Back to Journals » Drug Design, Development and Therapy » Volume 14
Emodin Reverses the Epithelial–Mesenchymal Transition of Human Endometrial Stromal Cells by Inhibiting ILK/GSK-3β Pathway
Authors Zheng Q, Wang J, Li W, Chen X, Chen S, Chen L
Received 25 May 2020
Accepted for publication 20 August 2020
Published 10 September 2020 Volume 2020:14 Pages 3663—3672
DOI https://doi.org/10.2147/DDDT.S262816
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
Qiaomei Zheng, Jinhua Wang, Wenwen Li, Xiaoyun Chen, Shaozhan Chen, Lihong Chen
Department of Obstetrics and Gynecology, The First Affiliated Hospital of Fujian Medical University, Fujian Medical University, Fuzhou, Fujian 350001, People’s Republic of China
Correspondence: Lihong Chen
Department of Obstetrics and Gynecology, The First Affiliated Hospital of Fujian Medical University, Fujian Medical University, 20 Chazhong Road, Fuzhou, Fujian 350001, People’s Republic of China
Tel +86-059187982061
Fax +86-059187981028
Email [email protected]
Purpose: To explore the exact mechanism through which emodin down-regulates the migration and invasion abilities of endometrial stromal cells. Moreover, to explore the theoretical basis of emodin in the treatment of endometriosis.
Patients and Methods: Endometriosis endometrial stromal cells (EESs) were cultured from 15 women with endometriosis and control endometrial stromal cells (CESs) were cultured from 12 women without endometriosis. The levels of proteins were evaluated by Western blot. The migration and invasion abilities of cells were detected by transwell assays.
Results: The abilities of migration and invasion of EESs were much stronger than those of CESs. After treated with emodin, the migration and invasion abilities of EESs and CESs were significantly down-regulated, and the levels of integrin-linked kinase (ILK) and p-GSK-3β were statistically down-regulated in EESs. Besides that, the expression of keratin was up-regulated while the expression of vimentin, β-catenin and slug were all down-regulated by emodin in a dose- and time-dependent manner. Silencing of ILK gene in EESs also achieved the above effects, which were strengthened by emodin. Conversely, exogenous expression of ILK in CESs increased the expression of p-GSK-3β, which were abrogated by emodin. Furthermore, SB216763 increased migration and invasion abilities of CESs by facilitating the epithelial–mesenchymal transition (EMT) through up-regulating levels of p-GSK-3β, β-catenin and slug, which were also abrogated by emodin.
Conclusion: Emodin inhibits the migration and invasion abilities of human endometrial stromal cells by reversing the EMT via ILK/GSK-3β pathway. So, emodin may be considered as a promising targeted therapy for endometriosis.
Keywords: emodin, endometriosis, EMT, ILK, GSK-3β
Introduction
Endometriosis is a common gynecological disease, characterized by the presence of functional endometrium tissues at extrauterine locations. It affects around 10% of women of reproductive age and results in dysmenorrhea, chronic pelvic pain, infertility and decreased quality of life.1,2 Due to the unclear pathogenesis of endometriosis, laparoscopic surgery is currently the first choice for endometriosis. However, the recurrence rate of endometriosis is more than 20% within 2 years after surgery.3 Up to now, it is widely accepted that endometriosis is caused by the reflux menstruation, indicating that endometrial tissues can regurgitate into pelvic cavity during menstruation and then survive and develop into endometriosis.4 Still, how endometrial tissues survive elsewhere and develop into endometriosis remains unclear.
Epithelial–mesenchymal transition (EMT), playing essential roles in the metastasis of various tumors, endows cells with invasive and metastatic properties. During EMT, epithelial markers are down-regulated while mesenchymal markers are up-regulated, making epithelial cells lose cell polarity and converted into mesenchymal cells. Published evidences indicate that EMT also plays a significant part in the initial formation of endometriosis for E-cadherin is proved to be down-regulated, while N-cadherin and vimentin are up-regulated in endometriosis.5–7 Additionally, our previous studies proved that the EMT plays a crucial role in the pathogenesis of endometriosis by enhancing the migration and invasion abilities of endometrial cells.8,9
Integrin-linked kinase (ILK) is a serine-threonine kinase, which plays essential roles in mediating the relationship between cell-cell and extracellular matrix (ECM)-cell interaction.10 Overexpression of ILK facilitates cell proliferation, migration, invasion and EMT, as well as tumor angiogenesis and vascular development in many cancers.11,12 Due to its kinase activity, ILK can directly phosphorylate several downstream targets (Akt, NF-κB or GSK-3β) to mediate cell-ECM and intracellular processes.13–15 Down-regulated the ILK expression can significantly reduce the expression of EMT associated transcription factors (Twist, Zeb, slug or β-catenin in the nucleus), and then inhibited the EMT process.16,17 Exogenous expression of ILK facilitated the migration and invasion of cancer cells, while ILK inhibition suppressed tumor growth and invasion.18,19 Similar to that, our previous study proved that ILK enhances migration and invasion abilities of human endometrial stromal cells by facilitating the EMT.9
Emodin (1,3,8-trihydroxy-6-methylanthraquinone) is a naturally occurring anthraquinone, presenting in the roots and barks of numerous plants. As an active ingredient of various Chinese herbs, emodin possesses various biological activities, including antibacterial, anti-inflammatory, immunosuppressive and anticancer effects.20 Way et al reported that emodin repressed the TWIST1-induced EMT in head and neck squamous cell carcinoma cells.21 Chen et al demonstrated that emodin inhibited the EMT of high glucose induced-podocyte through ILK pathway.22 Notably, with almost no toxic effect on normal cells, emodin showed excellent cytotoxicity against cancer cells.23,24 Besides that, our previous study indicated that emodin inhibited the migration and invasion abilities of endometrial stromal cells by facilitating the mesenchymal–epithelial transition (MET).25
Given that emodin can repress the EMT of cancers, we investigated and proved that emodin inhibited the migration and invasion abilities of human endometrial stromal cells.25 However, the exact pathway through which emodin inhibited the migration and invasion abilities of endometrial stromal cells is still unclear. The current study was undertaken to explore the exact mechanism through which emodin inhibited the migration and invasion abilities of human endometrial stromal cells.
Methods
Sample Collection and Cell Culture
The study included 15 women with endometriosis and 12 women without endometriosis. Endometriosis was visually diagnosed during the laparoscopy for ovarian cysts and then ascertained by pathological examination. Control endometrium samples were from women who had surgery for other benign ovarian cysts. All samples were taken at the proliferative phase of menstrual cycle. All of the participants were at reproductive age, had regular menstruation and received no hormonal therapy for at least 6 months before the study, chosen from the Department of Obstetrics and Gynecology, First Affiliated Hospital of Fujian Medical University, from April 2017 to November 2019. Informed consent was obtained from all participants prior to surgery. The Ethics Committee of the First Affiliated Hospital of Fujian Medical University approved the study (approval number: 2017[044]).
Endometriosis endometrial stromal cells (EESs) were cultured from women with endometriosis and control endometrial stromal cells (CESs) were cultured from women without endometriosis. Both EESs and CESs were generated from eutopic endometrium. As described in our previous study, EESs and CESs were isolated, cultured and evaluated the purity over 95% for the following experiments.8
Western Blot Analysis
Procedures of Western blot were described previously.8 Primary antibodies used for immunodetection were anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH), anti-ILK, anti-GSK-3β, anti-p-GSK-3β, anti-β-catenin, anti-slug, anti-vimentin as well as anti-keratin (Cell Signaling Technology, Danvers, Massachusetts, USA). Secondary antibodies were anti-rabbit and anti-mouse IgG peroxidase conjugate (Millipore, Massachusetts, USA). GAPDH was used as a loading control. The results were quantified by densitometry, using Image J software (NIH, Bethesda, Maryland, USA).
Transwell Migration and Invasion Assays
After treatment, cells were digested for migration and invasion assays as described in our previous study.8 For the migration and invasion assay, 4 × 104 cells were seeded into each transwell. Pictures (200×) were taken by the Olympus IX51 inverted microscope (Olympus Corporation, Tokyo, Japan). Cells were counted in four random fields of each chamber.
Silencing of the ILK Gene in EESs
Showing a higher level of ILK,9 EESs were transfected with small-interfering RNA sequences targeting human ILK (siRNA-ILK) to down-regulate the expression of ILK. The siRNA-ILK sequences (Table 1) were designed by GenePharma Company (Shanghai, China). EESs were seeded in 6-well plates without antibiotics treated and grown to 5060% confluence. Then, blank sequence (negative control, N.C.) or siRNA-ILK (50 nmol/L), was co-transfected into EESs using lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad, California, USA). Cells were digested for following experiments after 48 hours of transfection. ILK-homo-755 was used in the remaining tests for its strongest transfection effect on the silencing of ILK.
 |
Table 1 The Sequences of ILK siRNA |
Transient Transfection of the ILK Gene in CESs
Showing a lower level of ILK,9 CESs were transfected with ILK overexpression vectors (pEGFP-C1-ILK) to up-regulate the expression of ILK.6 The control vector (pEGFP-C1) and pEGFP-C1-ILK were also designed by GenePharma Company (Shanghai, China). CESs were seeded into 6-well plates without antibiotics treated and grown to 7080% confluence. Then, pEGFP-C1 (negative control) or pEGFP-C1-ILK (5 μg/well) was co-transfected into CESs using lipofectamine 2000 (5 μg/well). After 48 hours of transfection, cells were digested for the following experiments.
Statistical Analysis
GraphPad Prism Version 5.01 (GraphPad Software, San Diego, California, USA) was used for statistical analysis. Student’s t-test and one-way ANOVA analysis were conducted, respectively, to analyze the differences between groups and among groups. Data were shown as mean ± standard error of the mean (SEM). P value <0.05 was considered statistically significant (*#P < 0.05, **##P < 0.005 and ***###P < 0.001).
Results
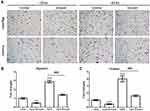
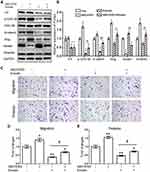
Emodin Inhibited the Migration and Invasion Abilities of Endometrial Stromal Cells
Transwell migration and invasion assays were conducted to confirm the migration and invasion abilities of EESs and CESs. As expected, the abilities of migration and invasion of EESs were much stronger than those of CESs (Figure 1). Then, we treated cells with emodin (Sigma, St Louis, Missouri, USA). As shown in Figure 1, emodin significantly down-regulated the migration and invasion abilities of EESs and CESs.
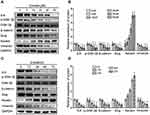
Emodin Decreased the Expression of ILK, p-GSK-3β and Reversed the EMT of Endometrial Stromal Cells
To explore the potential mechanism, we examined the expression of ILK and its downstream targets, including p-Akt, p-GSK-3β, p-Erk and p-NF-κB, in EESs. After treatment with emodin, the expression of ILK was decreased in a dose- and time-dependent manner. However, only one of the ILK downstream targets, p-GSK-3β, was decreased in a dose- and time-dependent manner (Figure 2, Supplementary figure 1). Besides that, the expression of keratin was up-regulated while the expression of vimentin, β-catenin and slug were all down-regulated by emodin in a dose- and time-dependent manner. Thus, emodin reversed the EMT of EESs.
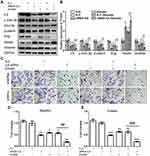
Emodin Reversed the EMT of Endometrial Stromal Cells Through ILK/GSK-3β Pathway
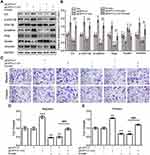
To verify whether emodin reversed the EMT of endometrial stromal cells by targeting ILK, we silenced the ILK by transfecting EESs with siRNA-ILK and over-expressed the ILK by transfecting CESs with pEGFP-C1-ILK. After transfection of siRNA-ILK, the levels of ILK, p-GSK-3β, β-catenin, slug and vimentin were all down-regulated while vimentin was up-regulated in EESs (Figure 3A and B). At the same time, the migration and invasion abilities of EESs were down-regulated after the transfection (Figure 3CE). Moreover, all the above effects were strengthened by the addition of emodin. Consistently, exogenous expression of ILK up-regulated the level of ILK, p-GSK-3β, β-catenin, slug and vimentin, but down-regulated the level of keratin in CESs (Figure 4A and B). At the same time, exogenous expression of ILK enhanced the migration and invasion abilities of CESs (Figure 4CE). However, all the above effects were abrogated by emodin (Figure 4).
Then, we tested whether ILK targeted GSK-3β to regulate the migration and invasion abilities of endometrial stromal cells by using GSK-3β inhibitor SB216763 (Sigma Aldrich, St. Louis, MO, USA), which can phosphorylate GSK-3β to an inactive protein. Regarding the lower proportion of p-GSK-3β in CESs, we treated the CESs with SB216763. After treated CESs with SB216763 (10μM) for 48 h, the levels of p-GSK-3β, β-catenin, slug and vimentin were all up-regulated, while the expression of keratin was down-regulated (Figure 5A and B). Furthermore, the migration and invasion abilities of CESs were both increased by SB216763 (Figure 5CE). Besides that, the effects of SB216763 and emodin on CESs could be abrogated by each other (Figure 5). Taken together, emodin inhibited the migration and invasion abilities of human endometrial stromal cells by reversing the EMT through ILK/GSK-3β pathway.
Discussion
Emodin, with almost no toxic effect on normal cells, shows excellent cytotoxicity against cancer cells and inhibits the migration and invasion abilities of several kinds of cancer cells.26–28 However, the mechanism through which emodin inhibits the migration and invasion abilities of cancers remains to be elucidated. Gu et al reported that emodin inhibited cell proliferation and invasion via regulating EMT-related genes.27 Way et al showed that emodin inhibited the migration and invasion of head and neck squamous cell carcinoma cells by repressing the Twist1-induced EMT through inhibiting the β-catenin and Akt pathways.21 A more recent study demonstrated that emodin repressed the EMT of high glucose induced-podocyte by inhibiting the ILK expression.22 Thus, EMT may account for the inhibition of migration and invasion abilities of emodin for its crucial role in the invasion-metastasis cascade. Consistent with these studies, our present study demonstrated that emodin inhibited the migration and invasion abilities of human endometrial stromal cells by reversing the EMT through ILK/GSK-3β pathway.
ILK was previously reported to facilitate cell proliferation, migration and invasion in many cancers.29 Emerging studies proved that ILK facilitated the abilities of migration and invasion of cancer cells by inducing the EMT program.30 Additionally, knockout of ILK decreased the invasion and metastasis abilities of cells through inhibiting the EMT process.31,32 Considering the crucial roles of EMT in modulating cell migration and invasion, ILK may be a promising treatment target for various diseases. Tang et al discovered that emodin inhibited the expression of ILK through the crosstalk of AMPKα and ERK1/2 signaling.33 Similarly, Chen et al demonstrated that emodin suppressed the EMT of podocyte through ILK pathway both in-vitro and in-vivo.22 In our previous study, we observed significantly up-regulated expression of ILK and increased abilities of migration and invasion in EESs of endometriosis.34 Further study confirmed that ILK increased the migration and invasion abilities of EESs by facilitating the EMT of EESs.9
As an important kinase, ILK can directly phosphorylate Akt, GSK-3β, Erk or NF-κB to mediate cell-ECM and cell–cell interaction. Que et al demonstrated that ILK enhanced EMT by up-regulating the expression of Snail, Slug and Twist2 through phosphorylating its downstream signaling targets Akt and GSK-3β.35 Similarly, overexpression of ILK promoted migration and invasion of colorectal cancer cells by inducing EMT via NF-κB signaling.36 In the present study, we detected the expression of p-Akt, p-GSK-3β, p-Erk and p-NF-κB in EESs, but found only p-GSK-3β was decreased along with ILK in EESs after treated with emodin. Likewise, emodin was reported to induce neurite outgrowth through GSK-3β signaling pathways in Neuro2a cells.37 Knockout of the ILK gene inhibited the EMT program in human peritoneal mesothelial cells through phosphorylation of GSK-3β.32 Moreover, inhibition of GSK-3β is crucial for EGF-induced EMT in human prostate and lung cancer.38 Previous studies indicated that GSK-3β played a crucial role in controlling the expression of β-catenin in the cytoplasm.39 As an inactivated protein, p-GSK-3β stabilized both β-catenin and slug, resulting in EMT.40,41 Lu et al demonstrated that emodin decreased the invasion and metastasis abilities of epithelial ovarian cancer cells by inhibiting the EMT via ILK/GSK-3β/Slug signaling pathway.42 Consistent with this, our study demonstrated that GSK-3β down-regulated the expression of β-catenin and slug and played essential roles in reversing the EMT procedure through which emodin inhibited the migration and invasion abilities of endometrial stromal cells. Thus, we supposed that emodin inhibited the migration and invasion abilities of EESs by reversing the EMT of endometrial stromal cells through ILK/GSK-3β/β-catenin/slug pathway.
Conclusion
Our study verified that emodin inhibited the migration and invasion abilities of EESs by reversing the EMT of endometrial stromal cells through ILK/GSK-3β signaling pathway. Given that emodin shows almost no toxic effect on normal cells, emodin may be considered as a promising targeted therapy for endometriosis. However, further in vivo studies are required to verify the potency of emodin in the treatment of endometriosis.
Ethics Approval and Informed Consent
The Ethics Committee of the First Affiliated Hospital of Fujian Medical University approved the study (approval number: 2017[044]). Informed consent was obtained from all participants prior to surgery.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med. 2020;382(13):1244–1256.
2. Peiris AN, Chaljub E, Medlock D. Endometriosis. JAMA. 2018;320(24):2608.
3. Bozdag G. Recurrence of endometriosis: risk factors, mechanisms and biomarkers. Women’s Health. 2015;11(5):693–699.
4. Vinatier D, Orazi G, Cosson M, Dufour P. Theories of endometriosis. Eur J Obstet Gynecol Reprod Biol. 2001;96(1):21–34.
5. Xiong W, Zhang L, E LH. -mediated EMT by activation of beta-catenin/Snail signalling during the development of ovarian endometriosis. J Cell Mol Med. 2019;23(12):8035–8045.
6. Yang YM, Yang WX. Epithelial-to-mesenchymal transition in the development of endometriosis. Oncotarget. 2017;8(25):41679–41689.
7. Wang S, Zhang M, Zhang T, Deng J, Xia X, Fang X. microRNA-141 inhibits TGF-beta1-induced epithelial-to-mesenchymal transition through inhibition of the TGF-beta1/SMAD2 signalling pathway in endometriosis. Arch Gynecol Obstet. 2020;301(3):707–714.
8. Zheng QM, Lu JJ, Zhao J, Wei X, Wang L, Liu PS. Periostin facilitates the epithelial-mesenchymal transition of endometrial epithelial cells through ILK-Akt signaling pathway. Biomed Res Int. 2016;2016:9842619.
9. Zheng QM, Chen XY, Bao QF, Yu J, Chen LH. ILK enhances migration and invasion abilities of human endometrial stromal cells by facilitating the epithelial-mesenchymal transition. Gynecol Endocrinol. 2018;34(12):1091–1096.
10. Zheng CC, Hu HF, Hong P, et al. Significance of integrin-linked kinase (ILK) in tumorigenesis and its potential implication as a biomarker and therapeutic target for human cancer. Am J Cancer Res. 2019;9(1):186–197.
11. Tsoumas D, Nikou S, Giannopoulou E, et al. ILK expression in colorectal cancer is associated with EMT, cancer stem cell markers and chemoresistance. Cancer Genomics Proteomics. 2018;15(2):127–141.
12. Zhou W, Peng Z, Zhang C, Liu S, Zhang Y. ILK-induced epithelial-mesenchymal transition promotes the invasive phenotype in adenomyosis. Biochem Biophys Res Commun. 2018;497(4):950–956.
13. Zhang Y, Huang W. Transforming Growth Factor beta1 (TGF-beta1)-stimulated Integrin-Linked Kinase (ILK) regulates migration and epithelial-mesenchymal transition (EMT) of human lens epithelial cells via nuclear factor kappaB (NF-kappaB). Med Sci Monit. 2018;24:7424–7430.
14. Pang M, Wang H, Rao P, et al. Autophagy links beta-catenin and Smad signaling to promote epithelial-mesenchymal transition via upregulation of integrin linked kinase. Int J Biochem Cell Biol. 2016;76:123–134.
15. Shen H, Ma JL, Zhang Y, et al. Integrin-linked kinase overexpression promotes epithelial-mesenchymal transition via nuclear factor-kappaB signaling in colorectal cancer cells. World J Gastroenterol. 2016;22(15):3969–3977.
16. Gil D, Ciolczyk-Wierzbicka D, Dulinska-Litewka J, Laidler P. Integrin-linked kinase regulates cadherin switch in bladder cancer. Tumour Biol. 2016;37(11):15185–15191.
17. Yang J, Hou Y, Zhou M, et al. Twist induces epithelial-mesenchymal transition and cell motility in breast cancer via ITGB1-FAK/ILK signaling axis and its associated downstream network. Int J Biochem Cell Biol. 2016;71:62–71.
18. Ning Z, Zhu X, Jiang Y, et al. Integrin-linked kinase is involved in the proliferation and invasion of esophageal squamous cell carcinoma. J Cancer. 2020;11(2):324–333.
19. Liang F, Zhang S, Wang B, Qiu J, Wang Y. Overexpression of integrin-linked kinase (ILK) promotes glioma cell invasion and migration and down-regulates E-cadherin via the NF-kappaB pathway. J Mol Histol. 2014;45(2):141–151.
20. Dong X, Fu J, Yin X, et al. Emodin: a review of its pharmacology, toxicity and pharmacokinetics. Phytother Res. 2016;30(8):1207–1218.
21. Way TD, Huang JT, Chou CH, Huang CH, Yang MH, Ho CT. Emodin represses TWIST1-induced epithelial-mesenchymal transitions in head and neck squamous cell carcinoma cells by inhibiting the beta-catenin and Akt pathways. Eur J Cancer. 2014;50(2):366–378.
22. Chen T, Zheng LY, Xiao W, Gui D, Wang X, Wang N. Emodin ameliorates high glucose induced-podocyte epithelial-mesenchymal transition in-vitro and in-vivo. Cell Physiol Biochem. 2015;35(4):1425–1436.
23. Song Y, Sheng Z, Xu Y, et al. Magnetic liposomal emodin composite with enhanced killing efficiency against breast cancer. Biomaterials sci. 2019;7(3):867–875.
24. Zheng XY, Yang SM, Zhang R, Wang SM, Li GB, Zhou SW. Emodin-induced autophagy against cell apoptosis through the PI3K/AKT/mTOR pathway in human hepatocytes. Drug Des Devel Ther. 2019;13:3171–3180.
25. Zheng Q, Xu Y, Lu J, Zhao J, Wei X, Liu P. Emodin inhibits migration and invasion of human endometrial stromal cells by facilitating the mesenchymal-epithelial transition through targeting ILK. Reprod Sci. 2016;23(11):1526–1535.
26. Dai G, Ding K, Cao Q, et al. Emodin suppresses growth and invasion of colorectal cancer cells by inhibiting VEGFR2. Eur J Pharmacol. 2019;859:172525.
27. Gu J, Cui CF, Yang L, Wang L, Jiang XH. Emodin inhibits colon cancer cell invasion and migration by suppressing epithelial-mesenchymal transition via the wnt/beta-catenin pathway. Oncol Res. 2019;27(2):193–202.
28. Lu J, Xu Y, Zhao Z, et al. Emodin suppresses proliferation, migration and invasion in ovarian cancer cells by down regulating ILK in vitro and in vivo. Onco Targets Ther. 2017;10:3579–3589.
29. Wu PA, Xie LL, Zhao DY, et al. Integrin-linked kinase is overexpressed in laryngeal squamous cell carcinoma and correlates with tumor proliferation, migration and invasion. Eur Rev Med Pharmacol Sci. 2018;22(24):8740–8748.
30. Gil D, Zarzycka M, Ciolczyk-Wierzbicka D, Laidler P. Integrin linked kinase regulates endosomal recycling of N-cadherin in melanoma cells. Cell Signal. 2020;72:109642.
31. Xing Y, Cui L, Kang Q. Silencing of ILK attenuates the abnormal proliferation and migration of human Tenon’s capsule fibroblasts induced by TGF-beta2. Int J Mol Med. 2016;38(2):407–416.
32. Luo L, Liu H, Dong Z, Sun L, Peng Y, Liu F. Small interfering RNA targeting ILK inhibits EMT in human peritoneal mesothelial cells through phosphorylation of GSK3beta. Mol Med Rep. 2014;10(1):137–144.
33. Tang Q, Zhao S, Wu J, et al. Inhibition of integrin-linked kinase expression by emodin through crosstalk of AMPKalpha and ERK1/2 signaling and reciprocal interplay of Sp1 and c-Jun. Cell Signal. 2015;27(7):1469–1477.
34. Xu X, Zheng Q, Zhang Z, Zhang X, Liu R, Liu P. Periostin enhances migration, invasion, and adhesion of human endometrial stromal cells through integrin-linked kinase 1/Akt signaling pathway. Reprod Sci. 2015;22(9):1098–1106.
35. Que L, Zhao D, Tang XF, et al. Effects of lentivirus-mediated shRNA targeting integrin-linked kinase on oral squamous cell carcinoma in vitro and in vivo. Oncol Rep. 2016;35(1):89–98.
36. Yan Z, Yin H, Wang R, et al. Overexpression of integrin-linked kinase (ILK) promotes migration and invasion of colorectal cancer cells by inducing epithelial-mesenchymal transition via NF-kappaB signaling. Acta Histochem. 2014;116(3):527–533.
37. Park SJ, Jin ML, An HK, et al. Emodin induces neurite outgrowth through PI3K/Akt/GSK-3beta-mediated signaling pathways in Neuro2a cells. Neurosci Lett. 2015;588:101–107.
38. Liu ZC, Chen XH, Song HX, et al. Snail regulated by PKC/GSK-3beta pathway is crucial for EGF-induced epithelial-mesenchymal transition (EMT) of cancer cells. Cell Tissue Res. 2014;358(2):491–502.
39. Doble BW, Patel S, Wood GA, Kockeritz LK, Woodgett JR. Functional redundancy of GSK-3alpha and GSK-3beta in Wnt/beta-catenin signaling shown by using an allelic series of embryonic stem cell lines. Dev Cell. 2007;12(6):957–971.
40. Zhou BP, Deng J, Xia W, et al. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol. 2004;6(10):931–940.
41. Wu ZQ, Li XY, Hu CY, Ford M, Kleer CG, Weiss SJ. Canonical Wnt signaling regulates slug activity and links epithelial-mesenchymal transition with epigenetic breast cancer 1, early onset (BRCA1) repression. Proc Natl Acad Sci U S A. 2012;109(41):16654–16659.
42. Lu J, Xu Y, Wei X, Zhao Z, Xue J, Liu P. Emodin inhibits the epithelial to mesenchymal transition of epithelial ovarian cancer cells via ILK/GSK-3beta/slug signaling pathway. Biomed Res Int. 2016;2016:6253280.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.