Back to Journals » OncoTargets and Therapy » Volume 13
Emerging Role of Immunotherapy for Colorectal Cancer with Liver Metastasis
Authors Yu X, Zhu L, Liu J, Xie M, Chen J, Li J
Received 13 July 2020
Accepted for publication 29 October 2020
Published 13 November 2020 Volume 2020:13 Pages 11645—11658
DOI https://doi.org/10.2147/OTT.S271955
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Leo Jen-Liang Su
Xianzhe Yu,1,* Lingling Zhu,2,* Jiewei Liu,2,* Ming Xie,1 Jiang Chen,3 Jianguo Li1
1Gastrointestinal Department, The Affiliated Hospital of Zunyi Medical University, Zunyi, Guizhou Province, People’s Republic of China; 2Lung Cancer Center, West China Hospital of Sichuan University, Chengdu, Sichuan Province, People’s Republic of China; 3Department of General Surgery, Sir Run Run Shaw Hospital, Zhejiang University, Hangzhou, Zhejiang Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jianguo Li
Gastrointestinal Department, The Affiliated Hospital of Zunyi Medical University, Huichuan Area, Dalian Road 149, Zunyi 563000, Guizhou, People’s Republic of China
Tel +86 851-28608241
Fax +86 22 6036 3013
Email [email protected]
Jiang Chen
Department of General Surgery, Sir Run Run Shaw Hospital, Zhejiang University, Hangzhou 310016, Zhejiang Province, People’s Republic of China
Email [email protected]
Abstract: Colorectal cancer (CRC) is the third most common malignant tumor in the world and the second leading cause of cancer-related deaths, with the liver as the most common site of distant metastasis. The prognosis of CRC with liver metastasis is poor, and most patients cannot undergo surgery. In addition, conventional antitumor approaches such as chemotherapy, radiotherapy, targeted therapy, and surgery result in unsatisfactory outcomes. In recent years, immunotherapy has shown good prospects in the treatment of assorted tumors by enhancing the host’s antitumor immune function, and it may become a new effective treatment for liver metastasis of CRC. However, challenges remain in applying immunotherapy to CRC with liver metastasis. This review examines how the microenvironment and immunosuppressive landscape of the liver favor tumor progression. It also highlights the latest research advances in immunotherapy for colorectal liver metastasis and identifies immunotherapy as a treatment regimen with a promising future in clinical applications.
Keywords: immunotherapy, colorectal cancer, liver metastasis, tumor immune microenvironment, immune checkpoint inhibitors, deficient DNA mismatch repair
Introduction
Colorectal cancer (CRC) ranked third with 1.8 million new cases (10.2%), and second (9.2%) in mortality with an estimated 881,000 deaths recorded worldwide in 2018.1 Due to its atypical clinical symptoms in the early stages, CRC is often ignored, leading to a delay in diagnosis and treatment.2 Considering that the liver is the main filter for intestinal venous drainage, CRC patients often develop colorectal liver metastases (CLM).3 Synchronous CLM occurs in approximately 15% of patients at the initiation of therapy, and approximately 50% of patients during follow-up.4 Surgical resection is the standard treatment for patients with limited disease CLM, but most patients are not suitable for surgery because of extensive disease, tumor multiplicities, concomitant major systemic diseases, or poor hepatic functional reserve.5 Only 20–30% of patients have operable tumors with a chance of cure.6,7 In untreated CLM, the median survival duration is 7.5 months, and the 5-year survival rate is <1%. Even after surgery, the 5-year survival rate is between 20 and 45%, with a recurrence rate of 60%.8
Patients with CLM who are not eligible for resection have a poor clinical outcome, highlighting the need for novel therapeutic strategies. The combination of radiotherapy, chemotherapy, and traditional Chinese medicine has improved the prognosis and prolonged the median survival rate in these patients.9 Although the introduction of cytotoxic agents have improved the survival in CRC patients and provided good symptomatic relief, the prognosis in the setting of metastatic CRC (mCRC) remains poor.10 A systematic review of combination treatment (5-fluorouracil, oxaliplatin, and irinotecan) for mCRC led to a conclusion that conventional chemotherapeutic agents had limited utility.11 Patients with mCRC who were administered this triple chemotherapy regimen in conjunction with bevacizumab had a 3-year overall survival of approximately 40%.12 Therefore, there is a dilemma on choosing the best treatment regimen among the currently available conventional therapeutic strategies that can improve the unfavorable long-term outcomes in patients with mCRC.
In recent years, immunotherapy has achieved a certain degree of success in the treatment of advanced solid tumors.13 The purpose of immunotherapy is to enhance the anti-tumor effect of patient’s own immune system by augmenting the innate immunity and antitumor function of T cells, and by targeting immunosuppressive tumor-associated macrophages.14 Immunotherapies include immune checkpoint inhibitors (ICIs); cancer vaccines; and other biotherapeutics such as chimeric antigen receptor (CAR) T cells. These therapies are effective in treating a variety of cancers,15 and mCRC, especially mCRC with deficient DNA mismatch repair (dMMR) genes.16 DNA mismatch repair (MMR) is an evolutionarily conserved DNA excision-resynthesis that preserves genomic integrity by correcting mismatched bases that have evaded the proofreading activity of DNA polymerase during DNA replication.17 Therefore, when this system is deficient, replication errors, including frameshift mutations and single-nucleotide variants accumulate,18 resulting in the production of microsatellite instability (MSI).19 Mechanistically, dMMR contributes to an accumulation of numerous genomic mutations, which generates multiple neoantigens and obvious response to immune therapy.18,20 It has been reported that reported that patients with dMMR colon cancer showed a stronger response to immunotherapy, including regimes containing programmed cell death 1 (PD-1) plus cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) inhibitors, than those with MMR-proficient (pMMR) tumors.21 The US Food and Drug Administration (FDA) approved PD-1 antibodies for CRC with high MSI or dMMR levels, including pembrolizumab and nivolumab.22 In May 2017, the FDA further approved pembrolizumab for patients with microsatellite instability-high (MSI-H) or those with dMMR metastatic solid tumors after the failure of the initial treatment.23
However, immunotherapy provides limited or no clinical benefits to most patients owing to the inhibitory impact of tumor immune microenvironment (TME).24 Some patients fail to develop an initial response to treatment with ICIs. Although the mechanism of drug resistance has been proposed to be due to functional changes in many signaling pathways, this process is still not fully understood. Currently known mechanisms of tumor cells evading immune surveillance include the destruction of the original processing mechanism of the antigen presentation machinery (APM) or the expression of HLA complexes (HLA class I heavy chains or 2-microglobulin [B2M]), resulting in defects in antigen processing. Studies have shown that MSI tumors with B2M mutations in CRC patients were resistant to anti-PD-1 monoclonal antibodies. Mutations in the JAK1 and JAK2 genes are another mechanism that leads to immune evasion. These genes encode kinases downstream of the IFN-γ receptor and are necessary to mediate the IFN-γ signaling pathway. Mutations in these genes were found in CRC patients resistant to PD-1 inhibitors.25,26 There is also a view that resistance to immunotherapy is a special form of Darwin’s natural selection, which originated from the selection of genetic or epigenetic features in tumor masses before therapeutic intervention. The main driver of tumor cell immune resistance mutations generated by this mechanism appears to be the genomic instability in transformed cells.27 Here, we will discuss the emerging role and potential challenges of immunotherapies in patients with CLM.
Biology of the Metastatic Colorectal Cancer
Colorectal carcinogenesis is a process that is accompanied by high heterogeneity and accumulation of somatic molecular mutations, which is impacted by several factors, including exposures to intestinal pathogens and host immunity.28 As previously noted, a major determinant of CRC-related morbidity and mortality is distant metastasis,29 with liver metastasis being the most common metastatic event and a dominant factor influencing survival. Immune escape is responsible for impairment of antitumor immunity during the initiation and metastasis in CRC.30 The tumor immune microenvironment (TME) consists of the neighboring tissues that surround the tumor, which include blood vessels, immune cells, fibroblasts, the extracellular matrix, and signaling molecules. The synergistic effect of malignant cells, immune and non-immune stromal cells together with soluble and insoluble factors in TME is the basis for the progression of CRC. The TME can trigger CRC progression by either recruiting immunosuppressive immune cells, inducing other immunosuppressive mechanisms, or inducing chronic inflammation.30,31 It has been proposed that metastatic spread of CRC can be mediated by multiple interactive mechanisms in the TME, which includes acquisition of aberrant immune phenotypes (NT5E/CD73, CD68, and CD163) via generation of immunosuppressive mediators.30
Desmoplastic, pushing, replacement, and two rarer patterns (sinusoidal and portal) are the histologic growth patterns (HGPs) in liver metastases. The different interfaces between tumor cells and adjacent normal liver parenchyma define the HGPs.32 A recent study showed high HGP concordance within metastasis (95%) when classifying HGPs as desmoplastic (dHGP) or non-desmoplastic (non-dHGP).33 The dHGP is characterized by angiogenesis and a peripheral fibrotic rim, whereas non-angiogenic HGP is characterized by the co-option of endogenous sinusoidal hepatic vasculature. The main characteristics of tumors with dHGP include angiogenesis and the fibrotic reaction (desmoplasia) that surround the metastases.34 Another critical step in tumor metastasis and invasion is thought to be through epithelial-mesenchymal transition (EMT).35 EMT is a developmental program in cancer cells that can activate cancer cells for invasion and metastasis. During the EMT process, epithelial cells undergo morphological changes, leading to an aggressive migration phenotype that is characteristic of mesenchymal cells.36 CRC characterized by EMT is known to have an increased vascular invasion and metastasis and a poor prognosis.35
Additionally, the expression level of PD-1 in CD8+ T cells in CRC specimens TME is higher than that in CD8+ T cells in tumor-free lymph nodes.37 Therefore, the PD-1/programmed death-ligand 1 (PD-L1) inhibitors have become a treatment option for CRC.37 Furthermore, an increasing number of clinical data have demonstrated that immune cell populations can influence the immune response and clinical outcomes in CLM patients.38 For example, the progression of CRC is closely related to tumor-infiltrating lymphocytes (TILs). Compared with the widely used tumor staging and lymph node metastasis, TILs can provide more accurate clinical predictions.39 A greater density of TILs in the primary tumor was correlated with better survival40 in CRC patients, particularly in those with dMMR tumors.41
Additionally, the formation of a pre-metastasis niche provides a supporting microenvironment for tumor cells to spread from the outside. The Features of the pre-metastasis niche include inflammation, immunosuppression, angiogenesis, etc.42 Tumor-derived secreted factors, extracellular vesicles, bone marrow-derived cells, immunosuppressive cells, and host stromal cells constitute the pre-metastasis niche. Tumor exosomes can inhibit the recruitment of immune cells, increase angiogenesis in the pre-metastatic niche, and increase vascular permeability. Exosomes secreted by CRC cells promote angiogenesis and destroy vascular endothelial cell connections.43 Recent evidence confirmed that miR-19a can inhibit CRC angiogenesis by targeting KRAS and VEGFA; exosomes secreted by CRC cells can trigger the downstream regulator PEAK1 of the EGFR/KRAS pathway to reduce cell proliferation, migration, and invasion.44 In CRC, tumor-derived exosomes facilitate the creation of pre-metastatic niche by modulating immune surveillance. Moreover, the liver inflammation microenvironment can be induced by CRC exosomes. Exosomes activate a pro-inflammatory phenotype in macrophages, which form an inflammatory pre-metastatic niche and promote liver metastasis.45 Although the important role of pre-metastasis niche formation in CRC metastasis has been confirmed by many studies, the mechanism of CRC cells, inducing pre-metastasis niche formation, still needs further research.42
The immune system plays an essential role in the occurrence of tumors, suggesting a promising healing strategy for treating cancers, including CRC.46 The clinical outcomes in all solid tumors, including CRC, are mainly impacted by the host’s immune system.47 A complicated interaction exists between immune cells and malignant cells within the TME of mCRC. The immune infiltrating cells in mCRC constitute both immune-supportive and immunosuppressive cells, and their relative ratio impacts the overall immune state of the tumor.48 Increased level of immunosuppressive cells, such as myeloid derived suppressive cells (MDSCs), T-regulating cells (Tregs), type 2 (M2) macrophages, N2 neutrophils, and alternative cancer-related cell brands, including reduced antigen presentation, contribute to immunosuppression and eventual immune evasion in CRC.49 For example, tumor‐infiltrating neutrophils suppressed in vitro activated T cell proliferation via activation of the TGFβ signaling pathway in CRC.50 Notably, neutrophil subsets can have either a protumor (N2) or antitumor (N1) phenotype by mediating TGFβ signaling.30 Additionally, enhanced tumor invasion and growth is induced via MDSCs, extracellular matrix remodeling, and angiogenesis mediated by M2 tumor-associated macrophages (M2-TAMs) in CRC.30 Collectively, all of these cells are linked to cancer progression and unfavorable prognosis.51
Based on the genetic alterations, CRC can be divided into two clinically relevant subgroups: pMMR/microsatellite-stable (MSS) and dMMR/MSI.52 In CRC, MMR deficiency leads to the accumulation of numerous insertions/deletions at DNA microsatellites, the repetitive DNA sequences with repeated units of 1–6 base pairs. This defect and the resulting MSI lead to genomic mutations that exhibit high levels of immunogenic tumor neoantigens targeted by the immune system.53 This is linked to a high mutational burden in MSI cancers, the mutation rate of MSI is usually 10–50 times higher than that of MSS cancer.54 Approximately 15% of CRC cases display MSI.55 DMMR or MSI-H CRC are more responsive to ICIs because there are more somatic mutations; therefore, these patients experience more clinical benefit.22,56,57 MSI-H tumors also possess a high level of TILs.58 This is likely because MMR defects produce frame shift mutations, which result in the synthesis of truncated proteins contributing to antitumor adaptive immunity mediated by T cells, and this leads to a better response to immunotherapies and better prognosis.59 Therefore, the altered mutation spectrum and molecular heterogeneity can be considered for therapy modification and patient stratification to avoid treatment-related toxicities.
However, the biological properties of liver metastases are reported to be distinct from those of the primary colorectal tumor.60 CLM, a selective, non-random process, constitutes multiple steps, including local remodeling of the liver microenvironment.44 MCRC cells enter the circulation by undergoing EMT to evade the host’s defense system, survive in the blood circulation, and then disseminate and grow in the liver.61 Immune dysfunction and immune evasion are the key mechanisms of CRC tumorigenesis and response to therapy.62
Therefore, from the available evidence, it can be concluded that the recruitment of immunosuppressive cells and other mechanisms of CRC tumorigenesis are pivotal to remodeling the TME and programming malignant cells toward a metastatic phenotype (Figure 1). This emphasizes the importance of the host CRC microenvironment in liver metastasis and highlights the potential of immunotherapy, including ICIs, in CRC, especially in dMMR CRC.
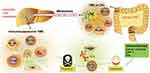 |
Figure 1 The immunosuppressive microenvironment of the liver and colorectal cancer contributes to liver metastasis and poor survival in colorectal cancer, which can be treated using immunotherapy. |
Immunotherapy as an Emerging Treatment for Cancer
Tumor cells have the capacity of growing exponentially and spreading rapidly, partly through inhibiting, evading, and exploiting the host immunity.63 In the recent few years, immunotherapy has shown impressive efficacy and has revolutionized the therapeutic landscape of multiple malignancies by strengthening immune responses.64 Unlike conventional cancer therapies, immunotherapy potentiates the patient’s immune system or modulates the TME instead of directly targeting tumor cells.13 Malignant cells can escape the antitumor immunity; therefore, the major aim of immunotherapy, such as ICIs, is to block the immune evasion mechanisms of tumor cells,65 thereby suppressing tumor progression, relapse, and metastasis.
PD-1 and CTLA-4 are receptors that inhibit T cell responses to maintain peripheral tolerance, thereby permitting the tumor cells to grow rather than being eliminated by the immune system. ICIs can potentiate anticancer immune responses via suppression of the inhibitory receptors expressed on T lymphocytes and on the surface of malignant cells (PD-L1), including CRC cells.66 PD-L1 interacts with PD-1 to induce rapid phosphorylation of SHP-1 and SHP-2 to exert an inhibitory effect.15 ICIs are the antibodies against CTLA-4, PD-1, or PD-L1 that can restore antitumor immune responses, leading to impressive clinical responses in various cancers.67,68 The most successful immunotherapeutic strategy is blocking the pD-1/PD-L1,69 and this is linked to strong clinical responses in many cancers, such as skin carcinomas, head and neck carcinoma, advanced non-small cell lung cancer, and renal cell carcinoma.70 CTLA-4 modulates T cell function by competing for shared ligands with CD28 receptors expressed by both CD4+ and CD8+ T cells. Accordingly, CTLA-4 blockade by anti-CTLA-4 antibodies can render CD28 ligands more available, permitting the activation of effector T cells and suppression the Tregs that modulate homeostasis,71 thereby inhibiting tumor progression.72
It has been confirmed that higher levels of lymphocyte activation gene-3 (LAG-3), T cell immunoglobulin mucin-3 (TIM-3), and T cell immunoreceptors with Ig and ITIM domains (TIGIT) were expressed in colorectal tumor tissues.73 Therefore, they are potential therapeutic targets for immune-mediated therapy because of their important role in tumor immune evasion.73 They are highly expressed in T cells that are stimulated by persistent antigens.74 TIM-3 is considered to be a key immune checkpoint for regulating T cell responses. The expression of TIM-3 on Tregs can form an immunosuppressive tumor microenvironment and promote immune evasion and tumor progression. TIM-3 in colorectal cancer tissues is significantly related to tumor lymph node/distant metastasis. The expression of TIM-3 in CRC TME predicts T cell failure and promotes tumor metastasis. Accurate characterization of TIM-3+ T cells should contribute to specific targeted therapy, enhance anti-tumor immunity, and improve clinical response.75 The proliferation and invasion of CRC cells are directly related to TIM-3.76
LAG-3 is another important immune checkpoint of the immunoglobulin superfamily. It is expressed in a variety of immune cells and inhibits the proliferation and activation of T cells. It encodes a surface molecule that is selectively upregulated in Treg and is a key regulator that controls the maximum Treg activity.77 LAG-3 binds to a major histocompatibility complex II (MHC class II) and causes a decrease in CD4+ T cell activity.78 Many preclinical studies have confirmed that LAG-3-mediated signaling led to cytotoxic CD8+ T cells exhaustion. PD-1/PD-L1 treatment can activate LAG3 block and restore anti-tumor immunity.79 In the colorectal adenocarcinoma model, the combination of anti-PD-L1 and anti-TIM-3 can enhance the anti-tumor activity of T cells. Similarly, in the MC38 colorectal adenocarcinoma model, the combination of anti-PD-1 and anti-LAG3 antibodies inhibited tumor advancement. Therefore, treatment regimens targeting LAG-3 may benefit patients with CRC.80
The inhibitory receptor TIGIT is a new target for immunotherapy. It is expressed in immune cells in colorectal cancer, including natural killer (NK) cells and CD8+ TILs.81 TIGHT can inhibit NK cells and effector T cells, and also inhibit the immune response by promoting Treg.82 The combined effect of anti-TIGIT antibodies and ICIs can enhance the function of T cells in tumors, thereby promoting tumor clearance. Many clinical studies are currently investigating the therapeutic effects of TIGIT block alone or in combination with PD-1 on various cancers.83 Blocking TIGIT in NK cells can restore the NK cells’ powerful effect in vivo and reverse their functional failure.84
The response rates to anti-PD-1/PD-L1 antibodies and anti-CTLA-4 antibodies in CRC patients were far from satisfactory. However, these new-generation immune checkpoints are promising therapeutic targets for clinical application.85
Adoptive T cell therapy (ACT) is applied to anti-tumor therapy through the treatment of transferred T cells.86 This process includes isolation of TILs with antitumor activity, followed with in vitro extensive expansion, activation, and subsequent administration to the patients.87 In an adaptation of this method, T cells with genetically engineered neoantigen-reactive T cell receptors (TCRs) were generated to enhance the efficiency of this strategy.88 The T cells used for ACT can be endogenous CD8+ T cells isolated from TILs or autologous circulating CD8+ T cells with genetically engineered antigen receptors.89 TILs, a crucial component of the TME, have been reported to be related to therapeutic responses and clinical outcomes.90 TILs capable of recognizing tumor antigens are responsible for the highly specific antitumor immune response. TILs are also less toxic than TCR-modified T cells or CAR-T cells. Moreover, TILs have heterogeneous specificity, indicating a pivotal advantage for counteracting immune escape.63 Therefore, TILs can function as an ideal source of tumor-reactive TCRs for personalized cancer immunotherapy and can also act as vehicles for ACT therapies and TCR gene therapies.91 Using TILs in ACT correlated with favorable overall response rates and sustained remission.92 In addition, immune therapies targeting toll-like receptors exhibited antitumor activity in CRC via activation of anticancer immunity or suppression of oncogenic signaling pathways.93
The use of CAR-T cells as an ACT method, or obtaining antigen-specific T cells through genetic engineering,94 holds an edge over the previous approaches using these modifications.87 CAR-T cells have shown promising curative effect, especially in hematological malignancies.95 CAR-T therapy has been approved in patients harboring B cell malignancies,96 and the FDA recently approved anti-CD19 targeting CAR-T cell therapy for both acute lymphoblastic leukemia and diffuse large B cell lymphoma.97 The capacity of CAR-T cells, including their improved expansion to tumor loci, long-lasting in vivo persistence, and synergy with the endogenous immune response, may permit this living, replicating therapy to trigger prolonged antitumor control in patients.98 Outside of the hematological malignancies, the therapeutic potential of CAR-T cells in solid cancers has recently been confirmed. However, further research is required to thoroughly utilize CAR-T cells in the management of solid cancers. Solid cancers are more complicated than hematological malignancies, which makes it difficult for T cells to eradicate tumor masses and maintain lasting regression.99
Recently, CAR-T cell therapy has produced great success in the treatment of CLM in preclinical models.100 In a carcinoembryonic (CEA) positive mouse model of colon cancer, recombinant lentivirus-modified peripheral lymphocytes with a chimeric T cell receptor demonstrated antitumor efficacy.101 In another CRC mouse model, intraperitoneal administration of CAR-T cells in conjunction with reduction of MDSCs and Tregs also showed antitumor potential.100 CAR-T cells against other targets, CD133, CYAD-101, EGFR, and IL-12, have also demonstrated potential antitumor activity in preclinical and clinical environments.100 In a Phase I clinical study, CART72 cells, the first-generation CAR-T cells, showed a good safety profile by intravenous injection or direct hepatic artery administration in patients with CLM.102 Another Phase I study reported that CEA CAR-T cell therapy was tolerable and had efficacy in 8 of 10 refractory and relapsed CEA-positive patients with CLM.103 However, a durable response to CAR-T cell therapy is only observed in a small number of CRC cases harboring CLM due to limitations such as augmented toxicities, recurrence, limited trafficking, and an unfavorable TME.
After the first successful attempt at cancer immunotherapy by Dr. William Coley, bacillus Calmette-Guérin, a live attenuated vaccine against Mycobacterium tuberculosis, was evaluated for treatment of bladder cancer.104 This encouraged the investigation and approval of cancer vaccines as a type of cancer immunotherapy by the FDA.105 Cancer vaccines are designed to induce an intense immune response to one or more tumor‐specific antigens, thereby driving antitumor cytotoxicity.106 This is achieved by injecting cancer-specific elements into patients to stimulate an immune attack that is highly specific to the patient’s tumor cells.107 Notably, vaccines can influence the TME by modifying antigen-specific B and T cell responses.65,108 The different impacts of varied levels of neoantigen clonality on TME might offer opportunities to refine the targets used in the vaccines, indicating the valuable role of adjuncts in the treatment of CRC.109 Other potential therapeutic strategies, such as hepatic macrophages and Tregs, were also found to be rational targets for antitumor intervention.30,110
Despite the establishment of multiple emerging therapeutic strategies in CLM, the lack of consistently successful clinical outcomes highlights the necessity of further research to develop novel treatment approaches or enhance the efficacy of existing strategies to improve clinical benefits.
The Hepatic Tumor Microenvironment
A tumor is a complex tissue constituting tumor cells and non-malignant components such as stromal fibroblasts, inflammatory cells, vasculature, normal epithelia, extracellular matrices and secreted factors; all of these are considered to be the TME.111 In the TME of the liver, non-malignant cells can promote immunosuppression and favor tumor growth by contributing to proliferation, invasion, migration, and metastasis of malignant cells;112 that is, the liver is a unique organ in which a characteristic TME facilitates the capability of cancer cells to evade natural host defenses and augments their ability to grow and migrate, contributing to a skew toward tolerance.113 The quantity and differentiation of infiltrated immune cells in the TME, especially immunosuppressive cells,114 is closely associated with tumorigenesis and metastasis, as well as the cellular response to therapy.115 Eynde and his colleagues noted that the densities of T/B cell infiltration differed in 603 distinct CLMs from the same case,116 suggesting diverse patterns of genetic variations within these metastatic sites. With the increasingly wide utilization of immunotherapy, including ICIs, assessing the heterogeneity of the TME landscape and remodeling the TME have emerged as promising avenues to improve treatment efficiency and patient prognosis.117 Furthermore, lymphocyte infiltration and plasma cell infiltration are linked to prolonged patient survival in CLM.118,119
Several key factors contribute to the extravasation of CRC cells into the liver.120 First, the blood circulation system in the liver is complicated and enriched. For example, CRC cells metastasize to the liver through the portal circulation, which accounts for 75% of the blood flow in the liver.121 Simultaneously, the slow microcirculation in the hepatic sinusoidal vessels permits retention of malignant cells in the liver. Second, the lack of a basement membrane in the liver endothelium allows cancer cells to efficiently attach to microvascular endothelium for developing micrometastases. Finally, high levels of some surface molecules expressed on liver-resident cells contribute to malignant cell migration from the vascular lumen into the space of Disse.
The cell types in tumors mainly include cancer cells, endothelial cells, hepatic stellate cells, mesenchymal stem cells, cancer-related stromal fibroblasts, and immune cells.122,123 Infiltration of immune cells, including macrophages, monocytes, lymphocytes, NK cells, and dendritic cells (DCs), into the tumor tissue at early stages of tumor progression is essential for targeting cancer. However, the antitumoral immune effects could be counteracted by the action of immunosuppressive cells within the developing TME, including MDSCs, Tregs, tumor-associated neutrophils, cancer-associated fibroblasts, and M2-TAMs.112,122
The hepatic microenvironment also constitutes various other cell types like liver sinusoidal endothelial cells (LSECs), hepatocytes, Kupffer cells (KCs), liver-associated lymphocytes, and hepatic stellate cells.124 Because 75% of the blood supply of the liver comes from the intestine via the portal vein,125 the liver is enriched with antigens and microbial products from the digestive tract. Moreover, due to its anatomical site, the liver is frequently exposed to pathogens as well as non-pathogenic stimuli.126 The TME of the liver exerts a key effect in antitumor response by activating naïve T cells via presenting antigens by LSECs, KCs, DCs, and hepatocytes. However, the liver’s immunosuppressive microenvironment promotes tolerance to numerous endogenous and exogenous intestinal bacteria and antigens and other endogenous and exogenous stimuli.127 As a result, the liver cannot attack cancer cells.
LSECs, the initial cells encountered by metastatic cancer cells, can function with KCs as a physical platform for recruiting and anchoring immunological cells and are the gatekeepers for liver immunomodulation.128 Hepatocytes can activate naïve CD8 T cells and promote the apoptosis and elimination of T cells in a Bim-dependent way, thereby triggering antigen-specific immunological tolerance.129 Other mechanisms include defects in antigen presentation, recruitment of immunosuppressive cells, inhibition of NK cells, compromised function of CD4+ T cells, and upregulation of immune checkpoint signaling.130 In CLM patients, the tumor-specific T cell response constitutes an expanded population of activated Tregs and MDSCs.37 MDSCs are considered as the most important cell types for the organization and maintenance of local immune suppression in mCRC.131
Hepatic macrophages are a heterogenic population including both resident KCs and recruited monocyte-derived macrophages.110 KCs, a subpopulation of macrophages with an immunosuppressive phenotype, control liver metastasis via regulating Dectin-2, a C-type lectin innate receptor.132 Another critical determinant of CLM, TAMs, nurture malignant cell growth and metastasis by mediating immunosuppression and secreting pro-inflammatory factors, including IL-10 and TGFβ, eventually leading to poor prognosis.38 TAMs can also express inhibitory receptors including PD-L1/2, B7-1/2, and the non-typical MHC-I proteins.133 Therefore, TAMs may be able to synergize with chemotherapy, radiotherapy, and unconventional targeted therapies.38
Secretory proteins can exert both antitumor and protumor effects during CLM.61 For example, the tumoricidal activity of LSECs is partly determined by cytokines. Additionally, activated LSECs and KCs can also promote metastasis by triggering the expression of cytokines (namely, TNF-α and IL-1) and adhesion molecules (CD44, CD15s, and mucin among many others).60 In addition, activated NK cells can stimulate KCs to release granulocyte macrophage colony stimulating factor and IFN-γ.134 Multiple chemokine signaling pathways have been reported to be linked to liver metastasis, including those that involve CXCL1–CXCR2, CXCL12–CXCR, CCL2–CCR2, CCL15–CCR1, and CX3CL1–CX3CR1 chemokines.135 Therefore, secreted factors, such as chemokines and cytokines, can remodel the TME of CLM by recruiting host-derived myeloid cells to promote metastasis and immune escape.
In conclusion, the immunosuppressive nature of the intrahepatic milieu might be responsible for the establishment of CLM and the aggressive pathology of the disease.136 Therefore, targeting TME components, including immune checkpoints, immunoregulatory cells and their secretory factors, and the tumor structure,122 may be an option to prevent and treat CLM.
Immunotherapy Solutions for Liver Metastases
Metastases have been and remain the main obstacle to a successful management of malignancies. Even among cancers that are sensitive to radiotherapy or chemotherapy, one of the principal pathways of treatment failure is metastasis. The previous treatments for CRC showed minimal improvement in the 5‐year survival rate, even with relapses that followed a complete surgical resection,137 especially among the patients with liver metastasis. Therefore, new treatment strategies are desperately needed. One such approach is immunotherapy.106 Previous findings have confirmed that blocking protumorigenic interactions and cancer-stromal interplay via the accumulation of myeloid cells and T cells and blocking protumorigenic cytokine signaling by targeting CCR5, achieved clinical benefits among patients with advanced/metastatic CRC.64
Liver metastasis occurs in multiple cancers and is related to an unfavorable prognosis.132 Among the main metastatic sites of CRC are also the liver and lungs. The liver’s immunosuppressive microenvironment supports the generation of both pre- and pro-metastatic niches to develop hepatic metastases.138 For instance, the treatment efficacy of CAR-T cells engineered against hepatic metastases can be undermined by the accumulation of MDSCs. Hepatic MDSCs can express PD-L1, and PD-L1 can inhibit the activation and proliferation of T cells by interacting with PD-1 on T cells. Reduced MDSC accumulation within the liver via PD-L1 blockade synergizes with CAR-T cell therapy to achieve antitumor responses. Moreover, blockade of molecules involved in MDSC biology and function augmented the efficiency of ACT against CRC metastases.139
Many ICIs are being evaluated in prospective trials of various cancer types, including CLM.140 Clinical trials with pembrolizumab have confirmed that patients with dMMR mCRC pretreatment benefited from ICIs.141 The immune-related objective response rates (ORRs) and progression-free survival rates in patients with MSI-H CRCs were 40% and 78%, respectively, and 0% and 11%, respectively, in those with MSS/pMMR CRCs.142 Compared to MSI cancers, MSS cancers are less sensitive to immunotherapy alone, and novel combination approaches are being investigated to enhance therapeutic efficiency in patients who might be sensitive to combination treatments.143 DMMR CRCs are prone to be heavily infiltrated by CD8+ T cells and highly expressed immune checkpoint molecules, including PD-L1.144 Response Evaluation Criteria In Solid Tumors (RECIST) assessment was performed in 10 patients with dMMR CRC, with an objective response rate (ORR) of 40% and an objective remission rate of 0% in MMR-proficient (MMR-p) CRC. In dMMR CRC and pMMR CRC, the disease control rate at 12 weeks was 90% and 11%, respectively.145 This might partly explain why anti-PD-1/PD-L1 therapies are correlated to antitumor responses in MSI-H or dMMR mCRC. The FDA has approved pembrolizumab and nivolumab for cases with dMMR or MSI-H mCRC.146 Indeed, pembrolizumab is now approved by the FDA for the treatment of all dMMR-MSI-H metastatic solid tumors.144
The Phase II CheckMate 142 clinical trial evaluated the nivolumab in conjunction with or without ipilimumab in dMMR or MSI-H mCRC. Preliminary data revealed an ORR and disease control rate of 31% and 69%, respectively. When ipilimumab was added to nivolumab, the ORR and disease control rates of 41% and 78%, respectively, were noted.141 Nivolumab has been approved for patients harboring dMMR/MSI-H mCRC who experience disease progression following treatment of fluoropyrimidine, oxaliplatin, and irinotecan.147 Targeting immune checkpoints has achieved good response in multiple tumor types, but response rates varied in CRC with distinct genomic alterations.148 Among mCRC patients with MSI‐H tumors, ICIs resulted in good responses to some extent. The ORR of pembrolizumab or nivolumab varies between 30% and 40%, and 50% of cases are controlled. These drugs have been approved for the second-line treatment of mCRC patients with dMMR/MSI-H tumors.149 Additionally, ipilimumab plus nivolumab improved the ORR to 55% among these patients. Based on this data, ICIs have even been investigated as monotherapy in MSI‐H mCRC.149 Even in MSS CRCs, mutational/neoantigen load has demonstrated some association with immune infiltration and survival, showing promise for successful exploration of immunotherapies.150
The median PFS in patients with advanced CRC treated with pembrolizumab combined with chemotherapy was 16.9 months, and the median OS was 18.8 months (median follow-up 7.9 months).151 In phase Ib/II trials of RAS wild-type mCRC patients treated with pembrolizumab plus cetuximab, seven of nine patients were stable and had good tolerance.151
Adoptive cell therapy (ACT) using TILs has shown significant efficacy in patients with metastatic melanoma. Striking responses have been observed in individual patients with CRC after ACT.152 In order to improve NK cell tumor response, CAR was designed to fuse the extracellular domain of NK cell receptor NKG2D with DAP12. NKG2D ED and DAP12 make up the CAR. A study confirmed that NKG2Dp CAR-NK cells could indeed recognize tumor cells and exhibit anti-tumor effects in CLM patients.153 This is an achievable way to combat liver metastasis by using new techniques developed in recent years. Because it can be harvested aseptically and does not contaminate the intestinal flora, liver metastasis may be an ideal source of TILs for the treatment of ACT in CRC patients.154 TILs have been isolated from CRC patients in several studies and their potential as an immunotherapy modality has been evaluated. TILs expanded in vitro mainly contain effector memory T cells, and have been found to trigger antitumor responses.155
Cancer vaccines have been used to prevent relapse in patients suffering from CRC.156 Approximately 61.3% of patients with advanced CRC who received vaccination developed cell-mediated immunity. However, whether vaccinated or not, the same overall survival rate of 48% in CRC patients was observed in all groups.41
Overall, immunotherapy might be a feasible option for most CRC patients with liver metastases. Standard treatment of CRC also includes antiangiogenic drugs. When combined with immunotherapy, anti-angiogenic drugs may also show synergistic effects.157 As monotherapy and in combination therapy, the role of immunotherapy will undoubtedly evolve.
A variety of current immunotherapies for CLM are shown in (Table 1).
 |
Table 1 Summary of Immunotherapy Approaches for Colorectal Cancer with Liver Metastasis |
Conclusions
Treatment of mCRC, especially CLM, is challenging because of its anatomical location and the immunosuppressive microenvironment. Immunotherapy is a novel effective therapeutic strategy. Despite increasing clinical data regarding the therapeutic role of ICIs among dMMR or MSI-H mCRC, most patients harboring pMMR or MSS tumors still do not benefit from immunotherapeutic agents.158 This may partly be explained by the inhibitory impact of multiple suppressive networks on effector immune cells to enable CRC to develop and form metastases within the TME.122 This necessitates further research to develop novel therapeutic approaches or identify biomarkers for personalized modulation of the TME to reverse immunosuppression, thus improving clinical outcomes.106 Specifically, the immune cells in the TME, together with the soluble factors, might also be potential targets to treat CLM. Still, immunotherapy holds promise as a rational treatment option, either as a monotherapy or as combinational therapy.159 This is especially true when immunotherapy is combined with other therapies to address the problem of low mutational load by increasing tumor immunogenicity, thus overcoming the immunosuppressive microenvironment. This is also the case for the treatment of CLM. Outside of the discovery of new immunotherapy targets, further directions should be focused on relieving CLM from the inhibitory networks and activation hurdles constituting the TME by combining immunotherapies with other therapies.
Abbreviations
ACT, adoptive T cell therapy; APM, antigen processing machinery; BMDCs, bone marrow-derived cells; CAR, chimeric antigen receptor; CAR-T, chimeric antigen receptor T cell; CLM, colorectal liver metastasis; CRC, colorectal cancer; CTLA-4, cytotoxic T-lymphocyte-associated antigen 4; DCs, dendritic cells; dMMR, deficient DNA mismatch repair; EMT, epithelial‐mesenchymal transition; EVs, extracellular vesicles; FDA, Food and Drug Administration; ICIs, immune checkpoint inhibitors; IFN, interferon; HGPs, histological growth patterns; KCs, Kupffer cells; LAG-3, Lymphocyte activation gene-3; LSECs, liver sinusoidal endothelial cells; mCRC, metastatic colorectal cancer; MDSCs, myeloid‐derived suppressor cells; MMR, DNA mismatch repair; MSI, microsatellite instability; MSI-H, microsatellite instability-high; MSS, microsatellite-stable; M2-TAMs, M2 tumor-associated macrophages; NK, natural killer; ORRs, objective response rates; PD-1, programmed cell death 1; PD-L1, programmed death-ligand 1; pMMR, mismatch repair-proficient; TDSFs, tumor-derived secreted factors; TIGIT, T cell immunoreceptors with Ig and ITIM domains; TILs, tumor-infiltrating lymphocytes; TIM-3, T cell immunoglobulin mucin-3; TME, tumor immune microenvironment; TNF, tumor necrosis factor; Tregs, T regulatory cells; TCRs, T cell receptors.
Acknowledgments
This work was funded by the National Natural Science Foundation of China (grant number 31301141) and the Science and Technology Planning Fund of Guizhou Province (Guizhou Science and Technology Cooperation LH [2014] No. 7594).
Disclosure
The authors report no conflicts of interest for this work.
References
1. Castejón M, Plaza A, Martinez-Romero J, Fernandez-Marcos PJ, Cabo R, Diaz-Ruiz A. Energy restriction and colorectal cancer: a call for additional research. Nutrients. 2020;12(1):114. doi:10.3390/nu12010114
2. Sun JJ, Fan GL, Wang XG, Xu K. The research on the influences of hyperthermal perfusion chemotherapy combined with immunologic therapy on the immunologic function and levels of circulating tumor cells of the advanced colorectal cancer patients with liver metastasis. Eur Rev Med Pharmacol Sci. 2017;21(13):3139–3145.
3. Donadon M, Cortese N, Marchesi F, Cimino M, Mantovani A, Torzilli G. Hepatobiliary surgeons meet immunologists: the case of colorectal liver metastases patients. Hepatobiliary Surg Nutr. 2019;8(4):370–377. doi:10.21037/hbsn.2019.03.06
4. Matsuoka H, Morise Z, Tanaka C, et al. Repeat hepatectomy with systemic chemotherapy might improve survival of recurrent liver metastasis from colorectal cancer-a retrospective observational study. World J Surg Oncol. 2019;17(1):33. doi:10.1186/s12957-019-1575-y
5. Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25(29):4575–4580. doi:10.1200/JCO.2007.11.0833
6. Dendy MS, Ludwig JM, Kim HS. Predictors and prognosticators for survival with Yttrium-90 radioembolization therapy for unresectable colorectal cancer liver metastasis. Oncotarget. 2017;8(23):37912–37922. doi:10.18632/oncotarget.16007
7. Qian Y, Zeng ZC, Ji Y, Xiao YP. Microinvasion of liver metastases from colorectal cancer: predictive factors and application for determining clinical target volume. Radiat Oncol. 2015;10(1). doi:10.1186/s13014-015-0428-2
8. Wang Y, Zheng J, Chen H, et al. A prognostic nomogram for colorectal cancer liver metastases after percutaneous thermal ablation. Int J Hyperthermia. 2018;34(6):853–862. doi:10.1080/02656736.2017.1368095
9. Wang C, Park J, Ouyang C, et al. A pilot feasibility study of yttrium-90 liver radioembolization followed by durvalumab and tremelimumab in patients with microsatellite stable colorectal cancer liver metastases. Oncologist. 2020;25(5):382–e776. doi:10.1634/theoncologist.2019-0924
10. Xiong YX, Ren L, Wang ZQ, Huang XW, Zhou YJ. The role of angiogenesis inhibitors re-challenge in colorectal cancer previously treated with bevacizumab: a meta-analysis of randomized controlled trials. Eur Rev Med Pharmacol Sci. 2017;21(7):1489–1494.
11. Arnold D, Prager GW, Quintela A, et al. Beyond second-line therapy in patients with metastatic colorectal cancer: a systematic review. Ann Oncol. 2018;29(4):835–856. doi:10.1093/annonc/mdy038
12. Naito A, Kagawa Y, Kawai K, et al. Surgical resection of colorectal cancer with distant metastases to other than liver or lung. In Vivo (Athens, Greece). 2019;33(5):1605–1608.
13. Tai D, Choo SP, Chew V. Rationale of immunotherapy in hepatocellular carcinoma and its potential biomarkers. Cancers. 2019;11(12):1926. doi:10.3390/cancers11121926
14. Koi M, Carethers JM. The colorectal cancer immune microenvironment and approach to immunotherapies. Future Oncol. 2017;13(18):1633–1647. doi:10.2217/fon-2017-0145
15. Lingling Z, Jiewei L, Li W, et al. Molecular regulatory network of PD-1/PD-L1 in non-small cell lung cancer. Pathol Res Pract. 2020;216(4):152852. doi:10.1016/j.prp.2020.152852
16. Kim JH, Kim SY, Baek JY, et al. A phase II study of avelumab monotherapy in patients with mismatch repair-deficient/microsatellite instability-high or pole-mutated metastatic or unresectable colorectal cancer. Cancer Res Treat. 2020. doi:10.4143/crt.2020.218
17. Richman S. Deficient mismatch repair: read all about it (review). Int J Oncol. 2015;47(4):1189–1202. doi:10.3892/ijo.2015.3119
18. Germano G, Amirouchene-Angelozzi N, Rospo G, Bardelli A. The clinical impact of the genomic landscape of mismatch repair-deficient cancers. Cancer Discov. 2018;8(12):1518–1528. doi:10.1158/2159-8290.CD-18-0150
19. Evrard C, Tachon G, Randrian V, Karayan-Tapon L, Tougeron D. Microsatellite instability: diagnosis, heterogeneity, discordance, and clinical impact in colorectal cancer. Cancers. 2019;11(10):1567. doi:10.3390/cancers11101567
20. Franke AJ, Skelton WP, Starr JS, et al. Immunotherapy for colorectal cancer: a review of current and novel therapeutic approaches. J Natl Cancer Inst. 2019;111(11):1131–1141. doi:10.1093/jnci/djz093
21. Chalabi M, Fanchi LF, Dijkstra KK, et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med. 2020;26(4):566–576. doi:10.1038/s41591-020-0805-8
22. Hamada T, Zhang X, Mima K, et al. Fusobacterium nucleatum in colorectal cancer relates to immune response differentially by tumor microsatellite instability status. Cancer Immunol Res. 2018;6(11):1327–1336. doi:10.1158/2326-6066.CIR-18-0174
23. Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15(5):325–340. doi:10.1038/nrclinonc.2018.29
24. Binnewies M, Roberts EW, Kersten K, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24(5):541–550.
25. Picard E, Verschoor CP, Ma GW, Pawelec G. Relationships between immune landscapes, genetic subtypes and responses to immunotherapy in colorectal cancer. Front Immunol. 2020;11(369). doi:10.3389/fimmu.2020.00369
26. Burr ML, Sparbier CE, Chan KL, et al. An evolutionarily conserved function of polycomb silences the MHC class i antigen presentation pathway and enables immune evasion in cancer. Cancer Cell. 2019;36(4):385–401.e388. doi:10.1016/j.ccell.2019.08.008
27. Gang W, Wang JJ, Guan R, et al. Strategy to targeting the immune resistance and novel therapy in colorectal cancer. Cancer Med. 2018;7(5):1578–1603. doi:10.1002/cam4.1386
28. O’Keefe SJ. Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol. 2016;13(12):691–706. doi:10.1038/nrgastro.2016.165
29. Kim TM, Jung SH, An CH, et al. Subclonal genomic architectures of primary and metastatic colorectal cancer based on intratumoral genetic heterogeneity. Clin Cancer Res. 2015;21(19):4461–4472. doi:10.1158/1078-0432.CCR-14-2413
30. Pancione M, Giordano G, Remo A, et al. Immune escape mechanisms in colorectal cancer pathogenesis and liver metastasis. J Immunol Res. 2014;2014:686879. doi:10.1155/2014/686879
31. Gessani S, Belardelli F. Immune dysfunctions and immunotherapy in colorectal cancer: the role of dendritic cells. Cancers. 2019;11(10):1491. doi:10.3390/cancers11101491
32. Wu JB, Sarmiento AL, Fiset PO, et al. Histologic features and genomic alterations of primary colorectal adenocarcinoma predict growth patterns of liver metastasis. World J Gastroenterol. 2019;25(26):3408–3425. doi:10.3748/wjg.v25.i26.3408
33. Höppener DJ, Nierop PMH, Herpel E, et al. Histopathological growth patterns of colorectal liver metastasis exhibit little heterogeneity and can be determined with a high diagnostic accuracy. Clin Exp Metastasis. 2019;36(4):311–319. doi:10.1007/s10585-019-09975-0
34. Galjart B, Nierop PMH, van der Stok EP, et al. Angiogenic desmoplastic histopathological growth pattern as a prognostic marker of good outcome in patients with colorectal liver metastases. Angiogenesis. 2019;22(2):355–368. doi:10.1007/s10456-019-09661-5
35. Zhang Z, Tan X, Luo J, et al. GNA13 promotes tumor growth and angiogenesis by upregulating CXC chemokines via the NF-κB signaling pathway in colorectal cancer cells. Cancer Med. 2018;7(11):5611–5620. doi:10.1002/cam4.1783
36. Tsoumas D, Nikou S, Giannopoulou E, et al. ILK expression in colorectal cancer is associated with EMT, cancer stem cell markers and chemoresistance. Cancer Genomics Proteomics. 2018;15(2):127–141.
37. Jacobs J, Smits E, Lardon F, Pauwels P, Deschoolmeester V. Immune checkpoint modulation in colorectal cancer: what’s new and what to expect. J Immunol Res. 2015;2015:158038. doi:10.1155/2015/158038
38. Cortese N, Soldani C, Franceschini B, et al. Macrophages in colorectal cancer liver metastases. Cancers. 2019;11(5):633. doi:10.3390/cancers11050633
39. Iseki Y, Shibutani M, Maeda K, et al. A new method for evaluating tumor-infiltrating lymphocytes (TILs) in colorectal cancer using hematoxylin and eosin (H-E)-stained tumor sections. PLoS One. 2018;13(4):e0192744.
40. Shibutani M, Maeda K, Nagahara H, et al. A comparison of the local immune status between the primary and metastatic tumor in colorectal cancer: a retrospective study. BMC Cancer. 2018;18(1):371. doi:10.1186/s12885-018-4276-y
41. Fletcher R, Wang YJ, Schoen RE, Finn OJ, Yu J, Zhang L. Colorectal cancer prevention: immune modulation taking the stage. Biochim Biophys Acta Rev Cancer. 2018;1869(2):138–148. doi:10.1016/j.bbcan.2017.12.002
42. Zeng Z, Li Y, Pan Y, et al. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat Commun. 2018;9(1):5395. doi:10.1038/s41467-018-07810-w
43. Guo Y, Ji X, Liu J, et al. Effects of exosomes on pre-metastatic niche formation in tumors. Mol Cancer. 2019;18(1):39. doi:10.1186/s12943-019-0995-1
44. Balacescu O, Sur D, Cainap C, et al. The impact of miRNA in colorectal cancer progression and its liver metastases. Int J Mol Sci. 2018;19(12):3711. doi:10.3390/ijms19123711
45. Herrera M, Galindo-Pumariño C, García-Barberán V, Peña C. A snapshot of the tumor microenvironment in colorectal cancer: the liquid biopsy. Int J Mol Sci. 2019;20(23):6016. doi:10.3390/ijms20236016
46. Koliarakis I, Psaroulaki A, Nikolouzakis TK, et al. Intestinal microbiota and colorectal cancer: a new aspect of research. J BUON. 2018;23(5):1216–1234.
47. Kather JN, Charoentong P, Suarez-Carmona M, et al. High-throughput screening of combinatorial immunotherapies with patient-specific in silico models of metastatic colorectal cancer. Cancer Res. 2018;78(17):5155–5163. doi:10.1158/0008-5472.CAN-18-1126
48. Lazarus J, Maj T, Smith JJ, et al. Spatial and phenotypic immune profiling of metastatic colon cancer. JCI Insight. 2018;3(22). doi:10.1172/jci.insight.121932.
49. Toor SM, Murshed K, Al-Dhaheri M, Khawar M, Abu NM, Elkord E. Immune checkpoints in circulating and tumor-infiltrating CD4(+) T cell subsets in colorectal cancer patients. Front Immunol. 2019;10:2936.
50. Germann M, Zangger N, Sauvain MO, et al. Neutrophils suppress tumor-infiltrating T cells in colon cancer via matrix metalloproteinase-mediated activation of TGFbeta. EMBO Mol Med. 2020;12(1):e10681. doi:10.15252/emmm.201910681
51. Yaghoubi N, Soltani A, Ghazvini K, Hassanian SM, Hashemy SI. PD-1/PD-L1 blockade as a novel treatment for colorectal cancer. Biomed Pharmacother. 2019;110:312–318. doi:10.1016/j.biopha.2018.11.105
52. Kather JN, Halama N, Jaeger D. Genomics and emerging biomarkers for immunotherapy of colorectal cancer. Semin Cancer Biol. 2018;52(Pt 2):189–197. doi:10.1016/j.semcancer.2018.02.010
53. Bestvina CM, Vokes E. Correction to lancet oncol 2017. Lancet Oncol. 2017;18(9):510.
54. Sun X, Suo J, Yan J. Immunotherapy in human colorectal cancer: challenges and prospective. World J Gastroenterol. 2016;22(28):6362–6372. doi:10.3748/wjg.v22.i28.6362
55. Vilar E, Gruber SB. Microsatellite instability in colorectal cancer-the stable evidence. Nat Rev Clin Oncol. 2010;7(3):153–162. doi:10.1038/nrclinonc.2009.237
56. Baek SK, Lee KT, Bae SB, Lee SC. Evaluating the recent developments in palliative chemotherapy for metastatic colorectal cancer. Korean J Intern Med. 2019;34(6):1188–1196. doi:10.3904/kjim.2019.071
57. Wang J, Zhen W, Kang X. Pseudo progression in heterogeneity of right-sided metastatic colon carcinoma during nivolumab therapy: a case report. Medicine. 2019;98(32):e16490. doi:10.1097/MD.0000000000016490
58. Ronnekleiv-Kelly SM, Burkhart RA, Pawlik TM. Molecular markers of prognosis and therapeutic targets in metastatic colorectal cancer. Surg Oncol. 2016;25(3):190–199. doi:10.1016/j.suronc.2016.05.018
59. Nosho K, Sukawa Y, Adachi Y, et al. Association of Fusobacterium nucleatum with immunity and molecular alterations in colorectal cancer. World J Gastroenterol. 2016;22(2):557–566. doi:10.3748/wjg.v22.i2.557
60. Bird NC, Mangnall D, Majeed AW. Biology of colorectal liver metastases: a review. J Surg Oncol. 2006;94(1):68–80. doi:10.1002/jso.20558
61. Clark AM, Ma B, Taylor DL, Griffith L, Wells A. Liver metastases: microenvironments and ex-vivo models. Exp Biol Med (Maywood). 2016;241(15):1639–1652.
62. Walkowska J, Kallemose T, Andersen MH, Andersen O, Therkildsen C, Langkilde A. Immunoprofiles of colorectal cancer from Lynch syndrome. Oncoimmunology. 2019;8(1):e1515612. doi:10.1080/2162402X.2018.1515612
63. Marin-Acevedo JA, Soyano AE, Dholaria B, Knutson KL, Lou Y. Cancer immunotherapy beyond immune checkpoint inhibitors. J Hematol Oncol. 2018;11(1):8. doi:10.1186/s13045-017-0552-6
64. Tauriello DV, Calon A, Lonardo E, Batlle E. Determinants of metastatic competency in colorectal cancer. Mol Oncol. 2017;11(1):97–119.
65. Janiczek M, Szylberg Ł, Kasperska A, et al. Immunotherapy as a promising treatment for prostate cancer: a systematic review. J Immunol Res. 2017;2017:4861570. doi:10.1155/2017/4861570
66. Mercier J, Voutsadakis IA. The platelets-neutrophils to lymphocytes ratio: a new prognostic marker in metastatic colorectal cancer. J Gastrointest Oncol. 2018;9(3):478–486. doi:10.21037/jgo.2018.03.13
67. Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol. 2016;39(1):98–106. doi:10.1097/COC.0000000000000239
68. Zhao Y, Lee CK, Lin CH, et al. PD-L1: CD80Cis-heterodimer triggers the co-stimulatory receptor CD28 while repressing the inhibitory PD-1 and CTLA-4 pathways. Immunity. 2019;51(6):1059–1073.e1059. doi:10.1016/j.immuni.2019.11.003
69. Rotte A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J Exp Clin Cancer Res. 2019;38(1):255. doi:10.1186/s13046-019-1259-z
70. Luo C, Cen S, Ding G, Wu W. Mucinous colorectal adenocarcinoma: clinical pathology and treatment options. Cancer Commun. 2019;39(1):13. doi:10.1186/s40880-019-0361-0
71. Rowshanravan B, Halliday N, Sansom DM. CTLA-4: a moving target in immunotherapy. Blood. 2018;131(1):58–67.
72. Fiegle E, Doleschel D, Koletnik S, et al. Dual CTLA-4 and PD-L1 blockade inhibits tumor growth and liver metastasis in a highly aggressive orthotopic mouse model of colon cancer. Neoplasia. 2019;21(9):932–944. doi:10.1016/j.neo.2019.07.006
73. Sasidharan NV, Toor SM, Taha RZ, Shaath H, Elkord E. DNA methylation and repressive histones in the promoters of PD-1, CTLA-4, TIM-3, LAG-3, TIGIT, PD-L1, and galectin-9 genes in human colorectal cancer. Clin Epigenetics. 2018;10(1):104. doi:10.1186/s13148-018-0539-3
74. Joller N, Kuchroo VK. Tim-3, Lag-3, and TIGIT. Curr Top Microbiol Immunol. 2017;410:127–156.
75. Sasidharan NV, Toor SM, Taha RZ, et al. Transcriptomic profiling of tumor-infiltrating CD4(+)TIM-3(+) T cells reveals their suppressive, exhausted, and metastatic characteristics in colorectal cancer patients. Vaccines. 2020;8(1).
76. Kuai W, Xu X, Yan J, et al. Prognostic impact of PD-1 and Tim-3 expression in tumor tissue in stage I-III colorectal cancer. Biomed Res Int. 2020;2020:5294043. doi:10.1155/2020/5294043
77. Wang W, Chen D, Zhao Y, et al. Characterization of LAG-3, CTLA-4, and CD8(+) TIL density and their joint influence on the prognosis of patients with esophageal squamous cell carcinoma. Ann Transl Med. 2019;7(23):776. doi:10.21037/atm.2019.11.38
78. Lecocq Q, Zeven K, De VY, et al. Noninvasive imaging of the immune checkpoint LAG-3 using nanobodies, from development to pre-clinical use. Biomolecules. 2019;9(10):548. doi:10.3390/biom9100548
79. Zeng H, Zhou Q, Wang Z, et al. Stromal LAG-3+ cells infiltration defines poor prognosis subtype muscle-invasive bladder cancer with immunoevasive contexture. J Immunother Cancer. 2020;8(1):e000651. doi:10.1136/jitc-2020-000651
80. Gestermann N, Saugy D, Martignier C, et al. LAG-3 and PD-1+LAG-3 inhibition promote anti-tumor immune responses in human autologous melanoma/T cell co-cultures. Oncoimmunology. 2020;9(1):1736792. doi:10.1080/2162402X.2020.1736792
81. Reches A, Ophir Y, Stein N, et al. Nectin4 is a novel TIGIT ligand which combines checkpoint inhibition and tumor specificity. J Immunother Cancer. 2020;8(1):e000266. doi:10.1136/jitc-2019-000266
82. Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity. 2016;44(5):989–1004. doi:10.1016/j.immuni.2016.05.001
83. Jin HS, Choi DS, Ko M, et al. Extracellular pH modulating injectable gel for enhancing immune checkpoint inhibitor therapy. J Control Release. 2019;315:65–75. doi:10.1016/j.jconrel.2019.10.041
84. Lupo KB, Matosevic S. CD155 immunoregulation as a target for natural killer cell immunotherapy in glioblastoma. J Hematol Oncol. 2020;13(1):76. doi:10.1186/s13045-020-00913-2
85. Qin S, Xu L, Yi M, Yu S, Wu K, Luo S. Novel immune checkpoint targets: moving beyond PD-1 and CTLA-4. Mol Cancer. 2019;18(1):155.
86. Busch DH, Fräßle SP, Sommermeyer D, Buchholz VR, Riddell SR. Role of memory T cell subsets for adoptive immunotherapy. Semin Immunol. 2016;28(1):28–34. doi:10.1016/j.smim.2016.02.001
87. Benmebarek MR, Karches CH, Cadilha BL, Lesch S, Endres S, Kobold S. Killing mechanisms of chimeric antigen receptor (CAR) T cells. Int J Mol Sci. 2019;20(6):1283. doi:10.3390/ijms20061283
88. Parkhurst M, Gros A, Pasetto A, et al. Isolation of T-cell receptors specifically reactive with mutated tumor-associated antigens from tumor-infiltrating lymphocytes based on CD137 expression. Clin Cancer Res. 2017;23(10):2491–2505. doi:10.1158/1078-0432.CCR-16-2680
89. Jiang X, Xu J, Liu M, et al. Adoptive CD8(+) T cell therapy against cancer: challenges and opportunities. Cancer Lett. 2019;462:23–32. doi:10.1016/j.canlet.2019.07.017
90. Chen B, Khodadoust MS, Liu CL, Newman AM, Alizadeh AA. Profiling tumor infiltrating immune cells with CIBERSORT. Methods Mol Biol. 2018;1711:243–259.
91. Shitaoka K, Hamana H, Kishi H, et al. Identification of tumoricidal TCRs from tumor-infiltrating lymphocytes by single-cell analysis. Cancer Immunol Res. 2018;6(4):378–388. doi:10.1158/2326-6066.CIR-17-0489
92. Ollé HM, Wolbert J, Fisher J, et al. Tumor infiltrating lymphocytes expanded from pediatric neuroblastoma display heterogeneity of phenotype and function. PLoS One. 2019;14(8):e0216373. doi:10.1371/journal.pone.0216373
93. Lucas C, Barnich N, Nguyen HTT. Microbiota, Inflammation and colorectal cancer. Int J Mol Sci. 2017;18(6).
94. Boyiadzis MM, Dhodapkar MV, Brentjens RJ, et al. Chimeric antigen receptor (CAR) T therapies for the treatment of hematologic malignancies: clinical perspective and significance. J Immunother Cancer. 2018;6(1):137. doi:10.1186/s40425-018-0460-5
95. Yu S, Yi M, Qin S, Wu K. Next generation chimeric antigen receptor T cells: safety strategies to overcome toxicity. Mol Cancer Ther. 2019;18(1):125.
96. Cho JH, Collins JJ, Wong WW. Universal chimeric antigen receptors for multiplexed and logical control of T cell responses. Cell. 2018;173(6):1426–1438.e1411. doi:10.1016/j.cell.2018.03.038
97. Yu S, Yi M, Qin S, Wu K. Next generation chimeric antigen receptor T cells: safety strategies to overcome toxicity. Mol Cancer. 2019;18(1):125. doi:10.1186/s12943-019-1057-4
98. Feins S, Kong W, Williams EF, Milone MC, Fraietta JA. An introduction to chimeric antigen receptor (CAR) T-cell immunotherapy for human cancer. Am J Hematol. 2019;94(S1):S3–s9. doi:10.1002/ajh.25418
99. Teng R, Zhao J, Zhao Y, et al. Chimeric antigen receptor-modified T cells repressed solid tumors and their relapse in an established patient-derived colon carcinoma xenograft model. J Immunother. 2019;42(2):33–42.
100. Sur D, Havasi A, Cainap C, et al. Chimeric antigen receptor T-cell therapy for colorectal cancer. J Clin Med. 2020;9(1):182. doi:10.3390/jcm9010182
101. Simmons A, Whitehead RP, Kolokoltsov AA, Davey RA. Use of recombinant lentivirus pseudotyped with vesicular stomatitis virus glycoprotein G for efficient generation of human anti-cancer chimeric T cells by transduction of human peripheral blood lymphocytes in vitro. Virol J. 2006;3(1):8. doi:10.1186/1743-422X-3-8
102. Hege KM, Bergsland EK, Fisher GA, et al. Safety, tumor trafficking and immunogenicity of chimeric antigen receptor (CAR)-T cells specific for TAG-72 in colorectal cancer. J Immunother Cancer. 2017;5:22.
103. Zhang C, Wang Z, Yang Z, et al. Phase I escalating-dose trial of CAR-T therapy targeting CEA(+) metastatic colorectal cancers. Mol Ther. 2017;25(5):1248–1258. doi:10.1016/j.ymthe.2017.03.010
104. Pagano F, Bassi P, Milani C, Piazza N, Meneghini A, Garbeglio A. BCG in superficial bladder cancer: a review of Phase III European trials. Eur Urol. 1992;21(Suppl 2):7–11. doi:10.1159/000474914
105. Temizoz B, Kuroda E, Ishii KJ. Vaccine adjuvants as potential cancer immunotherapeutics. Int Immunol. 2016;28(7):329–338. doi:10.1093/intimm/dxw015
106. Sarvizadeh M, Ghasemi F, Tavakoli F, et al. Vaccines for colorectal cancer: an update. J Cell Biochem. 2019;120(6):8815–8828. doi:10.1002/jcb.28179
107. Desrichard A, Snyder A, Chan TA. Cancer neoantigens and applications for immunotherapy. Clin Cancer Res. 2016;22(4):807–812. doi:10.1158/1078-0432.CCR-14-3175
108. Singh M, Ramos I, Asafu-Adjei D, et al. Curcumin improves the therapeutic efficacy of Listeria(at)-Mage-b vaccine in correlation with improved T-cell responses in blood of a triple-negative breast cancer model 4T1. Cancer Med. 2013;2(4):571–582. doi:10.1002/cam4.94
109. Sillo TO, Beggs AD, Morton DG, Middleton G. Mechanisms of immunogenicity in colorectal cancer. Br J Surg. 2019;106(10):1283–1297. doi:10.1002/bjs.11204
110. Keirsse J, Van DH, Geeraerts X, Beschin A, Raes G, Van GJA. The role of hepatic macrophages in liver metastasis. Cell Immunol. 2018;330:202–215. doi:10.1016/j.cellimm.2018.03.010
111. Tang H, Qiao J, Fu YX. Immunotherapy and tumor microenvironment. Cancer Lett. 2016;370(1):85–90. doi:10.1016/j.canlet.2015.10.009
112. Lu C, Rong D, Zhang B, et al. Current perspectives on the immunosuppressive tumor microenvironment in hepatocellular carcinoma: challenges and opportunities. Mol Cancer. 2019;18(1):130.
113. Reha J, Katz SC. Regional immunotherapy for liver and peritoneal metastases. J Surg Oncol. 2017;116(1):46–54. doi:10.1002/jso.24641
114. Fu Y, Liu S, Zeng S, Shen H. From bench to bed: the tumor immune microenvironment and current immunotherapeutic strategies for hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;38(1):396.
115. Ge P, Wang W, Li L, et al. Profiles of immune cell infiltration and immune-related genes in the tumor microenvironment of colorectal cancer. Biomed Pharmacother. 2019;118:109228. doi:10.1016/j.biopha.2019.109228
116. Van den Eynde M, Mlecnik B, Bindea G, et al. The link between the multiverse of immune microenvironments in metastases and the survival of colorectal cancer patients. Cancer Cell. 2018;34(6):1012–1026 e1013. doi:10.1016/j.ccell.2018.11.003
117. Zhou R, Zeng D, Zhang J, et al. A robust panel based on tumour microenvironment genes for prognostic prediction and tailoring therapies in stage I-III colon cancer. EBioMedicine. 2019;42:420–430. doi:10.1016/j.ebiom.2019.03.043
118. Hamada T, Nowak JA, Masugi Y, et al. Smoking and risk of colorectal cancer sub-classified by tumor-infiltrating T cells. J Natl Cancer Inst. 2019;111(1):42–51. doi:10.1093/jnci/djy137
119. Kurebayashi Y, Kubota N, Sakamoto M. Immune microenvironment of hepatocellular carcinoma, intrahepatic cholangiocarcinoma and liver metastasis of colorectal adenocarcinoma: relationship with histopathological and molecular classifications. Hepatol Res. 2020. doi:10.1111/hepr.13539
120. Khazali AS, Clark AM, Wells A. A pathway to personalizing therapy for metastases using liver-on-a-chip platforms. Stem Cell Rev Rep. 2017;13(3):364–380.
121. Jun HY, Lee YH, Juhng SK, Lee MS, Oh J, Yoon KH. Micro-CT measurements of tumoral vessels supplied by portal circulation in hepatic colorectal metastasis mouse model. Microsc Res Tech. 2014;77(6):415–421. doi:10.1002/jemt.22361
122. Pitt JM, Marabelle A, Eggermont A, Soria JC, Kroemer G, Zitvogel L. Targeting the tumor microenvironment: removing obstruction to anticancer immune responses and immunotherapy. Ann Oncol. 2016;27(8):1482–1492. doi:10.1093/annonc/mdw168
123. Ridge SM, Sullivan FJ, Glynn SA. Mesenchymal stem cells: key players in cancer progression. Mol Cancer. 2017;16(1):31. doi:10.1186/s12943-017-0597-8
124. Shiraha H, Iwamuro M, Okada H. Hepatic stellate cells in liver tumor. Adv Exp Med Biol. 2020;1234:43–56.
125. Lei H, Reinke P, Volk HD, Lv Y, Wu R. Mechanisms of immune tolerance in liver transplantation-crosstalk between alloreactive T cells and liver cells with therapeutic prospects. Front Immunol. 2019;10:2667. doi:10.3389/fimmu.2019.02667
126. Hilmi M, Neuzillet C, Calderaro J, Lafdil F, Pawlotsky JM, Rousseau B. Angiogenesis and immune checkpoint inhibitors as therapies for hepatocellular carcinoma: current knowledge and future research directions. J Immunother Cancer. 2019;7(1):333. doi:10.1186/s40425-019-0824-5
127. He Y, Dang Q, Li J, et al. Prediction of hepatocellular carcinoma prognosis based on expression of an immune-related gene set. Aging. 2020;12(1):965–977. doi:10.18632/aging.102669
128. Soydemir S, Comella O, Abdelmottaleb D, Pritchett J. Does mechanocrine signaling by liver sinusoidal endothelial cells offer new opportunities for the development of anti-fibrotics? Front Med. 2020;6:312. doi:10.3389/fmed.2019.00312
129. Wohlleber D, Knolle PA. The role of liver sinusoidal cells in local hepatic immune surveillance. Clin Transl Immunology. 2016;5(12):e117. doi:10.1038/cti.2016.74
130. Hato T, Goyal L, Greten TF, Duda DG, Zhu AX. Immune checkpoint blockade in hepatocellular carcinoma: current progress and future directions. Hepatology. 2014;60(5):1776–1782. doi:10.1002/hep.27246
131. Yagiz K, Rodriguez-Aguirre ME, Lopez EF, et al. A retroviral replicating vector encoding cytosine deaminase and 5-FC induces immune memory in metastatic colorectal cancer models. Mol Ther Oncolytics. 2018;8:14–26. doi:10.1016/j.omto.2017.12.001
132. Kimura Y, Inoue A, Hangai S, et al. The innate immune receptor Dectin-2 mediates the phagocytosis of cancer cells by Kupffer cells for the suppression of liver metastasis. Proc Natl Acad Sci U S A. 2016;113(49):14097–14102. doi:10.1073/pnas.1617903113
133. Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14(7):399–416.
134. Timmers M, Vekemans K, Vermijlen D, et al. Interactions between rat colon carcinoma cells and Kupffer cells during the onset of hepatic metastasis. Int J Cancer. 2004;112(5):793–802. doi:10.1002/ijc.20481
135. Itatani Y, Kawada K, Inamoto S, et al. The role of chemokines in promoting colorectal cancer invasion/metastasis. Int J Mol Sci. 2016;17(5):643. doi:10.3390/ijms17050643
136. Katz SC, Burga RA, McCormack E, et al. Phase I hepatic immunotherapy for metastases study of intra-arterial chimeric antigen receptor-modified T-cell therapy for CEA+ liver metastases. Clin Cancer Res. 2015;21(14):3149–3159. doi:10.1158/1078-0432.CCR-14-1421
137. Al BMH, Kim NK. Current status and future perspectives on treatment of liver metastasis in colorectal cancer (review). Oncol Rep. 2017;37(5):2553–2564. doi:10.3892/or.2017.5531
138. Milette S, Sicklick JK, Lowy AM, Brodt P. Molecular pathways: targeting the microenvironment of liver metastases. Clin Cancer Res. 2017;23(21):6390–6399. doi:10.1158/1078-0432.CCR-15-1636
139. Medina-Echeverz J, Eggert T, Han M, Greten TF. Hepatic myeloid-derived suppressor cells in cancer. Cancer Immunol Immunother. 2015;64(8):931–940. doi:10.1007/s00262-015-1736-y
140. Zarour LR, Anand S, Billingsley KG, et al. Colorectal cancer liver metastasis: evolving paradigms and future directions. Cell Mol Gastroenterol Hepatol. 2017;3(2):163–173. doi:10.1016/j.jcmgh.2017.01.006
141. Ciombor KK, Bekaii-Saab T. A comprehensive review of sequencing and combination strategies of targeted agents in metastatic colorectal cancer. Oncologist. 2018;23(1):25–34. doi:10.1634/theoncologist.2017-0203
142. Martini G, Troiani T, Cardone C, et al. Present and future of metastatic colorectal cancer treatment: a review of new candidate targets. World J Gastroenterol. 2017;23(26):4675–4688. doi:10.3748/wjg.v23.i26.4675
143. Vogel A, Hofheinz RD, Kubicka S, Arnold D. Treatment decisions in metastatic colorectal cancer - beyond first and second line combination therapies. Cancer Treat Rev. 2017;59:54–60. doi:10.1016/j.ctrv.2017.04.007
144. Ganesh K, Stadler ZK, Cercek A, et al. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol. 2019;16(6):361–375. doi:10.1038/s41575-019-0126-x
145. Golshani G, Zhang Y. Advances in immunotherapy for colorectal cancer: a review. Therap Adv Gastroenterol. 2020;13:1756284820917527. doi:10.1177/1756284820917527
146. Lee JJ, Chu E. Recent advances in the clinical development of immune checkpoint blockade therapy for mismatch repair proficient (pMMR)/non-MSI-H metastatic colorectal cancer. Clin Colorectal Cancer. 2018;17(4):258–273. doi:10.1016/j.clcc.2018.06.004
147. Overman MJ, Lonardi S, Wong KYM, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol. 2018;36(8):773–779. doi:10.1200/JCO.2017.76.9901
148. Guo J, Yu Z, Das M, Huang L. Nano codelivery of oxaliplatin and folinic acid achieves synergistic chemo-immunotherapy with 5-fluorouracil for colorectal cancer and liver metastasis. ACS Nano. 2020;14(4):5075–5089. doi:10.1021/acsnano.0c01676
149. Winer A, Ghatalia P, Bubes N, et al. Dual checkpoint inhibition with ipilimumab plus nivolumab after progression on sequential PD-1/PDL-1 inhibitors pembrolizumab and atezolizumab in a patient with lynch syndrome, metastatic colon, and localized urothelial cancer. Oncologist. 2019;24(11):1416–1419. doi:10.1634/theoncologist.2018-0686
150. Osumi H, Shinozaki E, Yamaguchi K, Zembutsu H. Clinical utility of circulating tumor DNA for colorectal cancer. Cancer Sci. 2019;110(4):1148–1155. doi:10.1111/cas.13972
151. Morse MA, Hochster H, Benson A. Perspectives on treatment of metastatic colorectal cancer with immune checkpoint inhibitor therapy. Oncologist. 2020;25(1):33–45. doi:10.1634/theoncologist.2019-0176
152. Lu YC, Jia L, Zheng Z, Tran E, Robbins PF, Rosenberg SA. Single-cell transcriptome analysis reveals gene signatures associated with T-cell persistence following adoptive cell therapy. Cancer Immunol Res. 2019;7(11):1824–1836. doi:10.1158/2326-6066.CIR-19-0299
153. Xiao L, Cen D, Gan H, et al. Adoptive transfer of NKG2D CAR mRNA-engineered natural killer cells in colorectal cancer patients. Mol Ther. 2019;27(6):1114–1125. doi:10.1016/j.ymthe.2019.03.011
154. Fan J, Shang D, Han B, Song J, Chen H, Yang JM. Adoptive cell transfer: is it a promising immunotherapy for colorectal cancer? Theranostics. 2018;8(20):5784–5800. doi:10.7150/thno.29035
155. Kitsou M, Ayiomamitis GD, Zaravinos A. High expression of immune checkpoints is associated with the TIL load, mutation rate and patient survival in colorectal cancer. Int J Oncol. 2020;57(1):237–248. doi:10.3892/ijo.2020.5062
156. Jiang S, Good D, Wei MQ. Vaccinations for colorectal cancer: progress, strategies, and novel adjuvants. Int J Mol Sci. 2019;20(14):3403. doi:10.3390/ijms20143403
157. Coupez D, Hulo P, Touchefeu Y, Bossard C, Bennouna J. Pembrolizumab for the treatment of colorectal cancer. Expert Opin Biol Ther. 2020;20(3):219–226. doi:10.1080/14712598.2020.1718095
158. Ciardiello D, Vitiello PP, Cardone C, et al. Immunotherapy of colorectal cancer: challenges for therapeutic efficacy. Cancer Treat Rev. 2019;76:22–32. doi:10.1016/j.ctrv.2019.04.003
159. Lichtenstern CR, Ngu RK, Shalapour S, Karin M. Immunotherapy, inflammation and colorectal cancer. Cells. 2020;9(3):618. doi:10.3390/cells9030618
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
