Back to Journals » Infection and Drug Resistance » Volume 14
Emerging Carbapenem-Resistant Enterobacteriaceae Infection, Its Epidemiology and Novel Treatment Options: A Review
Authors Tilahun M , kassa Y, Gedefie A , Ashagire M
Received 11 September 2021
Accepted for publication 13 October 2021
Published 21 October 2021 Volume 2021:14 Pages 4363—4374
DOI https://doi.org/10.2147/IDR.S337611
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Mihret Tilahun, Yeshimebet kassa, Alemu Gedefie, Melaku Ashagire
Department of Medical Laboratory Sciences, College of Medicine and Health Science, Wollo University, Dessie, Ethiopia
Correspondence: Mihret Tilahun
Department of Medical Laboratory Science, College of Medicine and Health Sciences, Wollo University, PO.BOX: 1145, Dessie, Ethiopia
Tel +251 920988307
Fax +251 333115250
Email [email protected]
Abstract: Infections due to multidrug-resistant Enterobacteriaceae have become major international public health problem due to the inadequate treatment options and the historically lagged pace of development of novel antimicrobial drugs. Inappropriate antimicrobial use in humans and animals coupled with increased global connectivity aided to the transmission of drug-resistant Enterobacteriaceae infections. Carbapenems are the medications of choice for extended-spectrum beta-lactamase and AmpC producers, but alternatives are currently needed because carbapenem resistance is increasing globally. This review pointed to discuss emerging drug-resistant Enterobacteriaceae, its epidemiology and novel treatment options for infections, which date back from 2010 to 2019 by searching Google Scholar, PubMed, PMC, Hinari and other different websites. The occurrence of carbapenem-resistant Enterobacteriaceae is reported worldwide with great regional variability. The rise of carbapenem-resistant Enterobacteriaceae poses a threat to all nations. Enzyme synthesis, efflux pumps, and porin mutations are the main methods by which Enterobacteriaceae acquire resistance to carbapenems. The major resistance mechanism among these is enzyme synthesis. Most carbapenem resistance is caused by three enzyme groups: Klebsiella pneumoniae carbapenemase (Ambler class A), metallo-ß-lactamases (Ambler class B), and oxacillinase-48 (Ambler class D). Ceftazidime–avibactam, which was newly licensed for carbapenemase producers, is the most common treatment option for infections. Meropenem–vaborbactam, imipenem–relebactam, plazomicin, cefiderocol, eravacycline, and aztreonam–avibactam are recently reported to be active against carbapenem-resistant Enterobacteriaceae; and are also in ongoing trials for different populations and combinations with other antibacterial agents. Overall, treatment must be tailored to the patient’s susceptibility profile, type and degree of infection, and personal characteristics.
Keywords: multidrug resistance, antimicrobial therapy, carbapenemase, Enterobacteriaceae
Introduction
The causative agent of an infectious disease whose incidence is increasing following its appearance in a new host population or whose incidence is increasing in an existing population as a result of long-term changes in its underlying epidemiology or the development of antimicrobial resistance has been defined as an emerging antibiotic-resistant pathogen.1 Carbapenem-resistant Enterobacteriaceae (CRE) infections result in longer hospital admissions, higher healthcare costs, and increased mortality than carbapenem-susceptible bacterial infections.2
The Enterobacteriaceae family includes many bacteria that are commonly isolated from clinical cultures, including Escherichia coli, Klebsiella spp., and Enterobacter spp. Because Enterobacteriaceae are a common cause of both community and healthcare-related diseases, they are particularly important in the context of antibiotic resistance.3 The Enterobacteriaceae family has developed through time and has an innate ability to proliferate by developing resistance to antimicrobial chemicals provided, rendering existing antibacterial ineffective, and emerging as resistant to carbapenem, a last-line antibiotic.4,5
In the human and animal colons, Enterobacteriaceae is mostly found as part of the normal flora. According to the World Health Organization’s antimicrobial resistance report, Enterobacteriaceae resistant to carbapenem are classified as a critical group and developing drug-resistant infections.6,7 According Centers for Disease Control (CDC) description of the antimicrobial-resistant pathogens, CRE such as Klebsiella species, Escherichia coli (E.coli) and Enterobacter species are the most crucial emerging resistance threats in the global.8
Bacterial infections caused by CRE, which are resistant to all classes of current antimicrobial drugs, have become a serious endpoint in the fight against bacterial infections in public health.9–11 Furthermore, the rise of carbapenem resistance and rapid dissemination of the Enterobacteriaceae family are referred to as “superbugs bacteria”.12,13
Because of the emergence of the CRE family, therapeutic approaches are no longer effective, and infections are more difficult to cure with the commonly used medications. As a result, infections would spread, longer hospital admissions would increase economic and social expenses, and death would rise.14 Carbapenem-resistant bacteria pneumonia, urinary tract infections, septicemia, endocarditis, meningitis, and severe intra-abdominal infections are only a few of the illnesses caused by Enterobacteriaceae.15 Due to the synthesis of extended spectrum beta-lactamase (ESBL), Enterobacteriaceae have developed resistance to third-generation cephalosporins, making therapy very challenging.16 The introduction and spread of bacteria resistant to carbapenems, on the other hand, puts their effectiveness in threat.17,18 To combat this threat, the medical community turned to broader spectrum agents and carbapenems, which in turn contributed to resistance due to selection and increased the emergence of increasingly resistant organisms.19
Carbapenem resistance in Enterobacteriaceae is mostly expressed by the synthesis of carbapenemase enzymes, which are encoded by numerous genotypes and can be transferred among Enterobacteriaceae via transferable genetic elements. Commonly pronounced enzymes include Class A Klebsiella pneumoniae carbapenemase, Class B metallo-β- lactamases, and Class D OXA β-lactamases.20 Resistance can also be developed through efflux pumps, permeability changes caused by the loss of outer membrane porin, or target mutations.21 Resistance is enhancement to be a continuous evolutionary process in the Enterobacteriaceae family. Mutation is a type of genetic change that occurs frequently in nature. Mutations in genetic structures can affect a cell’s ability to grow and survive in the presence of environmental stresses like antimicrobials.22
However, novel medications for treating CRE infection have just been accessible, and several more are in the works. While traditional drugs such as fosfomycin, tigecycline, minocycline, polymyxin and other therapeutic alternatives are still being used as therapy for CRE infections, recently emerged drugs including meropenem–vaborbactam, imipenem–relebactam, plazomicin, cefiderocol, eravacycline, and aztreonam–avibactam are more potent against CRE infections with fewer side effects and toxicities. Moreover, copper oxide nanoparticles, phage therapy and varieties of candidate vaccines have also influenced the development of Shigellosis infection.23
This review aims to provide an update on the rising carbapenem-resistant Enterobacteriaceae, its epidemiology as well as novel treatment options against these increasingly resistant bacteria.24
Literature Review
Natural Habitats and Reservoirs of Antibiotic-Resistant Enterobacteriaceae
Carbapenem-resistant Enterobacteriaceae (CRE) infections can arise naturally from bacteria found in a healthy person’s intestinal tract, as well as soil, water, sewage, plants (fruit, vegetables, and herbs), dairy products, and raw meat, depending on the species.25 Colonized/infected patients, biofilms from medical equipment, sinks/hand washing basins, faucets, drains, sink traps, toilets with a rim, sewage/drainage system, and contaminated healthcare personnel’s stethoscopes are the main reservoirs or sources of infection in healthcare institutions.26 Shower heads, bars of soap, liquid soap, artificial fingernails, pools/hot tubs/water fountains, dialysis tubing, infusion pumps, breathing equipment, and cleaning mops all contribute to a damp humid environment.25
Drug-resistant Enterobacteriaceae and/or resistance genes can spread in a variety of ways, depending on the microbe’s robustness in the environment, virulence, and infectious dosage, among other factors. Contact with colonized patients, air, water, food, beverages, and contaminated equipment can all spread them. Furthermore, increased mobility, environmental changes, overcrowding, poor cleanliness, and poor infection management are all contributing to an increase in the rate of transmission.27
Risk Groups for Acquiring Carbapenem-Resistant Enterobacteriaceae
Prior and recent antibiotic use, residency in long-term acute care facilities, admission to an intensive care unit, presence of indwelling medical devices, poor functional status, increased age, solid organ or stem cell transplant, and receipt of health care in or travel to endemic areas are all risk factors for acquiring MDR Enterobacteriaceae.28 Patients who need ventilators (breathing machines), urinary (bladder) catheters, or intravenous (vein) catheters, as well as those on extended courses of particular medicines and those with weaker immune systems, are at risk for CRE infections.29
Emerging Carbapenem-Resistant Enterobacteriaceae and Mechanism of Resistance
Global antimicrobial resistance reports show the rising frequency of resistance in species belonging to the Enterobacteriaceae family in various sources including health facilities and the community. The emergence and dissemination of CRE is the most recent and alarming report globally.30 Carbapenem-resistant bacteria in the Enterobacteriaceae family have spread widely across numerous fronts. On a biological level, resistance genes are frequently carried on plasmids, which are easily transmitted among Enterobacteriaceae. This resistant gene exchange can take place both within a host and in the environment.31,32
Antimicrobial resistance can also be caused by the production of antibiotic-inactivating enzymes and non-enzymatic mechanisms, which could be intrinsic or acquired.33 Increased production of intrinsic resistance mechanisms (either antibiotic-inactivating enzymes or efflux pumps) due to chromosomal gene mutations, permeability changes due to loss of outer membrane porin, or target modifications.21 Horizontal transfers of mobile genetic elements such as plasmids carrying resistance genes producing beta-lactamase enzymes, aminoglycoside-modifying enzymes (AMEs), or non-enzymatic mechanisms such fluoroquinolone resistance in Enterobacteriaceae are among the common mechanisms. Because these plasmids frequently include numerous resistance determinants, a single plasmid conjugation may be enough to transmit multidrug resistance to the recipient strain.34
Carbapenem resistance in Enterobacteriaceae can be caused by a variety of methods, including the synthesis of Carbapenemase enzymes, extended spectrum beta-lactamases (ESBLs), AmpC enzymes (mainly plasmid-mediated), and porin loss, which reduces drug permeability.35
Enterobacteriaceae can develop resistance to one or more antibiotic classes that are normally effective against them. Klebsiella pneumoniae carbapenemase (KPC), imipenem’s metallo-beta-lactamase (IMP), New Delhi metallo-beta-lactamase (NDM), Verona integron-encoded metallo-beta-lactamase (VIM), and oxacillin carbapenemase (OXA) are among the most important acquired resistances.36
Acquisition and Spread of Carbapenem Resistance Among Enterobacteriaceae
Resistance genes can also be obtained from bacteria that are not resistant to antibiotics. They can be passed between bacteria of the same species as well as bacteria of different species or genera.21 Transduction, transformation, and conjugation are examples of horizontal gene transfer mechanisms. Plasmids (resistance plasmid 1 is a common example in Enterobacteriaceae), transposons, and other vectors can carry one or more resistance genes (eg, Tn5053).37 Resistance determinants for other antimicrobial classes, such as aminoglycosides and fluoroquinolones, may be carried by ESBLs and carbapenemase expressing plasmids.21
For ESBLs genes in Enterobacteriaceae families, the possibility of plasmid-mediated horizontal resistance gene transmission between livestock and humans (eg, via the food chain) has been observed.38–40 Furthermore, excessive use of antibiotic agents, usage without prescriptions, and use of antibiotics in both the health care system and livestock production may encourage the diffusion of resistance genes, which has a direct effect on the expansion of antibiotic resistance.41,42
Epidemiology
Multidrug-resistant Enterobacteriaceae (MDR-E) is thought to have initially arisen in the 1980s, shortly after the widespread use of cephalosporins and other broad-spectrum ß-lactam antibiotics. SHV and TEM were among the first ESBLs found, followed by CTX-M, which have now expanded to become the most common plasmid-mediated ß-lactamases worldwide. Different carbapenemase-producing Enterobacteriaceae are becoming more prevalent in different parts of the world, according to epidemiological data. Carbapenem-resistant strains were initially discovered in the 1980s and rapidly spread over the world.43
New Delhi metallo-ß-lactamase-1 is the most common carbapenemase generating resistance in India, Pakistan, and Sri Lanka. KPC-producing Enterobacteriaceae, on the other hand, are found in the United States, Colombia, Argentina, Greece, and Italy, and OXA-48-like enzyme-producers are found in Turkey, Malta, the Middle East, and North Africa.44 Another study from 2011 to 2014 in the United States found 10% carbapenem resistance in Klebsiella pneumoniae and 16–36% third-generation cephalosporin (3rd GC) resistance in Escherichia coli.45 Another study using clinical isolates in Europe found that K. pneumonia is resistant to third GC in 31% of the cases, while E. coli is resistant to carbapenem in 8% of the cases and third GC in 12% of the cases.46
Before 2001, the Greek System for Antibiotic Resistant Research showed a carbapenem resistance rate of 1%; by 2008, this had risen to 30% in hospital wards and 60% in intensive care units. According to data from the European Centre for Disease Prevention and Control’s EARS-Net, 678 (62.3%) of 1088 Greek K. pneumoniae isolates were carbapenem-resistant in 2014.47 In the state of Israel, in 2008 and 2013, two cross-sectional nationwide surveys of CP Enterobacteriaceae (CPE) in Israeli post-acute-care hospitals revealed a considerable drop in the overall incidence of carbapenem resistance among Enterobacteriaceae isolates (184 of 1147 isolates (16%)). KPC-carrying K. pneumoniae, on the other hand, remained the most common CPE, with a growing proportion of ST258 K. pneumoniae strains (120 of 184 (65%) in 2008 versus 91 of 113 (80%) in 2013).48
The Meropenem Annual Drug Susceptibility Data Gathering surveillance system mentioned 57 isolates in 2006, with 9.5% of the gathering characterized as clonal blaKPC-producing Klebsiella strains, representing a twofold increase from the previous year; most isolates were from states in the Mid-Atlantic US Census division, and hospital prevalence rates ranged from 2.4% in Ohio to 9.5% in Pennsylvania; most isolates were from states in the Mid-Atlantic.49,50 The spread of KPC-producing bacteria across the United States is clear; according to the same surveillance program, 28 of 195 Enterobacteriaceae isolates (14.4%) tested from 26 US medical centers had blaKPC-2 or blaKPC-3 by 2010, with 9 of the 28 discovered in Texas.51 In Egypt and other African countries, carbapenem resistance is common and spreading, with more than half of hospitals (64%) having at least one CRE isolate and half (47.9%) of Enterobacteriaceae isolates being CRE, which is greater than estimates from other Arab, African, and Asian countries.52
In general, all countries are at risk of being victims to the emergence of carbapenem-resistant Enterobacteriaceae infection with growing prevalence has been reported worldwide.74
Prevention and Control
Infections can be controlled by using a multidimensional, coordinated strategy and following infection prevention principles. Specifically, by reducing CRE transmission from person to person.75 Essentially, avoiding unnecessary or misuse of invasive equipment such as indwelling urinary catheters or IV lines, medical staff hand washing, greater barrier measures, and isolation of patients colonized or infected with carbapenemase producers. Infection management strategies are primarily focused on stopping the spread of such organisms from patient to patient. Apart from that, the main issues are healthcare professionals’ hand hygiene, the use and cleaning of medical equipment, and environmental colonization; early implementation of active surveillance through rectal screening for CRE carriage on hospital admission, environmental cleaning, staff education, case notification/flagging, and contact tracing are all necessary to prevent spreading and outbreaks of carbapenemase-producing bacteria spread and outbreaks.6
Generally, educating health care workers, using infection control and antimicrobial stewardship, and active surveillance for halting the chain of transmission should all be part of a proactive approach to tackling antimicrobial resistance at a regional, national, and international level.53
Principal Treatment Options
For infections caused by ESBL and AmpC producers, carbapenems are the preferred treatment options. CRE, on the other hand, are resistant to practically all beta-lactams, with a few exceptions, and older antibiotic groups that have acceptable action. As a result, CRE therapy options are more limited, with older treatments such as aminoglycosides, polymyxins, a glycylcycline, and Fosfomycin being particularly effective.76 Although polymyxins are effective against CRE, they have nephrotoxicity and neurotoxicity as adverse effects, as well as poor effectiveness against Proteus, Providencia, Morganella, and Serratia infections.77
Because tigecycline has a higher binding affinity with ribosomal sites than tetracycline, it has been shown to be an effective therapeutic against Enterobacteriaceae that are resistant to tetracycline.78 Tigecycline is effective against practically all ESBLs and multidrug-resistant (MDR) E. coli isolates, as well as the vast majority of ESBL and MDR Klebsiella isolates. However, their activity against Proteus, Providencia, and Morganella is limited.78 Nevertheless, due to its low penetration and quick tissue diffusion after being intravenously administered, tigecycline has unsatisfactory clinical effects in both urinary tract and main blood infections, making it ineffective.79
Fosfomycin is an antibacterial drug that works against CRE by inhibiting the enzyme UDP-N-acetylglucosamine enol pyruvyl transferase (MurA), which catalyzes one of the first steps in bacterial cell wall production. However, it has limited use to treatment for lower urinary tract infections. In vitro and retrospective investigations suggest that, at least for KPC infections, combined therapy with a carbapenem (eg, polymyxin–carbapenem or aminoglycoside–carbapenem) is more effective.80
In the case of OXA-48 and NDM infections, a recent retrospective observational analysis concluded that combined therapy with colistin is the best treatment option. Because existing antimicrobial medicines have limitations, new and effective antimicrobial drugs are required for CRE infections.81 Except for the monobactam aztreonam, aztreonam/avibactam MBLs can hydrolyze any beta-lactams (ATM). However, due to the fact that MBL-producing Enterobacteriaceae frequently produce serine-lactamases, which can hydrolyze aztreonam, aztreonam is only effective against about 30% of these isolates. As a result, combining ATM with a -lactam/-lactamase inhibitor like ceftazidime–avibactam (CAZ-AVI) has proven to be an appealing combination with synergistic in vitro efficacy, especially against infections that produce both metallo- and serine-lactamases.82 Cefiderocol is a new siderophore cephalosporin that enters the bacterial cell via iron transporters, avoiding resistance induced by porin channel mutations and efflux pump overproduction. Cefiderocol also has other chemical structural characteristics that offer greater efficacy against Gram-negative bacteria that are difficult to treat, as well as stability against hydrolysis by different beta-lactamases in vitro, including MBL.83
Scientists are concentrating on developing new antibacterial medications to address these pressing challenges b-lactamase inhibitor (NB-BLI) which is avibactam combination with ceftazidime. Ceftazidime–avibactam (CAZ-AVI) is an intravenous cephalosporin/beta-lactamase, which is currently available on the market, and it is also being tested in conjunction with aztreonam and ceftaroline.84
Contrasting to other β-lactamase inhibitors, avibactam is not a β-lactam. Rather, its diazabicyclooctane structure mimics a β-lactam and is capable of hindering β-lactamases belonging to Strider classes A and C, and some class D enzymes.85 Despite not possessing antibacterial activity on its own, avibactam broadens the spectrum of ceftazidime’s activity. In isolates of E. coli, Enterobacter spp., and Klebsiella spp. that are carbapenem-resistant or express extended-spectrum B-lactamases, AmpC, OXA-48, KPC, and other resistance mechanisms, this combination enhances sensitivity to ceftazidime by 16- to 1024-fold.85,86
Despite the fact that combination therapy is now the gold standard for treating CRE infections, resistance, treatment failure, and toxicity have prompted the development of new therapeutic approaches.87 Unfortunately, several drugs with anti-CRE activity are in late-stage research. Among these under pipelines agents are plazomicin, relebactam + imipenem, eravacycline, zidebactam and meropenem + novel β-lactamase inhibitor. Each drug is detailed in Table 2.
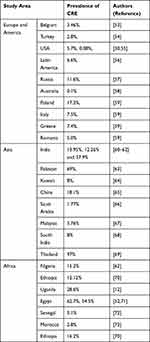 |
Table 1 Summary of the Global Prevalence of Carbapenem-Resistant Enterobacteriaceae |
 |
Table 2 List of Antibiotics Used for CRE Infection Treatment and Under Pipeline Development for Future Treatment of CRE Infections |
Overall, therapy must be tailored to the patient’s susceptibility profile, type and degree of infection, and personal characteristics. Beyond those unique treatment options, nano-strategies (using nanoparticles) to combat multidrug-resistant bacteria, such as carbapenem-resistant Enterobacteriaceae, and other strategies, such as phage therapy and vaccine strategies, are being developed to overcome these types of resistance.96–98
Ceftazidime–avibactam (CAZ-AVI), ceftolozane–tazobactam (TOL-TAZ), meropenem–vaborbactam (MER-VAB) and imipenem/cilastatin–relebactam (IMI-REL) are recently reported to be active against Enterobacteriaceae, including ESBL-producing, AmpC-producing and carbapenemase producing isolates; and are also in ongoing trials for different populations and combinations with other antibacterial. Moreover, β-lactam–sulfone β-lactamase inhibitor combinations including cefepime–tazobactam and cefepime–enmetazobactam (AAI101), β-lactam–diazabicyclooctane β-lactamase inhibitor combinations including cefepime–zidebactam (WCK 5107), aztreonam–avibactam, sulbactam–durlobactam (ETX2514), meropenem–nacubactam (FPI-1465) and cefpodoxime proxetil-ETX0282; and β-lactam–boronate β-Lactamase inhibitor combinations such as cefepime–taniborbactam (VNRX-5133) and QPX7728 are in development processes and under clinical trials, which could be promising future treatment options against ESBL and carbapenemase-producing bacteria.99
Possibly in the future many clinical trials and investigations have looked into the efficacy and resistance patterns of CAZ/AVI. Interestingly, the International Network for Optimal Resistance Monitoring (INFORM) studied more than 30,000 strains of Enterobacteriaceae obtained from patients with diverse bacterial illnesses and found that these microbes were more susceptible to the CAZ/AVI combination (99.5%).100
Ceftazidime–avibactam is the primary treatment for CRE infections that produce OXA-48-like proteins. Ceftazidime–avibactam, meropenem–vaborbactam, and imipenem–cilastatin–relebactam have action against Enterobacteriaceae that produce KPC enzymes, which are the most frequent carbapenemase in the US.101
Nanoparticles
Because of their bactericidal properties, nanoparticles (NPs) have increased in popularity in recent years and have demonstrated broad-spectrum antibacterial efficacy against pathogenic microorganisms.96 Nanoparticles typically kill bacteria, causing damage to membrane load cells and their integrity, as well as the production of free oxygen radicals. They can usually be given effectively as antibacterial agents. The bactericidal action of graphene oxide/Cu/Ag NPs against E. coli and K. pneumonia has recently been discovered, possibly because to a synergy between several harmful pathways.102 Copper oxide nanoparticles have also been identified as a potential antibacterial agent for the treatment of Shigella spp.103
Phage Therapy
Phage therapy is increasing in popularity due to a number of benefits, including high specificity to target bacteria without affecting the human body’s normal microflora, replication at the infection site, bactericidal activity against antibiotic-resistant bacteria, and fewer side effects than other therapies. Because phages are self-limiting, they can linger on target sites at a low level after killing their bacterial targets.97 The use of phages as an alternative to antibiotics for treating MDR S. dysenteries isolated from wastewater has been studied104 despite the fact that there have been no reports of serious side effects during the long history of phage therapy in humans.
Vaccine Strategies
To prevent infection by Shigella spp., a number of candidate vaccines have been developed, the majority of which are still being tested for safety and immunogenicity. Vaccination provides protection in humans and animals, according to current studies.98 Glycoconjugate vaccines, such as recombinant glycoconjugate, synthetic glycoconjugate, and O-polysaccharide covalently coupled to immunogenic carrier proteins, are potential candidates for Shigella vaccinations,105 vir G-based live attenuated (WRSS1, WRSs3, WRSf3, WRSf2G12, WRSf2G15 and WRSd1) recombinant outer-membrane proteins, live attenuated vaccines,106 invasion-plasmid antigens B, C, and D.107 In addition to this, DNA-based vaccines, Ty21a typhoid vaccine expressing Shigella LPS,108 and whole-cell-killed and Shigella trivalent inactivated whole-cell and heat-killed multi serotype Shigella109 as well as novel antigen candidates, such as triacylated S-LPS, GMMA protein particles currently have been developed.98 However, there is no licensed vaccine available against this pathogen.
Conclusion and Recommendations
Finally, it is clear that carbapenem resistance in Enterobacteriaceae has developed substantially, posing a serious threat to global health. CRE have once again become a concern to civilization, as evidenced by a steady stream of publications describing resistant strains and sometimes novel resistance mechanisms. Dissemination and acquisition can go undetected, posing serious problems for infection control. Because the Enterobacteriaceae are a typical part of the gut microbiota, people might be colonized asymptomatically and unintentionally serve as a reservoir for spreading the bacteria to others; a subset of people will get illnesses as a result of these bacteria. Drug resistance mechanisms are varied and adaptable in these bacteria, making control and early identification of CRE infections difficult. As a result, a holistic approach must be adopted, which includes continued health-care professional education, infection control, interrupting the infection chain, antimicrobial stewardship in both humans and animals, and increased regional and international collaboration to slow the emergence of resistances. Existing antibiotics such as Fosfomycin, aminoglycosides, and colistin are currently used as therapeutic options for CRE infections, while Avibactam combined with carbapenem-containing regimens is a newly developed antibiotic. Plazomicin, eravacycline, cefiderocol, zidebactam, and nacubactam are among the novel agents in development as new agents for various Enterobacteriaceae.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report that they have no conflicts of interest.
References
1. Petrosillo N. Infections: the emergency of the new millennium. In: Nuclear Medicine in Infectious Diseases. Springer; 2020:1–8.
2. Lutgring JD. Carbapenem-resistant Enterobacteriaceae: an emerging bacterial threat. In: Seminars in Diagnostic Pathology. Elsevier; 2019.
3. Van Duin D, Paterson DL. Multidrug-resistant bacteria in the community: trends and lessons learned. Infect Dis Clin. 2016;30(2):377–390.
4. Spera AM, Esposito S, Pagliano P. Emerging antibiotic resistance: carbapenemase-producing enterobacteria. Bad new bugs, still no new drugs. Le Infezioni Medicina. 2019;27(4):357–364.
5. O’Malley PA. A most dangerous outbreak: New Delhi metallo-β-lactamase-1 carbapenemase-producing Enterobacteriaceae. Clin Nurse Special. 2020;34(1):13–16. doi:10.1097/NUR.0000000000000497
6. World Health Organization. WHO Priority Pathogens List for R&D of New Antibiotics. Geneva, Switzerland: World Health Organization; 2017.
7. Gordon MA, Feasey NA, Nyirenda TS, Graham SM. Nontyphoid salmonella disease. In: Hunter’s Tropical Medicine and Emerging Infectious Diseases. Elsevier; 2020:500–506.
8. Centres for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2019. US Department of Health and Human Services, Centres for Disease Control and Prevention; 2019.
9. Magiorakos AP, Srinivasan A, Carey R, et al. Multidrug‐resistant, extensively drug‐resistant and pandrug‐resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi:10.1111/j.1469-0691.2011.03570.x
10. Goic-Barisic I, Seruga Music M, Kovacic A, Tonkic M, Hrenovic J. Pan drug-resistant environmental isolate of Acinetobacter baumannii from Croatia. Microb Drug Resist. 2017;23(4):494–496. doi:10.1089/mdr.2016.0229
11. Li L, Yu T, Ma Y, et al. The genetic structures of an Extensively Drug Resistant (XDR) Klebsiella pneumoniae and its plasmids. Front Cell Infect Microbiol. 2018;8:446. doi:10.3389/fcimb.2018.00446
12. Okoche D, Asiimwe BB, Katabazi FA, Kato L, Najjuka CF. Prevalence and characterization of carbapenem-resistant Enterobacteriaceae isolated from Mulago National Referral Hospital, Uganda. PLoS One. 2015;10(8):e0135745. doi:10.1371/journal.pone.0135745
13. Boutal H, Vogel A, Bernabeu S, et al. A multiplex lateral flow immunoassay for the rapid identification of NDM-, KPC-, IMP-and VIM-type and OXA-48-like carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother. 2018;73(4):909–915. doi:10.1093/jac/dkx521
14. Lee C-M, Lai -C-C, Chiang H-T, et al. Presence of multidrug-resistant organisms in the residents and environments of long-term care facilities in Taiwan. J Microbiol Immunol Infect. 2017;50(2):133–144. doi:10.1016/j.jmii.2016.12.001
15. Rodríguez-Baño J, Gutiérrez-Gutiérrez B, Machuca I, Pascual A. Treatment of infections caused by extended-spectrum-beta-lactamase-, AmpC-, and carbapenemase-producing Enterobacteriaceae. Clin Microbiol Rev. 2018;31(2):e00079–17. doi:10.1128/CMR.00079-17
16. Pana ZD, Zaoutis T. Treatment of extended-spectrum β-lactamase-producing Enterobacteriaceae (ESBLs) infections: what have we learned until now? F1000Research. 2018;7:1347. doi:10.12688/f1000research.14822.1
17. Kohlenberg A, Weitzel-Kage D, Van der Linden P, et al. Outbreak of carbapenem-resistant Pseudomonas aeruginosa infection in a surgical intensive care unit. J Hosp Infect. 2010;74(4):350–357. doi:10.1016/j.jhin.2009.10.024
18. Cezário RC, De Morais LD, Ferreira JC, Costa-Pinto RM, da Costa Darini AL, Gontijo-Filho PP. Nosocomial outbreak by imipenem-resistant metallo-β-lactamase-producing Pseudomonas aeruginosa in an adult intensive care unit in a Brazilian teaching hospital. Enferm Infecc Microbiol Clin. 2009;27(5):269–274. doi:10.1016/j.eimc.2008.09.009
19. D’Angelo RG, Johnson JK, Bork JT, Heil EL. Treatment options for extended-spectrum beta-lactamase (ESBL) and AmpC-producing bacteria. Expert Opin Pharmacother. 2016;17(7):953–967. doi:10.1517/14656566.2016.1154538
20. Diene SM, Rolain J-M. Carbapenemase genes and genetic platforms in Gram-negative bacilli: Enterobacteriaceae, pseudomonas and Acinetobacter species. Clin Microbiol Infect. 2014;20(9):831–838. doi:10.1111/1469-0691.12655
21. Ruppé É, Woerther P-L, Barbier F. Mechanisms of antimicrobial resistance in Gram-negative bacilli. Ann Intensive Care. 2015;5(1):21. doi:10.1186/s13613-015-0061-0
22. Blanquart F, Lehtinen S, Lipsitch M, Fraser C. The evolution of antibiotic resistance in a structured host population. J Royal Soc Interface. 2018;15(143):20180040. doi:10.1098/rsif.2018.0040
23. Ranjbar R, Farahani A. Shigella: antibiotic-resistance mechanisms and new horizons for treatment. Infect Drug Resist. 2019;12:3137. doi:10.2147/IDR.S219755
24. Logan LK, Weinstein RA. The epidemiology of carbapenem-resistant Enterobacteriaceae: the impact and evolution of a global menace. J Infect Dis. 2017;215(suppl_1):S28–S36. doi:10.1093/infdis/jiw282
25. Jarvis KG, Daquigan N, White JR, et al. Microbiomes associated with foods from plant and animal sources. Front Microbiol. 2018;9:2540. doi:10.3389/fmicb.2018.02540
26. Vergara-López S, Domínguez M, Conejo M, Pascual Á, Rodríguez-Baño J. Wastewater drainage system as an occult reservoir in a protracted clonal outbreak due to metallo-β-lactamase-producing Klebsiella oxytoca. Clin Microbiol Infect. 2013;19(11):E490–E8. doi:10.1111/1469-0691.12288
27. Singh G. Hospital Infection Control Guidelines: Principles and Practice. Jaypee Brothers Publishers; 2012.
28. Tzouvelekis L, Markogiannakis A, Psichogiou M, Tassios P, Daikos G. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev. 2012;25(4):682–707. doi:10.1128/CMR.05035-11
29. Mehta Y, Gupta A, Todi S, et al. Guidelines for prevention of hospital acquired infections. Indian J Crit Care Med. 2014;18(3):149. doi:10.4103/0972-5229.128705
30. Logan LK, Medernach RL, Rispens JR, et al. Community origins and regional differences highlight risk of plasmid-mediated fluoroquinolone resistant Enterobacteriaceae infections in children. Pediatr Infect Dis J. 2019;38(6):595. doi:10.1097/INF.0000000000002205
31. Borgia S, Lastovetska O, Richardson D, et al. Outbreak of carbapenem-resistant Enterobacteriaceae containing bla NDM-1, Ontario, Canada. Clin Infect Dis. 2012;55(11):e109–e17. doi:10.1093/cid/cis737
32. Walsh TR, Weeks J, Livermore DM, Toleman MA. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: an environmental point prevalence study. Lancet Infect Dis. 2011;11(5):355–362. doi:10.1016/S1473-3099(11)70059-7
33. Blair JM, Webber MA, Baylay AJ, Ogbolu DO, Piddock LJ. Molecular mechanisms of antibiotic resistance. Nat Rev Microbiol. 2015;13(1):42–51. doi:10.1038/nrmicro3380
34. Miró E, Grünbaum F, Gómez L, et al. Characterization of aminoglycoside-modifying enzymes in Enterobacteriaceae clinical strains and characterization of the plasmids implicated in their diffusion. Microb Drug Resist. 2013;19(2):94–99. doi:10.1089/mdr.2012.0125
35. Codjoe FS, Donkor ES. Carbapenem resistance: a review. Med Sci. 2018;6(1):1. doi:10.3390/medsci6010001
36. Nordmann P, Poirel L. The difficult-to-control spread of carbapenemase producers among Enterobacteriaceae worldwide. Clin Microbiol Infect. 2014;20(9):821–830. doi:10.1111/1469-0691.12719
37. Siefert JL. Man and his spaceships: vehicles for extraterrestrial colonization? Mob Genet Elements. 2012;2(6):272–278. doi:10.4161/mge.23238
38. Liu -Y-Y, Wang Y, Walsh TR, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2016;16(2):161–168. doi:10.1016/S1473-3099(15)00424-7
39. Falgenhauer L, Waezsada S-E, Yao Y, et al. Colistin resistance gene mcr-1 in extended-spectrum β-lactamase-producing and carbapenemase-producing Gram-negative bacteria in Germany. Lancet Infect Dis. 2016;16(3):282–283. doi:10.1016/S1473-3099(16)00009-8
40. Smith H, Bossers A, Harders F, et al. Characterization of epidemic IncI1-Iγ plasmids harboring ambler class A and C genes in Escherichia coli and Salmonella enterica from animals and humans. Antimicrob Agents Chemother. 2015;59(9):5357–5365. doi:10.1128/AAC.05006-14
41. Exner M, Bhattacharya S, Christiansen B, et al. Antibiotic resistance: what is so special about multidrug-resistant Gram-negative bacteria? GMS Hygiene Infect Control. 2017;12. doi:10.3205/dgkh000290
42. McEwen SA, Collignon PJ. Antimicrobial resistance: a one health perspective. In: Antimicrobial Resistance in Bacteria from Livestock and Companion Animals. Wiley Online Libraray; 2018:521–547.
43. Vink JP, Otter JA, Edgeworth JD. Carbapenemase-producing Enterobacteriaceae–once positive always positive? Curr Opin Gastroenterol. 2020;36(1):9–16. doi:10.1097/MOG.0000000000000596
44. Bush K, Bradford PA. Epidemiology of β-lactamase-producing pathogens. Clin Microbiol Rev. 2020;33(2):e00047–19. doi:10.1128/CMR.00047-19
45. Weiner L, Webb A, Limbago B, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect Control Hosp Epidemiol. 2016;37(11):1288–1301. doi:10.1017/ice.2016.174
46. Weist K, Högberg LD. ECDC publishes 2015 surveillance data on antimicrobial resistance and antimicrobial consumption in Europe. Eurosurveillance. 2016;21(46):30401. doi:10.2807/1560-7917.ES.2016.21.46.30399
47. Souli M, Galani I, Antoniadou A, et al. An outbreak of infection due to β-lactamase Klebsiella pneumoniae carbapenemase 2–producing K. pneumoniae in a Greek University Hospital: molecular characterization, epidemiology, and outcomes. Clin Infect Dis. 2010;50(3):364–373. doi:10.1086/649865
48. Adler A, Hussein O, Ben-David D, et al. Persistence of Klebsiella pneumoniae ST258 as the predominant clone of carbapenemase-producing Enterobacteriaceae in post-acute-care hospitals in Israel, 2008–13. J Antimicrob Chemother. 2015;70(1):89–92. doi:10.1093/jac/dku333
49. Rhomberg PR, Deshpande LM, Kirby JT, Jones RN. Activity of meropenem as serine carbapenemases evolve in US medical centers: monitoring report from the MYSTIC program (2006). Diagn Microbiol Infect Dis. 2007;59(4):425–432. doi:10.1016/j.diagmicrobio.2007.05.009
50. Deshpande LM, Rhomberg PR, Sader HS, Jones RN. Emergence of serine carbapenemases (KPC and SME) among clinical strains of Enterobacteriaceae isolated in the United States Medical Centers: report from the MYSTIC Program (1999–2005). Diagn Microbiol Infect Dis. 2006;56(4):367–372. doi:10.1016/j.diagmicrobio.2006.07.004
51. Castanheira M, Farrell SE, Deshpande LM, Mendes RE, Jones RN. Prevalence of β-lactamase-encoding genes among Enterobacteriaceae bacteremia isolates collected in 26 US hospitals: report from the SENTRY Antimicrobial Surveillance Program (2010). Antimicrob Agents Chemother. 2013;57(7):3012–3020. doi:10.1128/AAC.02252-12
52. Kotb S, Lyman M, Ismail G, et al. Epidemiology of carbapenem-resistant Enterobacteriaceae in Egyptian intensive care units using National Healthcare–associated Infections Surveillance Data, 2011–2017. Antimicrob Resist Infect Control. 2020;9(1):1–9. doi:10.1186/s13756-019-0639-7
53. Huttner A, Harbarth S, Carlet J, et al. Antimicrobial resistance: a global view from the 2013 World Healthcare-Associated Infections Forum. Antimicrob Resist Infect Control. 2013;2(1):1–13. doi:10.1186/2047-2994-2-31
54. Baran I, Aksu N. Phenotypic and genotypic characteristics of carbapenem-resistant Enterobacteriaceae in a tertiary-level reference hospital in Turkey. Ann Clin Microbiol Antimicrob. 2016;15(1):1–11. doi:10.1186/s12941-016-0136-2
55. Mathers AJ, Cox HL, Kitchel B, et al. Molecular dissection of an outbreak of carbapenem-resistant Enterobacteriaceae reveals intergenus KPC carbapenemase transmission through a promiscuous plasmid. MBio. 2011;2(6):e00204–11. doi:10.1128/mBio.00204-11
56. Shortridge D, Castanheira M, Pfaller MA, Flamm RK. Ceftolozane-tazobactam activity against Pseudomonas aeruginosa clinical isolates from US hospitals: report from the PACTS Antimicrobial Surveillance Program, 2012 to 2015. Antimicrob Agents Chemother. 2017;61(7):e00465–17. doi:10.1128/AAC.00465-17
57. Partina I, Kalinogorskaya O, Kojima S, et al. Surveillance of antimicrobial susceptibility of Enterobacteriaceae pathogens isolated from intensive care units and surgical units in Russia. Jpn J Antibiot. 2016;69(1):41–51.
58. Bailey AL, Armstrong T, Dwivedi H-P, et al. Multicenter evaluation of the Etest gradient diffusion method for ceftolozane-tazobactam susceptibility testing of Enterobacteriaceae and Pseudomonas aeruginosa. J Clin Microbiol. 2018;56(9):e00717–18. doi:10.1128/JCM.00717-18
59. Sader HS, Castanheira M, Flamm RK, Mendes RE, Farrell DJ, Jones RN. Tigecycline activity tested against carbapenem-resistant Enterobacteriaceae from 18 European nations: results from the SENTRY surveillance program (2010–2013). Diagn Microbiol Infect Dis. 2015;83(2):183–186. doi:10.1016/j.diagmicrobio.2015.06.011
60. Chatterjee B, Khanduri N, Agarwal R. Comparative evaluation of rapid colorimetric methods for detecting carbapenemase enzymes in Gram-negative bacilli. Indian J Microbiol Res. 2017;4:263–266.
61. Nair PK, Vaz MS. Prevalence of carbapenem resistant Enterobacteriaceae from a tertiary care hospital in Mumbai, India. J Microbiol Infect Dis. 2013;3(04):207–210. doi:10.5799/ahinjs.02.2013.04.0110
62. Khare V, Gupta P, Haider F, Begum R. Study on MICs of tigecycline in clinical isolates of carbapenem resistant Enterobacteriaceae (CRE) at a tertiary care centre in North India. JCDR. 2017;11(3):DC18.
63. Amjad A, Mirza IA, Abbasi S, Farwa U, Malik N, Zia F. Modified Hodge test: a simple and effective test for detection of carbapenemase production. Iran J Microbiol. 2011;3(4):189.
64. Jamal WY, Albert MJ, Rotimi VO, Galdiero M. High prevalence of New Delhi metallo-β-lactamase-1 (NDM-1) producers among carbapenem-resistant Enterobacteriaceae in Kuwait. PLoS One. 2016;11(3):e0152638. doi:10.1371/journal.pone.0152638
65. Li Y, Sun Q-L, Shen Y, et al. Rapid increase in prevalence of carbapenem-resistant Enterobacteriaceae (CRE) and emergence of colistin resistance gene mcr-1 in CRE in a hospital in Henan, China. J Clin Microbiol. 2018;56(4):e01932–17. doi:10.1128/JCM.01932-17
66. Kandeel A. Epidemiology of carbapenemase producing Enterobacteriaceae in a general hospital. J Microbiol Infect Dis. 2015;5(2):57–62. doi:10.5799/ahinjs.02.2015.02.0177
67. Zaidah AR, Mohammad NI, Suraiya S, Harun A. High burden of Carbapenem-resistant Enterobacteriaceae (CRE) fecal carriage at a teaching hospital: cost-effectiveness of screening in low-resource setting. Antimicrob Resist Infect Control. 2017;6(1):1–6. doi:10.1186/s13756-017-0200-5
68. Almugadam B, Ali N, Ahmed A, Ahmed E, Wang L. Prevalence and antibiotics susceptibility patterns of carbapenem resistant Enterobacteriaceae. J Bacteriol Mycol Open Access. 2018;6(3):187–190.
69. Paveenkittiporn W, Lyman M, Biedron C, et al. Molecular epidemiology of carbapenem-resistant Enterobacterales in Thailand, 2016–2018. Antimicrob Resist Infect Control. 2021;10(1):1–8. doi:10.1186/s13756-021-00950-7
70. Legese MH, Weldearegay GM, Asrat D. Extended-spectrum beta-lactamase-and carbapenemase-producing Enterobacteriaceae among Ethiopian children. Infect Drug Resist. 2017;10:27. doi:10.2147/IDR.S127177
71. Khalil HS, Wahab MAAE. Risk factors, phenotypic and genotypic characterization of carbapenem resistant Enterobacteriaceae in Tanta University Hospitals, Egypt. Int J Infect Control. 2016;12(2). doi:10.3396/ijic.v12i2.15905
72. Camara M, Mane MT, Ba-Diallo A, et al. Extended-spectrum beta-lactamase-and carbapenemase-producing Enterobacteriaceae clinical isolates in a Senegalese teaching hospital: a cross sectional study. Afri J Microbiol Res. 2017;11(44):1600–1605. doi:10.5897/AJMR2017.8716
73. El Wartiti MA, Bahmani F-Z, Elouennass M, Benouda A. Prevalence of carbapenemase-producing Enterobacteriaceae in a University Hospital in Rabat, Morocco: a 19-months prospective study. Int Arab J Antimicrob Agents. 2012;2(3):1–6.
74. World Health Organization. Antimicrobial Resistance Global Report on Surveillance: 2014 Summary. World Health Organization; 2014.
75. Khabbaz RF, Moseley RR, Steiner RJ, Levitt AM, Bell BP. Challenges of infectious diseases in the USA. Lancet. 2014;384(9937):53–63. doi:10.1016/S0140-6736(14)60890-4
76. Elliott W, Chan J. Plazomicin injection (Zemdri). Intern Med Alert. 2018;40(15).
77. Tran TB, Velkov T, Nation RL, et al. Pharmacokinetics/pharmacodynamics of colistin and polymyxin B: are we there yet? Int J Antimicrob Agents. 2016;48(6):592–597. doi:10.1016/j.ijantimicag.2016.09.010
78. Shankar C, Nabarro LE, Anandan S, Veeraraghavan B. Minocycline and tigecycline: what is their role in the treatment of carbapenem-resistant gram–negative organisms? Microb Drug Resist. 2017;23(4):437–446. doi:10.1089/mdr.2016.0043
79. Kanj SS, Kanafani ZA, editors. Current concepts in antimicrobial therapy against resistant gram-negative organisms: extended-spectrum β-lactamase–producing Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae, and multidrug-resistant Pseudomonas aeruginosa.
80. Tumbarello M, Viale P, Viscoli C, et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase–producing K pneumoniae: importance of combination therapy. Clin Infect Dis. 2012;55(7):943–950.
81. Balkan II, Aygün G, Aydın S, et al. Blood stream infections due to OXA-48-like carbapenemase-producing Enterobacteriaceae: treatment and survival. Int J Infect Dis. 2014;26:51–56. doi:10.1016/j.ijid.2014.05.012
82. Tan X, Kim HS, Baugh K, et al. Therapeutic options for metallo-β-lactamase-producing enterobacterales. Infect Drug Resist. 2021;14:125. doi:10.2147/IDR.S246174
83. Sato T, Yamawaki K. Cefiderocol: discovery, chemistry, and in vivo profiles of a novel siderophore cephalosporin. Clin Infect Dis. 2019;69(Supplement_7):S538–S543. doi:10.1093/cid/ciz826
84. Li J, Learoyd M, Qiu F, Zhu L, Edeki T. A randomized, Phase I study to assess the safety, tolerability and pharmacokinetics of ceftazidime-avibactam in healthy Chinese subjects. Clin Drug Investig. 2016;36(2):119–126. doi:10.1007/s40261-015-0347-x
85. Zhanel GG, Lawson CD, Adam H, et al. Ceftazidime-avibactam: a novel cephalosporin/β-lactamase inhibitor combination. Drugs. 2013;73(2):159–177. doi:10.1007/s40265-013-0013-7
86. Zhanel GG, Cheung D, Adam H, et al. Review of eravacycline, a novel fluorocycline antibacterial agent. Drugs. 2016;76(5):567–588. doi:10.1007/s40265-016-0545-8
87. Karaiskos I, Lagou S, Pontikis K, Rapti V, Poulakou G. The “old” and the “new” antibiotics for MDR gram-negative pathogens: for whom, when, and how. Front Public Health. 2019;7:151. doi:10.3389/fpubh.2019.00151
88. Vardakas KZ, Legakis NJ, Triarides N, Falagas ME. Susceptibility of contemporary isolates to fosfomycin: a systematic review of the literature. Int J Antimicrob Agents. 2016;47(4):269–285. doi:10.1016/j.ijantimicag.2016.02.001
89. Ni W, Han Y, Liu J, et al. Tigecycline treatment for carbapenem-resistant Enterobacteriaceae infections: a systematic review and meta-analysis. Medicine. 2016;95(11):e3126. doi:10.1097/MD.0000000000003126
90. Bulik CC, Nicolau DP. Double-carbapenem therapy for carbapenemase-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2011;55(6):3002–3004. doi:10.1128/AAC.01420-10
91. de Jonge BL, Karlowsky JA, Kazmierczak KM, Biedenbach DJ, Sahm DF, Nichols WW. In vitro susceptibility to ceftazidime-avibactam of carbapenem-nonsusceptible Enterobacteriaceae isolates collected during the INFORM global surveillance study (2012 to 2014). Antimicrob Agents Chemother. 2016;60(5):3163–3169. doi:10.1128/AAC.03042-15
92. Landman D, Babu E, Shah N, et al. Activity of a novel aminoglycoside, ACHN-490, against clinical isolates of Escherichia coli and Klebsiella pneumoniae from New York City. J Antimicrob Chemother. 2010;65(10):2123–2127. doi:10.1093/jac/dkq278
93. Blizzard TA, Chen H, Kim S, et al. Discovery of MK-7655, a β-lactamase inhibitor for combination with Primaxin®. Bioorg Med Chem Lett. 2014;24(3):780–785. doi:10.1016/j.bmcl.2013.12.101
94. Saisho Y, Katsube T, White S, Fukase H, Shimada J. Pharmacokinetics, safety, and tolerability of cefiderocol, a novel siderophore cephalosporin for Gram-negative bacteria, in healthy subjects. Antimicrob Agents Chemother. 2018;62(3):e02163–17. doi:10.1128/AAC.02163-17
95. Barnes MD, Taracila MA, Good CE, et al. Nacubactam enhances meropenem activity against carbapenem-resistant Klebsiella pneumoniae producing KPC. Antimicrob Agents Chemother. 2019;63(8):e00432–19. doi:10.1128/AAC.00432-19
96. Saqib S, Munis MFH, Zaman W, et al. Synthesis, characterization and use of iron oxide nano particles for antibacterial activity. Microsc Res Tech. 2019;82(4):415–420. doi:10.1002/jemt.23182
97. Jamal M, Chaudhry WN, Hussain T, Das CR, Andleeb S. Characterization of new Myoviridae bacteriophage WZ1 against multi‐drug resistant (MDR) Shigella dysenteriae. J Basic Microbiol. 2015;55(4):420–431. doi:10.1002/jobm.201400688
98. Obiero CW, Ndiaye AG, Sciré AS, et al. A phase 2a randomized study to evaluate the safety and immunogenicity of the 1790GAHB generalized modules for membrane antigen vaccine against Shigella sonnei administered intramuscularly to adults from a shigellosis-endemic country. Front Immunol. 2017;8:1884. doi:10.3389/fimmu.2017.01884
99. Yahav D, Giske CG, Grāmatniece A, Abodakpi H, Tam VH, Leibovici L. New β-lactam–β-lactamase inhibitor combinations. Clin Microbiol Rev. 2020;34(1):e00115–20. doi:10.1128/CMR.00115-20
100. Vrancianu CO, Dobre EG, Gheorghe I, Barbu I, Cristian RE, Chifiriuc MC. Present and future perspectives on therapeutic options for carbapenemase-producing Enterobacterales infections. Microorganisms. 2021;9(4):730. doi:10.3390/microorganisms9040730
101. Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. Infectious Diseases Society of America guidance on the treatment of extended-spectrum β-lactamase producing Enterobacterales (ESBL-E), carbapenem-resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with difficult-to-treat resistance (DTR-P. aeruginosa). Clin Infect Dis. 2021;72(7):e169–e83.
102. Jankauskaitŀ V, Vitkauskienŀ A, Lazauskas A, Baltrusaitis J, Prosyŀevas I, Andruleviŀius M. Bactericidal effect of graphene oxide/Cu/Ag nanoderivatives against Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Staphylococcus aureus and Methicillin-resistant Staphylococcus aureus. Int J Pharm. 2016;511(1):90–97. doi:10.1016/j.ijpharm.2016.06.121
103. Babaei S, Bajelani F, Mansourizaveleh O, Abbasi A, Oubari F. A study of the bactericidal effect of copper oxide nanoparticles on Shigella sonnei and Salmonella typhimurium. J Babol Univ Med Sci. 2017;19(11):76–81.
104. Jin X, Chen Q, Shen F, et al. Resistance Evolution of Hypervirulent Carbapenem-resistant Klebsiella pneumoniae ST11 during Treatment with Tigecycline and Polymyxin. Emerg Microbes Infect. 2021;10(just–accepted):1–29.
105. Pozsgay V, Kubler-Kielb J, Schneerson R, Robbins JB. Effect of the nonreducing end of Shigella dysenteriae type 1 O-specific oligosaccharides on their immunogenicity as conjugates in mice. Proc Natl Acad Sci. 2007;104(36):14478–14482. doi:10.1073/pnas.0706969104
106. DeLaine BC, Wu T, Grassel CL, et al. Characterization of a multicomponent live, attenuated Shigella flexneri vaccine. FEMS Pathogens Dis. 2016;74(5):ftw034. doi:10.1093/femspd/ftw034
107. Mitobe J, Sinha R, Mitra S, et al. An attenuated Shigella mutant lacking the RNA-binding protein Hfq provides cross-protection against Shigella strains of broad serotype. PLoS Negl Trop Dis. 2017;11(7):e0005728. doi:10.1371/journal.pntd.0005728
108. Wu Y, Chakravarty S, Li M, Wai TT, Hoffman SL, Sim BKL. Development of a live attenuated bivalent oral vaccine against Shigella sonnei shigellosis and typhoid fever. J Infect Dis. 2017;215(2):259–268. doi:10.1093/infdis/jiw528
109. Kaminski R, Wu M, Turbyfill K, et al. Development and preclinical evaluation of a trivalent, formalin-inactivated Shigella whole-cell vaccine. Clin Vaccine Immunol. 2014;21(3):366–382. doi:10.1128/CVI.00683-13
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
