
Su YT, Chang HL, Shyue SK, Hsu SL. Emodin induces apoptosis in human lung adenocarcinoma cells through a reactive oxygen species-dependent mitochondrial signaling pathway. Biochem Pharmacol. 2005;70(2):229–241. | ||
Sun Y, Wang X, Zhou Q, et al. Inhibitory effect of emodin on migration, invasion and metastasis of human breast cancer MDA-MB-231 cells in vitro and in vivo. Oncol Rep. 2015;33(1):338–346. | ||
Way TD, Huang JT, Chou CH, Huang CH, Yang MH, Ho CT. Emodin represses TWIST1-induced epithelial-mesenchymal transitions in head and neck squamous cell carcinoma cells by inhibiting the β-catenin and Akt pathways. Eur J Cancer. 2014;50(2):366–378. | ||
Zou J, Luo H, Zeng Q, Dong Z, Wu D, Liu L. Protein kinase CK2α is overexpressed in colorectal cancer and modulates cell proliferation and invasion via regulating EMT-related genes. J Transl Med. 2011;9:97. | ||
Hu C, Dong T, Li R, Lu J, Wei X, Liu P. Emodin inhibits epithelial to mesenchymal transition in epithelial ovarian cancer cells by regulation of GSK-3β/β-catenin/ZEB1 signaling pathway. Oncol Rep. 2016;35(4):2027–2034. | ||
Lu J, Xu Y, Wei X, Zhao Z, Xue J, Liu P. Emodin inhibits the epithelial to mesenchymal transition of epithelial ovarian cancer cells via ILK/GSK-3beta/Slug signaling pathway. Biomed Res Int. 2016;2016:6253280. | ||
Ahmed N, Oliva K, Rice GE, Quinn MA. Cell-free 59 kDa immunoreactive integrin-linked kinase: a novel marker for ovarian carcinoma. Clin Cancer Res. 2004;10(7):2415–2420. | ||
Gil D, Ciołczyk-Wierzbicka D, Dulińska-Litewka J, Laidler P. Integrin-linked kinase regulates cadherin switch in bladder cancer. Tumour Biol. 2016;37(11):15185–15191. | ||
Shen H, Ma JL, Zhang Y, et al. Integrin-linked kinase overexpression promotes epithelial-mesenchymal transition via nuclear factor-κB signaling in colorectal cancer cells. World J Gastroenterol. 2016;22(15):3969–3977. | ||
Que L, Zhao D, Tang XF, et al. Effects of lentivirus-mediated shRNA targeting integrin-linked kinase on oral squamous cell carcinoma in vitro and in vivo. Oncol Rep. 2016;35(1):89–98. | ||
Pooja T, Karunagaran D. Emodin suppresses Wnt signaling in human colorectal cancer cells SW480 and SW620. Eur J Pharmacol. 2014;742:55–64. | ||
Bian Y, Chang X, Liao Y, et al. Promotion of epithelial-mesenchymal transition by Frizzled2 is involved in the metastasis of endometrial cancer. Oncol Rep. 2016;36(2):803–810. | ||
Tang Q, Zhao S, Wu J, et al. Inhibition of integrin-linked kinase expression by emodin through crosstalk of AMPKα and ERK1/2 signaling and reciprocal interplay of Sp1 and c-Jun. Cell Signal. 2015;27(7):1469–1477. | ||
Chen TF, Chen M, Qin JH, Wang MY. Interventional effect of emodin on the expression of intergern linked kinase in tubular epithelial-myofibroblast transdifferentiation. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2008;24(6):574–576. | ||
Chen T, Zheng LY, Xiao W, Gui D, Wang X, Wang N. Emodin ameliorates high glucose induced-podocyte epithelial-mesenchymal transition in-vitro and in-vivo. Cell Physiol Biochem. 2015;35(4):1425–1436. | ||
Zheng Q, Xu Y, Lu J, Zhao J, Wei X, Liu P. Emodin inhibits migration and invasion of human endometrial stromal cells by facilitating the mesenchymal-epithelial transition through targeting ILK. Reprod Sci. 2016;23(11):1526–1535. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.