Back to Journals » Drug Design, Development and Therapy » Volume 14
Emodin Protects Against Acute Pancreatitis-Associated Lung Injury by Inhibiting NLPR3 Inflammasome Activation via Nrf2/HO-1 Signaling
Authors Gao Z , Sui J, Fan R, Qu W, Dong X, Sun D
Received 23 January 2020
Accepted for publication 27 April 2020
Published 21 May 2020 Volume 2020:14 Pages 1971—1982
DOI https://doi.org/10.2147/DDDT.S247103
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Yan Zhu
Zhenming Gao,1,* Jidong Sui,1,* Rong Fan,2 Weikun Qu,1 Xuepeng Dong,1 Deguang Sun1
1Department of Hepatopancreatobiliary Surgery, The Second Affiliated Hospital of Dalian Medical University, Dalian, Liaoning 116027, People’s Republic of China; 2Department of International Medicine, The Second Affiliated Hospital of Dalian Medical University, Dalian, Liaoning 116027, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhenming Gao; Rong Fan
Department of Hepatopancreatobiliary Surgery, The Second Affiliated Hospital of Dalian Medical University
Department of International Medicine, The Second Affiliated Hospital of Dalian Medical University, 467 Zhongshan Road, Dalian, Liaoning 116027, People’s Republic of China
Email [email protected]; [email protected]
Aim: Lung injury is a common complication of acute pancreatitis (AP), which leads to the development of acute respiratory distress syndrome and causes high mortality. In the present study, we investigated the therapeutic effect of emodin on AP-induced lung injury and explored the molecular mechanisms involved.
Materials and Methods: Thirty male Sprague-Dawley rats were randomly divided into AP (n=24) and normal (n=6) groups. Rats in the AP group received a retrograde injection of 5% sodium taurocholate into the biliary-pancreatic duct and then randomly assigned to untreated, emodin, combined emodin and ML385, and dexamethasone (DEX) groups. Pancreatic and pulmonary injury was assessed using H&E staining. In in vitro study, rat alveolar epithelial cell line L2 cells were exposed to lipopolysaccharide and treated with emodin. Nrf2 siRNA pool was applied for the knockdown of Nrf2. The contents of the pro-inflammatory cytokines in the bronchoalveolar lavage fluid and lung were determined using enzyme-linked immunosorbent assay. The expressions of related mRNAs and proteins in the lung or L2 cells were detected using real-time polymerase chain reaction, Western blot, immunohistochemistry and immunofluorescence.
Key Findings: Emodin administration alleviated pancreatic and pulmonary injury of rats with AP. Emodin administration suppressed the production of proinflammatory cytokines, downregulated NLRP3, ASC and caspase-1 expressions and inhibited NF-κB nuclear accumulation in the lung. In addition, Emodin increased Nrf2 nuclear translocation and upregulated HO-1 expression. Moreover, the anti-inflammatory effect of emodin was blocked by Nrf2 inhibitor ML385.
Conclusion: Emodin effectively protects rats against AP-associated lung injury by inhibiting NLRP3 inflammasome activation via Nrf2/HO-1 signaling.
Keywords: emodin, acute pancreatitis, lung injury, Nrf2, NLRP3 inflammasome
Introduction
Acute pancreatitis (AP) is a severe inflammatory disease, manifesting as edema, hemorrhage and necrosis in exocrine pancreas. AP has broad manifestations and variable clinical processes ranging from mild to severe forms.1,2 The incidence rate of AP ranges from 4.9 to 73.4 per 100,000 people per year worldwide.3 Most cases of AP in developed countries are mainly attributable to gallstone disease and alcohol abuse.4
Acute lung injury is a common AP-associated multiple organ dysfunction.5 AP-associated lung injury accounts for approximately 60–70% mortality in AP patients.6 The primary treatment for AP-associated lung injury is drug therapy. However, only a limited number of drugs are available, and the efficiency is not satisfactory. Therefore, the investigation of an effective therapeutic drug is critical for the treatment of AP-associated lung injury in the clinic.
Accumulating evidence shows that the overproduction of certain cytokines and the excessive release of inflammatory mediators may result in the respiratory complications of AP.7 Nuclear factor erythrocyte-2 associated factor-2 (Nrf2) is originally characterized as a master regulator of redox homeostasis, and it emerges as a critical transcriptional regulator affecting cellular redox status through activating endogenous antioxidant systems.8 With the discovery of novel target genes, it has been found that Nrf2 not only plays a key role in redox homeostasis, but also affects mitochondrial function, DNA repair,9 and inflammation.10,11
Nucleotide-binding domain leucine-rich repeat family containing a pyrin domain 3 (NLRP3) inflammasome is essential for host immune defenses against exogenous infections.12 However, it has been linked to the pathogenesis of certain inflammatory disorders, including Alzheimer’s disease,13 diabetes14 and autoinflammatory diseases.15 NLRP3 inflammasome has been demonstrated to mediate caspase-1 activation and the secretion of proinflammatory cytokines interleukin-1β (IL-1β) and IL-18.16 NLRP3 inflammasome-mediated caspase-1 activation results in a form of programmed cell death known as pyroptosis.17 Wu XB et al demonstrated that plasma-derived exosomes contribute to pancreatitis-associated lung injury by triggering NLRP3-dependent pyroptosis in alveolar macrophages.18
Emodin (1,3,8-trihydroxy-6-methylindole) is a natural anthraquinone derivative extracted from many plants such as rhubarb,19 polygonum cuspidatum20 and polygonum multiflorum, with a wide spectrum of therapeutic functions, including anti-tumor, anti-inflammation, neuroprotection and immunosuppression.21–24 Previous studies have confirmed that emodin alleviates acute pancreatitis.25,26 However, the potential therapeutic mechanism is not fully understood. In the present study, the functions and mechanisms for the effect of emodin against AP-associated lung injury on regulating the Nrf2/NLRP3 signaling pathway were investigated.
Materials and Methods
Reagents and Antibodies
Sodium taurocholate and emodin were purchased from Aladdin Regents (Shanghai, China). ML385 was purchased from MedChemExpress (New Jersey, USA). Dexamethasone (DEX) was purchased from Yucheng Kelong Veterinary Medicine (Shanxi, China). NLRP3 and ASC antibodies were purchased from ABclonal (Wuhan, China). IL-18, cleaved-caspase 1, IκBα, p65, Nrf2 and HO-1 antibodies were purchased from Proteintech (Illinois, USA). IL-1β and p-IκBα antibodies were purchased from Bioss (Beijing, China). p-p65 antibody was purchased from MultiSciences Biotech (Hangzhou, China). Histone H3 antibody was purchased from ABGENT (California, USA). β-actin antibody was purchased from Santa Cruz Biotechnology (California, USA). Goat anti-Rabbit IgG and Goat anti-Mouse IgG were purchased from Beyotime Biotechnology (Shanghai, China).
Animal Experiment
All the experimental procedures in this study involving the use of live animals were approved by the Ethics Committee of Dalian Medical University and strictly adhered to the guidelines of the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health.
Male Sprague-Dawley rats (250–300 g) were fed with standard laboratory food and water for 1 week in a humidity-controlled room (45–55%) under a 12-h light: 12-h dark cycle at a temperature of 25 ± 1 °C. The rats were randomly divided into the following five groups: sham group, AP group, AP + Emodin group, AP + Emodin + ML385 group and AP + DEX group. All the rats were fasted overnight and anesthetized. Laparotomies were performed in the lower abdomen position under sterile conditions to expose the large papillary part of duodenum. Sodium taurocholate (5%, 1 mL/kg) was retrogradely injected into the biliary pancreatic duct with a 1 mL syringe at a constant rate (0.1 mL/min). Rats in the sham group were injected with the same amount of normal saline in the same way. Then, the abdominal walls of rats were carefully sutured. Rats were then treated as follows: Rats in the AP + Emodin group were administered with emodin (25 mg/kg) by gavage; Rats in the AP + Emodin + ML385 group were injected with ML385 (30 mg/kg) intraperitoneally, and then administered with emodin (25 mg/kg) orally 30 min later; Rats in the AP+DEX group were injected with dexamethasone (2 mg/kg) intraperitoneally; Rats in the group and AP groups were administered with an equal amount of emodin solvent. Animals were euthanized 6 hours after administration and samples were taken for subsequent testing.
Cell Culture, Transfection and Treatment
Rat alveolar epithelial cell line L2 cells were obtained from Procell Life Science & Technology (Wuhan, China) and cultured in Ham’s F-12K medium (Procell) containing 10% fetal bovine serum (Sigma-Aldrich, Missouri, USA) in an incubator at 37 °C, 5% CO2.
For transfection experiments, L2 cells at a confluency of 70–80% were plated into 6-well plates the day before transfection. After the cells were cultured for 24 h, the medium was replaced with serum-free medium. Then, 100 pmol of siNrf2 pool or siNC (Santa Cruz Biotechnology, California, USA) was mixed with 8 µL of Lipofectamine 2000 (Invitrogen, California, USA) in 200µL Opti-MEM (GIBCO, California, USA) medium at room temperature for 20 min to allow complex formation. The transfection mixture was slowly added to each well and the cells were then cultured in an incubator at 37 °C, 5% CO2 for 48 h.
The cells were divided into six groups: control, LPS, LPS + Emodin, LPS + Emodin + ML385, LPS + Emodin + siNrf2, LPS + Emodin + siNC. Administered concentrations were 1 µg/mL for LPS, 1 µM for ML385, and 40 µM for emodin. ML385 was added 30 min later after emodin was added. The tests were carried out 24 h or 48 h later.
MTT Proliferation Assay
The medium was replaced with MTT mixture at a concentration of 0.5 mg/mL. The cells were incubated in an incubator at 37 °C, 5% CO2 for 4 h. Then, the supernatants were carefully aspirated. Each well was added with 100 μL of dimethyl sulfoxide (DMSO) and incubated for 10 min in the darkness. The optical density (OD) value was measured on a microplate reader (BIOTEK, Vermont, USA) at a wavelength of 570 nm.
Serum Amylase Detection
Serum amylase levels were detected using the Amylase Kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacture’s instruction.
Enzyme-Linked Immunosorbent Assay (ELISA)
The levels of tumor necrosis factor-α (TNF-α), IL-6 and IL-1β in the alveolar lavage fluid, cell culture supernatant and tissue samples were detected using corresponding ELISA kits (USCN Life Science, Wuhan, China) according to the kit instructions.
Myeloperoxidase Activity Detection
Myeloperoxidase (MPO) activity was detected using kits from Nanjing Jiancheng Bioengineering Institute, following the manufacturer’s instruction.
Hematoxylin & Eosin (H&E) Staining
The samples were fixed in 4% paraformaldehyde for 24 h at 4 °C and dehydrated with a gradient of ethanol. The samples were then embedded in paraffin and sliced into 5-μm-thick sections. The sections were washed, rehydrated and stained with hematoxylin (Solarbio, Beijing, China) and eosin (Sangon Biotech, Shanghai, China) following the standard protocols. The staining was observed under a microscope (Olympus Corporation, Tokyo, Japan) and photographed at 200 × magnifications.
Immunohistochemical (IHC) Staining
The paraffin sections were processed through routine dewaxing and hydration. Antigen retrieval was performed in sodium citrate buffer using a microwave and the non-specific antibody binding sites were blocked with 3% H2O2. Then, the sections were incubated with the corresponding primary antibody (1:200) at 4 °C overnight, and with HRP-labeled goat-anti-rabbit IgG (Thermo Fisher, Massachusetts, USA) for 1 h at 37 °C. The staining was visualized with DAB (Solarbio) and co-stained with hematoxylin. The sections were observed and photographed under a microscope (Olympus) at 400× magnifications.
Immunofluorescence Assay
Paraffin sections were dewaxed and hydrated, and cell slides were fixed in 4% paraformaldehyde. The sections were incubated in 0.1% triton X-100 (Beyotime) for 30 min and in goat serum for 15 min. Then, the sections were incubated with Nrf2 antibody (1:200) at 4 °C overnight, and with Cy3-labeled goat-anti-rabbit IgG (1:200, Beyotime) at 37 °C for 1 h. DAPI (Beyotime) was added dropwise into the sections. The sections were then washed with phosphate buffered solution buffer and added with anti-fluorescence quencher (Solarbio) to slow down the fluorescence quenching speed. The staining was observed under a fluorescence microscope (Olympus) at 400× magnifications.
Quantitative Real-Time PCR
Total RNAs were extracted from the tissue and cell samples using TRIpure Reagent (Bio Teke Corporation, Beijing, China), and cDNAs were synthesized using Super M-MLV reverse transcriptase (BioTeke). The real-time PCR was performed according to the SYBR Green (Sigma) kit instructions on an ExicyclerTM 96 fluorescence quantitative assay system (Bioneer Corporation, Daejeon, Korea). The expression level of mRNA was quantitatively analyzed by the 2−ΔΔCT method. The primer sequences are listed in Table 1.
 |
Table 1 Primer Sequences for qRT-PCR |
Western Blot
Total protein was extracted from tissue and cell samples using Cell Lysis Buffer for Western and IP (Beyotime) and nuclear protein was isolated using Nuclear and Cytoplasmic Protein Extraction Kit (Beyotime). The concentration of protein was quantified using BCA quantitative kit (Beyotime). Protein samples were loaded, subjected to sodium dodecyl sulfate (SDS)-Polyacrylamide gel electrophoresis (PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Massachusetts, USA). After being blocked by 5% skim milk for 1 h, the membranes were incubated with the primary antibody at 4 °C overnight. Then, the PVDF membranes were washed in TBST buffer and incubated with IgG-HRP secondary antibody (1:5000) for 45 min at 37 °C. Proteins were visualized with electrochemiluminescence substrate (Beyotime), and the grey value was analyzed by using Gel-Pro-Analyzer system (Media Cybernetics, Maryland, USA).
Statistical Analysis
Statistical analyses were conducted using GraphPad Prism 8.0.2 (Version X; California, USA). Ordinary one-way analysis of variance (ANOVA) was conducted to evaluate the significance of the differences between the groups. Data were expressed as mean ± SD. P value <0.05 were considered as statistically significant.
Results
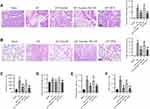
Emodin Attenuated Pancreatic and Pulmonary Damage in Rats with AP
We first evaluated the effects of emodin on the pancreas of rats with AP. ML385, a specific NRF2 inhibitor, was used to block the transduction of Nrf2.27 Dexamethasone (DEX), a glucocorticoid broadly used in the treatment of AP,33 was used as a positive control. H&E staining of the pancreas showed that obvious focal necrotic area, lobular structure destruction, inflammatory cell infiltration and hemorrhage were observed in AP model rats, while emodin administration effectively attenuated pancreatic damage (Figure 1A). Meanwhile, elevated serum amylase level is a common diagnostic criterion for AP clinically.28 The serum amylase levels of rats in the AP group were significantly higher than that in the Sham group, while emodin administration significantly lowered the levels (Figure 1C). ML385 abolished the effect of emodin on pancreatic damage.
Next, we evaluated the effects of emodin on pulmonary injury. H&E staining results showed obvious edema, hyperemia and inflammatory cell infiltration in pulmonary interstitial tissues of rats in the AP group, with a significantly increased inflammation score. Emodin effectively alleviated the pathological damage (Figure 1B). In addition, the magnitude of pulmonary edema was quantified by lung wet/dry weight ratio. A statistically significant difference was shown in the lung wet/dry ratio of rats in the AP group compared with the Sham group, while emodin obviously alleviated pulmonary enema (Figure 1D). Moreover, the count of inflammatory cells was reduced and the activity of MPO was inhibited greatly after emodin administration (Figure 1E and F). Similarly, ML385 reversed the effect of emodin on pulmonary damage.
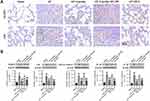
Emodin Inhibited Pulmonary Inflammation of AP Model Rats
As shown in Figure 2A–C, the expressions of pro-inflammatory cytokines TNF-α, IL-1β and IL-6 in the alveolar lavage fluid and in the lung of rats in the AP group were significantly promoted compared with the Sham group. Emodin administration reduced the secretion of these cytokines. Besides, the results of Western Blot (Figure 2D and E) showed an increased expression of p-IκBα and p-p65, while emodin administration inhibited the phosphorylation of IκBα and p65. Consistently, ML385 reversed the effect of emodin on pulmonary inflammation.
Emodin Inhibited NLRP3 Inflammasome Activation
It is well known that NLRP3 combines with ASC and caspase-1 to constitute NLRP3 inflammasome, which induces inflammatory by activating and production of IL-1β and IL-18.29 As detected by immunohistochemistry and Western Blot, the expressions of NLRP3, ASC, cleaved-caspase-1, IL-18 and IL-1β were significantly increased of rats in the AP group, while emodin significantly decreased the levels of these factors. Similarly, ML385 abolished the effect of emodin (Figure 3).
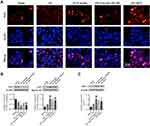
Emodin Activated the Nrf2/HO-1 Pathway in vivo
The dysregulation of Nrf2/HO-1 pathway accounts for a critical cause for developing AP.30 As shown by immunofluorescence staining and Western Blot, emodin administration promoted Nrf2 nuclear translocation and upregulated HO-1 expression, while ML385 reversed the effect of emodin on Nrf2/HO-1 signaling pathway (Figure 4).
Emodin Inhibited Alveolar Epithelial Cell Proliferation by Activating Nrf2
To further determine the mechanism of the therapeutic effect of emodin on AP-associated lung injury, we established an inflammatory cell model in vitro. As shown in Figure 5A and B, a Nrf2-knockdown cell model was successfully established. MTT assay (Figure 5C) showed that emodin at a concentration of 40 μg/mL significantly inhibited the proliferation of inflammatory cells. Knockdown of Nrf2 reversed the ability of emodin in inflammatory cell proliferation (Figure 5D).
Emodin Inhibited NLRP3 Inflammasome Activation of Alveolar Epithelial Cells
Results of Western Blot (Figure 6A) showed that the expressions of NLRP3, ASC, cleaved-caspase-1, IL-18 and IL-1β in LPS-treated alveolar epithelial cells were significantly up-regulated, while down-regulated after emodin intervention. Real-time PCR (Figure 6B) and ELISA (Figure 6C) results indicated that emodin decreased the expressions of TNF-α, IL-1β and IL-6. Moreover, results of Western Blot showed that emodin decreased the expressions of p-IκBα and p-p65 (Figure 6D and E). Both ML385 and Nrf2 siRNA abolished the effect of emodin.
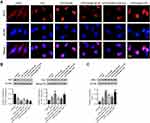
Emodin Activated the Nrf2/HO-1 Pathway in vitro
As shown by immunofluorescence staining and Western Blot, emodin treatment promoted Nrf2 nuclear translocation and upregulated HO-1 expression. Conversely, either ML385 or Nrf2 siRNA reversed the effect of emodin on Nrf2/HO-1 pathway (Figure 7).
Discussion
Studies have demonstrated that emodin is a potential candidate in the treatment of AP-associated lung injury,31,32 but the underlying mechanisms by which emodin performs its pharmacological activities remain incompletely known. The present study investigated the effect of emodin on Nrf2/NLRP3 signaling pathway in AP-associated lung injury.
In this study, a rat model of AP was successfully established by injecting with sodium taurocholate into the pancreatic duct and obvious lung injury was developed. The proinflammatory cytokines TNF-α, IL-1β, and IL-6 are well known as pivotal promoters for initiating lung injury. TNF-α and IL-6 are able to recruit leukocytes into lung tissues, while IL-1β is able to accelerate the lung damage process by inducing monocytes and macrophages.33,34 Moreover, MPO, a heme protein that is rich in neutrophils, is a marker widely linked with neutrophil infiltration.35 Our data demonstrated that emodin significantly lowered the expressions of these proinflammatory cytokines or the abundance of neutrophils, indicating that emodin greatly suppressed pulmonary inflammation. In addition, inhibition of the IκBα/NF-κB pathway also appears to be critically involved in acute lung injury.36 Our data showed that emodin treatment inhibited the activation of NF-κB and promoted the degradation of IκBα.
The NLRP3 inflammasome is a multiprotein cytosolic complex containing NLR proteins and adaptor protein ASC (apoptotic speck-like protein containing a caspase recruitment domain). The NLRP3 inflammasome is a vital component of the innate immune system, which can mediate caspase-1 activation and promote the secretion of proinflammatory cytokines IL-1β and IL-18, as in response to the exogenous infection or cellular damage. Our data demonstrated that emodin administration significantly decreased the expressions of NLRP3, ASC, cleaved caspase-1, IL-1β and IL-18. Intriguingly, pyroptosis, which mainly depends on caspase-1 activation, is known to be closely associated with acute lung injury, leading to the concomitant release of pro-inflammatory cytokines, including IL-1β and IL-18.37 A previous study demonstrated that the plasma-derived exosomes may contribute to pancreatitis-associated lung injury in alveolar macrophages by triggering NLRP3-dependent pyroptosis.18 Since emodin showed an inhibitory effect in the activation of NLRP3 inflammasome, it is very promising that emodin may exert its protective effects against AP-associated lung injury by inhibiting pyroptosis, which needs more persuasive evidence to prove.
To further explore the upstream regulators of NLRP3 inflammasome, we focus on Nrf2, a regulator of cellular redox balance, which has emerged as an important therapeutic target for various diseases. In a cerebral ischemia reperfusion injury model, Nrf2 was found to inhibit NLRP3 inflammasome activation through regulating Trx1/TXNIP complex.38 Moreover, Nrf2/ARE pathway was found to inhibit ROS-induced NLRP3 inflammasome activation in BV2 cells.39 However, whether emodin affects Nrf2 signaling in AP remains unknown. In this study, we hypothesized that emodin inhibited NLRP3 inflammasome activation via Nrf2 signaling. To further confirm this hypothesis, we used Nrf2 siRNA pool and ML385 to block the expression of Nrf2. ML385 has been confirmed to regulate the activity of the Nrf2 transcription factor by binding to Neh1, a CNC-bZIP domain that allows heterodimerization of Nrf2 with a small Maf protein, thereby blocking Nrf2 transcriptional activity.27 Our results showed that ML385 reversed the therapeutic effect of emodin on pancreatic and pulmonary injury and inflammation. Both Nrf2 siRNA and ML385 could reverse the effect of emodin on NLRP3 inflammasome activation. The results indicated that emodin exerted its protective effects against AP-induced lung injury by inhibiting NLRP3 inflammasome activation, which is specifically activated by Nrf2/HO-1 pathway.
In summary, this study presented a view that emodin protected against AP-associated lung injury by inhibiting NLRP3 inflammasome activation, which was mediated in part by Nrf2/HO-1 pathway. However, some limitations exist. The direct effect of emodin on pulmonary inflammation has not been confirmed, and the roles of other pulmonary functional cells have not been explored. Certainly, more work should be performed to clarify the detailed mechanisms.
Conclusion
Emodin treatment protects rats against AP-associated lung injury by inhibiting NLRP3 inflammasome activation via Nrf2/HO-1 signaling pathway. Nrf2 may become a potential therapeutic target for using emodin to treat AP-induced lung injury.
Acknowledgments
This project was supported by grants from the National Natural Science Foundation of China (No. 81773966) and the Traditional Chinese Medicine Related Foundation of Dalian (No. 17Z2014). ZG and RF conceived and designed the experiments. WQ, XD and DS performed the experiments. JS performed the statistical analysis and wrote the manuscript. All authors discussed the results and approved the final manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lankisch PG, Apte M, Banks PA. Acute pancreatitis. Lancet. 2015;386:85–96. doi:10.1016/S0140-6736(14)60649-8
2. Habtezion A, Gukovskaya AS, Pandol SJ. Acute pancreatitis: a multifaceted set of organelle and cellular interactions. Gastroenterology. 2019;156:1941–1950. doi:10.1053/j.gastro.2018.11.082
3. Barbara M, Tsen A, Rosenkranz L. Acute pancreatitis in chronic dialysis patients. Pancreas. 2018;47:946–951. doi:10.1097/MPA.0000000000001119
4. Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252–1261. doi:10.1053/j.gastro.2013.01.068
5. De Campos T, Deree J, Coimbra R. From acute pancreatitis to end-organ injury: mechanisms of acute lung injury. Surg Infect. 2007;8:107–120. doi:10.1089/sur.2006.011
6. Guice KS, Oldham KT, Johnson KJ, Kunkel RG, Morganroth ML, Ward PA. Pancreatitis-induced acute lung injury. An ARDS model. Ann Surg. 1988;208:71–77. doi:10.1097/00000658-198807000-00010
7. Wu D, Zeng Y, Fan Y, et al. Reverse-migrated neutrophils regulated by JAM-C are involved in acute pancreatitis-associated lung injury. Sci Rep. 2016;6:20545. doi:10.1038/srep20545
8. Dodson M, de la Vega MR, Cholanians AB, Schmidlin CJ, Chapman E, Zhang DD. Modulating NRF2 in disease: timing is everything. Annu Rev Pharmacol Toxicol. 2019;59:555–575. doi:10.1146/annurev-pharmtox-010818-021856
9. Tebay LE, Robertson H, Durant ST, et al. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic Biol Med. 2015;88:108–146. doi:10.1016/j.freeradbiomed.2015.06.021
10. Ahmed SM, Luo L, Namani A, Wang XJ, Tang X. Nrf2 signaling pathway: pivotal roles in inflammation. Biochim Biophys Acta. 2017;1863:585–597. doi:10.1016/j.bbadis.2016.11.005
11. Mohan S, Gupta D. Crosstalk of toll-like receptors signaling and Nrf2 pathway for regulation of inflammation. Biomed Pharmacother. 2018;108:1866–1878. doi:10.1016/j.biopha.2018.10.019
12. Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20:3328. doi:10.3390/ijms20133328
13. Olsen I, Singhrao SK. Inflammasome involvement in Alzheimer’s disease. J Alzheimers Dis. 2016;54:45–53. doi:10.3233/JAD-160197
14. Rovira-Llopis S, Apostolova N, Banuls C, Muntane J, Rocha M, Victor VM. Mitochondria, the NLRP3 inflammasome, and sirtuins in type 2 diabetes: new therapeutic targets. Antioxid Redox Signal. 2018;29:749–791. doi:10.1089/ars.2017.7313
15. Zhong Z, Sanchez-Lopez E, Karin M. Autophagy, NLRP3 inflammasome and auto-inflammatory/immune diseases. Clin Exp Rheumatol. 2016;34:12–16.
16. Yang Y, Wang H, Kouadir M, Song H, Shi F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019;10:128. doi:10.1038/s41419-019-1413-8
17. Qiu Z, Lei S, Zhao B, et al. NLRP3 inflammasome activation-mediated pyroptosis aggravates myocardial ischemia/reperfusion injury in diabetic rats. Oxid Med Cell Longev. 2017;2017:9743280. doi:10.1155/2017/9743280
18. Wu XB, Sun HY, Luo ZL, Cheng L, Duan XM, Ren JD. Plasma-derived exosomes contribute to pancreatitis-associated lung injury by triggering NLRP3-dependent pyroptosis in alveolar macrophages. Biochim Biophys Acta. 2020;1866:165685. doi:10.1016/j.bbadis.2020.165685
19. Huang Q, Lu G, Shen HM, Chung MC, Ong CN. Anti-cancer properties of anthraquinones from rhubarb. Med Res Rev. 2007;27:609–630. doi:10.1002/med.20094
20. Li L, Song X, Yin Z, et al. The antibacterial activity and action mechanism of emodin from Polygonum cuspidatum against Haemophilus parasuis in vitro. Microbiol Res. 2016;186–187:139–145. doi:10.1016/j.micres.2016.03.008
21. Wang Y, Yu H, Zhang J, et al. Anti-tumor effect of emodin on gynecological cancer cells. Cell Oncol. 2015;38:353–363. doi:10.1007/s13402-015-0234-8
22. Qiu F, Liu H, Liang CL, Nie GD, Dai Z. A new immunosuppressive molecule emodin induces both CD4(+)FoxP3(+) and CD8(+)CD122(+) regulatory T Cells and suppresses murine allograft rejection. Front Immunol. 2017;8:1519. doi:10.3389/fimmu.2017.01519
23. Tian SL, Yang Y, Liu XL, Xu QB. Emodin attenuates bleomycin-induced pulmonary fibrosis via anti-inflammatory and anti-oxidative activities in rats. Med Sci Monit. 2018;24:1–10. doi:10.12659/MSM.905496
24. Liu YW, Liu XL, Kong L, et al. Neuroprotection of quercetin on central neurons against chronic high glucose through enhancement of Nrf2/ARE/glyoxalase-1 pathway mediated by phosphorylation regulation. Biomed Pharmacother. 2019;109:2145–2154. doi:10.1016/j.biopha.2018.11.066
25. Xia XM, Wang FY, Wang ZK, Wan HJ, Xu WA, Lu H. Emodin enhances alveolar epithelial barrier function in rats with experimental acute pancreatitis. World J Gastroenterol. 2010;16:2994–3001. doi:10.3748/wjg.v16.i24.2994
26. Xu J, Huang B, Wang Y, et al. Emodin ameliorates acute lung injury induced by severe acute pancreatitis through the up-regulated expressions of AQP1 and AQP5 in lung. Clin Exp Pharmacol Physiol. 2016;43:1071–1079. doi:10.1111/1440-1681.12627
27. Singh A, Venkannagari S, Oh KH, et al. Small molecule inhibitor of NRF2 selectively intervenes therapeutic resistance in KEAP1-deficient NSCLC tumors. ACS Chem Biol. 2016;11:3214–3225. doi:10.1021/acschembio.6b00651
28. Rompianesi G, Hann A, Komolafe O, Pereira SP, Davidson BR, Gurusamy KS. Serum amylase and lipase and urinary trypsinogen and amylase for diagnosis of acute pancreatitis. Cochrane Database Syst Rev. 2017;4:Cd012010.
29. Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome–mediated inflammatory disease. Nat Med. 2015;21:263–269. doi:10.1038/nm.3804
30. Liu X, Zhu Q, Zhang M, et al. Isoliquiritigenin ameliorates acute pancreatitis in mice via inhibition of oxidative stress and modulation of the Nrf2/HO-1 pathway. Oxid Med Cell Longev. 2018;2018:7161592. doi:10.1155/2018/7161592
31. Li YY, Gao ZF, Dui DH. Therapeutic effect of qingyi decoction and tetrandrine in treating severe acute pancreatitis in miniature pigs and serum drug level determination. Zhongguo Zhong Xi Yi Jie He Za Zhi Zhongguo Zhongxiyi Jiehe Zazhi = Chin J Integr Tradit West Med. 2003;23:832–836.
32. Cui H, Li S, Xu C, Zhang J, Sun Z, Chen H. Emodin alleviates severe acute pancreatitis-associated acute lung injury by decreasing pre-B-cell colony-enhancing factor expression and promoting polymorphonuclear neutrophil apoptosis. Mol Med Rep. 2017;16:5121–5128. doi:10.3892/mmr.2017.7259
33. Xiping Z, Jun F, Jie Z, et al. Influence of dexamethasone on the expression levels of P-selectin protein in multiple organs of rats with severe acute pancreatitis. Inflamm Res. 2010;59:31–39. doi:10.1007/s00011-009-0067-x
34. Ye W, Zheng C, Yu D, et al. Lipoxin A4 ameliorates acute pancreatitis-associated acute lung injury through the antioxidative and anti-inflammatory effects of the Nrf2 pathway. Oxid Med Cell Longev. 2019;2019:2197017. doi:10.1155/2019/2197017
35. Cavallaro EC, Liang KK, Lawrence MD, Forsyth KD, Dixon DL. Neutrophil infiltration and activation in bronchiolitic airways are independent of viral etiology. Pediatr Pulmonol. 2017;52:238–246. doi:10.1002/ppul.23514
36. Kim DI, Kim SR, Kim HJ, et al. PI3K-γ inhibition ameliorates acute lung injury through regulation of IκBα/NF-κB pathway and innate immune responses. J Clin Immunol. 2012;32:340–351. doi:10.1007/s10875-011-9628-1
37. Hou L, Yang Z, Wang Z, et al. NLRP3/ASC-mediated alveolar macrophage pyroptosis enhances HMGB1 secretion in acute lung injury induced by cardiopulmonary bypass. Lab Invest. 2018;98:1052–1064. doi:10.1038/s41374-018-0073-0
38. Hou Y, Wang Y, He Q, et al. Nrf2 inhibits NLRP3 inflammasome activation through regulating Trx1/TXNIP complex in cerebral ischemia reperfusion injury. Behav Brain Res. 2018;336:32–39. doi:10.1016/j.bbr.2017.06.027
39. Xu X, Zhang L, Ye X, et al. Nrf2/ARE pathway inhibits ROS-induced NLRP3 inflammasome activation in BV2 cells after cerebral ischemia reperfusion. Inflamm Res. 2018;67:57–65. doi:10.1007/s00011-017-1095-6
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.