Back to Journals » Research and Reports in Urology » Volume 10
Emerging biomarkers for the diagnosis and monitoring of urothelial carcinoma
Authors Miyake M , Owari T, Hori S , Nakai Y, Fujimoto K
Received 13 October 2018
Accepted for publication 13 November 2018
Published 14 December 2018 Volume 2018:10 Pages 251—261
DOI https://doi.org/10.2147/RRU.S173027
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jan Colli
Makito Miyake, Takuya Owari, Shunta Hori, Yasushi Nakai, Kiyohide Fujimoto
Department of Urology, Nara Medical University, Kashihara-shi, Nara 634-8522, Japan
Abstract: Urothelial carcinoma (UC) arises extensively from the renal pelvis, ureter, urinary bladder, and urethra. UC represents a clinical and social challenge because of its incidence, post-treatment recurrence rate, and prognosis. Combinations of urine cytology, cystoscopy, and conventional imaging such as computed tomography are currently used for diagnosis and monitoring modalities of UC. Both the poor diagnostic accuracy of urine cytology and poor cost performance of cystoscopy and conventional imaging modalities emphasize the urgent need for advancement in clinical guidance for UC. Urine- and blood-based biomarkers for detection of UC of the bladder and upper urinary tract represent a considerable research area. Biomarkers can help to improve UC diagnosis with the aim of replacing cystoscopy and other imaging examinations in future and may enable individualizing risk stratification regarding therapy and follow-up. Over the decades, numerous studies have focused on the potential application of biomarkers for UC, including urine, circulating tumor DNA, RNAs, proteins, and extracellular vesicles. Although some biomarkers such as ImmunoCyt/uCyt+, UroVysion, NMP-22, bladder tumor antigen, CxBladder, and Xpert Bladder Cancer are currently available in clinical practice, few biomarkers achieve high sensitivity and specificity. Emerging biomarkers are continuously developed and reported in medical journals. However, there is a significant lack on following external validation using different cohorts. The positive results are needed to be confirmed by more studies with large-scale cohorts and long follow-up periods to prove the true value of novel biomarkers, followed by their adoption in clinical practice. The present paper provides an overview of the evidence based on high-impact studies regarding urine- and blood-based biomarkers and their clinical applications in bladder cancer and upper tract UC.
Keywords: urothelial carcinoma, bladder cancer, upper urinary tract cancer, biomarker, diagnosis, surveillance
Introduction: clinical issues in urothelial carcinoma of the bladder and upper urinary tract
Urothelium is the epithelial lining of renal collecting ducts, calyces, ureters, bladder, and urethra.1 Urothelial carcinoma (UC), previously referred to as transitional cell carcinoma, is a histopathologic type of cancer that typically arises from the urothelium. Majority of cases presenting UC are bladder cancers (BCa), whereas upper urinary tract urothelial cancer (UTUC) accounts for only 5%–10% of all urothelial malignancies.2 Primary urethral cancer is an extremely rare lesion, accounting for only <1% of the total incidence of malignancies. UC of the bladder (accounting for 90% of BCa) is the most common malignancy involving the urinary tract and the sixth most common cancer in the USA, with an estimated 79,030 cases diagnosed in 2017.2,3 The incidence of BCa is approximately four times higher in men than in women. Cigarette smoking is a significant risk factor for both BCa and UTUC, with the reported OR of 3.22 and 4–11, respectively.4,5 Occupational carcinogen exposure,6 infection with Schistosoma haematobium,7 phenacetin,4 pioglitazone,8 and thiazolidinediones9 have been reported as other risk factors for the incidence of UC.
Approximately 70%–80% of BCa are diagnosed as non-muscle invasive BCa (NMIBC), consisting of Ta (70%), T1 (20%), and primary Tis (10%).10 The clinical course of treated NMIBC is characterized by a favorable survival prognosis with a high intravesical recurrence rate. NMIBC is a heterogeneous subset with different treatment options, such as intravesical instillation and immediate cystectomy, follow-up schedules, and varying outcomes.11 Despite transurethral resection of the bladder tumor (TURBT) followed by adjuvant intravesical instillation with chemotherapeutics or bacillus Calmette–Guérin (BCG), up to 70% of cases with NMIBC will experience intravesical recurrences, whereas 10%–30% will progress to life-threatening muscle-invasive BCa (MIBC, ≥T2).12,13 Two major scoring systems for prediction of recurrence and progression after TURBT for NMIBC based on clinicopathologic parameters, the EORTC model (3), and the CUETO model (4) have been clinically available for the management of NMIBC.14,15 According to the risk stratification, patients with NMIBC should be under appropriate intensive surveillance following treatment. Once disease progression is observed, the prognosis significantly declines.16−18 The incredibly high rates of intravesical recurrence and disease progression require follow-up of treated NMIBC patients with cystoscopy and urine cytology at regular intervals (every 3–6 months during the next several years). Postoperative routine examinations make NMIBC one of the costliest malignancies from diagnosis to death, with an estimated cost of $187,000 per case in the USA.19 In addition, its total annual expenditure was estimated at $4 billion in 2010, which is expected to increase up to $5 billion by 2020.19,20 The current gold standard method for detection, diagnosis, and monitoring of BCa is still a combination of flexible cystoscopy and urine cytology. As to the medical cost, cystoscopy coupled with urine cytology is expensive, about $150 in Japan (with reference to the exchange rate against the US dollar value as of March 2016).11
UTUC is a rare and heterogenous carcinoma. Among UTUCs, pelvic cancer is four times as large as ureteral cancer.21 UCs involving the urinary tract have been supposed to have a similar carcinogenic mechanism and pathogenesis. However, recent studies have suggested that there are several differences, between UTUC and UC of the bladder such as tumor behavior and molecular mechanisms underlying tumor development and progression.22,23 The estimated annual incidence of UTUC is ~2 cases per 100,000 person-year and has slowly risen over the past three decades.24,25 Despite the improvements in diagnosis, surgery, and systemic chemotherapy, the prognosis of patients with UTUC has not improved over the past two decades.26 Radical nephroureterectomy (RNU) with bladder-cuff removal is the gold standard treatment for localized UTUC. However, 60% of UTUCs are invasive at diagnosis, and oncologic outcomes are unacceptable for patients with locally advanced-stage and/or lymph node involvement.27 The clinical issue of UTUC includes heterogenous clinical courses: intravesical recurrence, extra-urinary tract recurrence, and distant metastasis. The mortality after diagnosis and treatment is significantly associated with tumor stage (pathologic Ta-1, T2, T3, and T4 stages, representing 5-year survival rates of 92.1%–97.8%, 74.7%–84.1%, 54.0%–56.3%, and 0%–12.2%, respectively).28–30 Therefore, early detection of primary and recurrent UTUC is mandatory for improvement of clinical outcome. Several biomarkers with tissue-, blood-, and urine-based biomaterials have been investigated to date. Here, we review promising blood- and urine-based biomarkers, especially genomic biomarkers, for diagnosis and monitoring of UTUC.
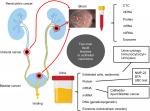
The definition of “liquid biopsy” is the sampling and analysis of biologic fluids such as urine, blood, pleural liquid, ascites, cerebrospinal fluid, and saliva. The target of molecular analysis includes circulating tumor cells (CTCs), circulating cell-free tumor DNA, proteins, mRNAs, miRNAs, long noncoding RNAs, and vesicles (Figure 1). These biomarkers in liquid biopsy hold extensive potential because they can help in diagnosis and monitoring of disease stage, recurrence, and treatment response without invasive intervention. Numerous studies have investigated the diagnostic impact of various urine- or blood-based biomarkers such as genomic assays in urothelial malignancies. The present paper provides an overview of the evidence based on high-impact studies regarding the urine- and blood-bound biomarkers and their clinical applications in BCa and UTUC.
Diagnostic and post-TURBT monitoring markers in BCa
Representative urine-based tests to diagnosis and post-TURBT monitoring for BCa are listed in Table 2.
Urine cytology and its derivatives
Cytology remains only one gold standard urine-based test in the clinical management of UC, with excellent specificity but unsatisfactory sensitivity for diagnosis and monitoring of NMIBC (Table 1).31−33 The sensitivity significantly depends on tumor grade, size, and stage, attaining a decent sensitivity of about 60% for high-grade tumors and carcinoma in situ.34 For screening recurrence of NMIBC, urine cytology has a high sensitivity for high-grade tumors but an especially low sensitivity in patients with low-grade tumors (16%–53%).32,33 Another problem faced by urine cytology is that its results depend on the expertise of the cytopathologist. ImmunoCyt/uCyt+ tests based on detection of three BCa antigens (M344, LDQ10, and 19A11) in urinary exfoliated cells improve the predictive values of cytology, with 73% sensitivity, but its specificity decreased to 66%.35
UroVysion bladder cancer kit is a multitarget fluorescence in situ hybridization (FISH) assay that detects aneuploidy for chromosomes 3, 7, and 17, and focal loss of 9p21 from exfoliated urothelial cells in urine.36 A meta-analysis from 14 studies demonstrated a diagnostic accuracy of 72% sensitivity and 83% specificity of the UroVysion kit (Table 1). Several studies demonstrated that the UroVysion has higher sensitivity than urine cytology.37,38 Positive UroVysion has proven to be a significant predictor of recurrence and progression in patients under NMIBC surveillance with suspicious cytology but negative cystoscopy.39 In addition, positive UroVysion at the end of intravesical treatment with BCG is a risk factor for progression to MIBC.40,41 Thus, surveillance with the UroVysion test can provide prognostic information for treated NMIBC patients. However, most previous studies have demonstrated that UroVysion has lower specificity than urine cytology.37,38
Over the last two decades, 5-aminolevulinic acid (5-ALA) or hexaminolevulinate-induced fluorescence cystoscopy has been established with the aim of detecting flat and/or small lesions that are hardly visible under conventional cystoscopy, leading to the decreased rate of post-TURBT residual tumor and recurrence.42 Since 2014, we have investigated the feasibility and usefulness of urine-based tests taking advantage of 5-ALA-derived fluorescence.43,44 Detection modalities are based on fluorescence microscopic cytology, fluorescence spectrophotometry, and flow cytometry. Although analyses using spectrophotometry and flow cytometry enable quantitative measurement of accumulated protoporphyrin IX (PPIX) leading to increased objectivity, they require cumbersome manual procedures. We have been developing an automated detection system for 5-ALA-derived intracellular fluorescence.
Protein markers
Two of the most extensively studied proteins as BCa urinary biomarkers are NMP-22 and BTA. There are currently two commercialized detection assays: a quantitative sandwich ELISA test (NMP-22 Test and BTA TRAK) and a qualitative point-of-care test (NMP-22 BladderChek and BTA stat). The diagnostic performance of NMP-22 exceeds conventional urine cytology in detection with regard to sensitivity (68% vs 44%), which was predominantly due to an improved detection rate of low-grade tumors.46
In contrast, diagnostic performance of the BTA test has been evaluated by several clinical studies, showing a sensitivity of 57%–83% and a specificity of 60%–92%.47,48
Urine bladder cancer (UBC) test is a quantitative immunoassay that detects soluble fragments of cytokeratins 8 and 18, which are derived from dead UC cells in urine supernatant. The UBC test enables the discrimination of patients with BCa from noncancer individuals with a sensitivity of 64% and specificity of 80%.49
A recent report demonstrated that the protein level of two subunits of collagens (4A1 and 13A1) in urine supernatant was increased in patients with BCa compared to healthy controls. The sensitivity and specificity of the combination of both collagens (COL4A1 + COL13A1) for BCa detection were 72.1% and 65.6%, respectively.50 In addition, high urinary COL4A1 + COL13A1 was found to be an independent risk factor for intravesical recurrence after TURBT. Urinary collagens could be potential diagnostic and prognostic biomarkers for BCa.50 In 2014, Rosser et al reported the potential of a urine-based biomarker panel consisting of ten proteins (ANG, APOE, CA9, IL8, matrix metalloproteinase 9 [MMP9], MMP10, SDC1, SERPINA1, SERPINE1, and VEGFA) to detect intravesical recurrence (79% sensitivity and 88% specificity).51 In 2016, the same group described a comparable study with a similar protein panel (including PAI-1 and A1AT instead of SERPINA1 and SERPINE1) that enabled detection of BCa patients from patients with benign disease and healthy controls (85% sensitivity and 81% specificity).52
Genetic detection markers
Various studies have demonstrated that DNA mutations derived from urothelial cells in urine samples could detect and predict the recurrence of NMIBC.53,54 While overexpression of FGFR3 protein has been observed in 29% of muscle MIBC and 49% of metastatic MIBC, FGFR3 mutation has been rarely observed in metastatic MIBC (6%–9%).55,56 In contrast, FGFR mutations are highly associated with low-grade NMIBC (66.2% of Ta tumors and 37.9% of T1 tumors).57 FGFR3 mutations detected in urine sediments by highly sensitive PCR assay were found to be a useful detection markers of recurrence in NMIBC and its sensitivity and specificity were 78% and 100%, respectively.53 Zuiverloon et al revealed that FGFR3 mutation-positive urine was associated with 3.8-fold (P<0.001) higher risk of development recurrence during surveillance in NMIBC.58 CertNDx™ Bladder Cancer Assay (Predictive Biosciences, Lexington, MA, USA) is a commercial urine-based FGFR3 genetic test (not approved by the US Food and Drug Administration [FDA]). CertNDx™ was based on a study by Fernandez et al,59 in which several coauthors were employees of Predictive Biosciences. When results of FGFR3 mutation analysis were combined with the results of other molecular tests including MMP2 and DNA methylation in Twist 1/Nid2, the diagnostic accuracy for detecting cancer recurrence was a 92% sensitivity and 51% specificity.
Roperch et al demonstrated that DNA hypermethylation of CpG island marker (HS3ST2, SEPTIN9, and SLIT2) combined with positive FGFR3 mutation improved the diagnostic accuracy of recurrence as compared to FGFR3 mutation assay alone, especially for Ta/low-grade tumors (96.4/93.6% vs 58.3/54.6%).60 A similar approach reported by Kandimalla et al revealed that DNA methylation CpG island markers (OTX1, ONECUT2, and OSR1) combined with FGFR3 mutations could improve the sensitivity of cytology alone and FGFR3 mutation alone for detection of recurrence of NMIBC.54 The DNA-based urine biomarker could increase the detection rate of recurrence and reduce the frequency of follow-up cystoscopy.
mRNA detection markers
Several mRNA-based urine biomarkers have been developed and have improved the accuracy of BCa diagnosis. The Cxbladder test measures five mRNA targets (IGFBP5, MDK, HOXA13, CDK1, and CXCR2) by quantitative real-time PCR.61 The former four biomarkers are associated with growth and propagation of the tumor tissue, whereas CXCR2 is an inflammation biomarker. Although the Cxbladder Monitor could improve the sensitivity compared to cytology (81.8% vs 56.1%), the sensitivity was slightly low (85.1% vs 94.5%).61 In an external validation study including 1,036 urine samples from 803 patients undergoing surveillance, the sensitivity was 91% and negative predictive value (NPV) was 96%.62 In addition, diagnostic accuracy (sensitivity and NPV) was not affected by past history of BCG treatment.63
The Xpert BC Monitor measures five target mRNAs (ABL1, CRH, IGF2, UPK1B, and ANXA10) using quantitative real-time PCR.64,65 These markers are related to proliferation and survival (IGF2), neuroendocrine stress response and inflammation (CRH), cell growth and signal transduction (ANXA10), and epigenetic dysregulation in BCa (UPK1B). Recently, the first prospective study was conducted to evaluate the efficiency of the Xpert BC Monitor.64,65 In this study including 155 urine samples obtained from 140 patients with history of NMIBC, the sensitivity (84%) and specificity (91%) of the Xpert BC Monitor were significantly higher than bladder washing cytology (33% and 76%). The sensitivity of the Xpert BC Monitor was superior to that of cytology in low-grade (77%) and Ta tumors (82%). The sensitivity of the Xpert BC Monitor (91%) was not inferior to that of cytology (94%). This mRNA-based urinary test could increase the accuracy of diagnosis of recurrence in patients with NMIBC even for low-grade tumors and reduce invasive surveillance. Further prospective validation studies and more cost effectiveness are required for widespread use of this promising test.
Circulating biomarkers
The existence of tumor-derived epithelial cells in peripheral blood obtained from cancer patients is associated with distant metastasis. The CellSearch system is a widely used technique that was approved by the FDA for CTC detection in patients with metastatic breast, colorectal, and prostate cancer.66 However, only a few studies analyzing CTCs using the CellSearch system have been reported in patients with BCa. As to NMIBC, CTCs were detected in 18% (8/44) patients and presence of CTCs was associated with shorter time to first recurrence.67 Importantly, CTC-positive NMIBC patients showed significantly worse overall, progression-free, and cancer-specific survival.67 A meta-analysis of patients with BCa demonstrated that overall sensitivity of CTC detection was 35.1% and specificity was 89.4%.68 CTCs may be useful for detection of residual tumors after surgery, monitoring subsequent recurrence and metastasis, or decision-making for perioperative chemotherapy. Given that there is low overall sensitivity, CTC should not be used for initial screening test for BCa.
Diagnostic and post-RNU monitoring markers in UTUC
Representative urine-based tests to diagnosis and post-RNU monitoring for UTUC are listed in Table 2.
Urine cytology and its derivatives
Urine cytology is a gold standard diagnostic tool for UTUC as well as BCa. Voided urine cytology provides high specificity but low sensitivity.69,70 Contrarily, cytology of urine or lavage fluid obtained from upper tract showed high sensitivity and low specificity compared with that using voided urine.70,71 With regard to ImmunoCyt/uCyt+ test for UTUC, the sensitivity of voided urine is 50% for cytology, 75% for ImmunoCyt/uCyt+, and 87% for both methods combined. In addition, the sensitivity of urine obtained from the upper tract is 82% for cytology, 91% for ImmunoCyt/uCyt+, and 100% for both methods combined.69 The potential of UroVysion test is reported to diagnose UTUC. The overall sensitivity of FISH analysis is superior to that of cytology (77% vs 36%), and the specificities of FISH and cytology are 94.7% and 100%, respectively.72
Protein detection markers
NMP-22 test was evaluated as a detection tool of upper urinary tract recurrence in patients having a history of NMIBC, resulting in disappointing accuracy even in high-grade tumors.73 The overall sensitivity and specificity of urine obtained from the upper tract are 82% and 89% for BTA test, 11% and 54% for voided urine cytology, and 48% and 33% for the diagnosis of UTUC.74
mRNA/miRNA detection markers
miRNAs/miRs in serum have the potential to diagnose UTUC and predict prognosis of patients with UTUC. Ten miRNAs (miR-664a-3p, miR-431-5p, miR-423-5p, miR-191-5p, miR-33b-3p, miR-26a-5p, miR-22-3p, miR-16-5p, let-7b-5p, and let-7c) were reported for the first time to have the potential to distinguish UTUC from controls (areas under the curve [AUC] >0.8).75 Furthermore, miRNA-141 could be used as a diagnostic biomarker for UTUC (AUC =0.73), and miRNA-151b was significantly associated with tumor progression and cancer-specific survival (HR =0.33, P<0.001 and HR =0.25, P<0.001, respectively).76,77
Genetic/epigenetic risk factors and detection markers
Cyclooxygenase 2 (COX2) and caveolin-1 (CAV1) genotyping assays have showed that polymorphic genotypes (COX2; G-765C and intron 5, CAV1; rs3807987 and rs7804372) were significantly different between patients with UTUC and healthy controls. COX2 G-765C/intron 5 carrying GG/AT variants have a significantly increased risk of UTUC (OR =4.83, 95% CI =1.79–13.06), whereas those carrying CG/TT variants have a decreased risk (OR =0.26, 95% CI =0.14–0.49) than those carrying GG/TT haplotype. CAV1 rs3807987 and rs7804372 were significantly different between patients with UTUC and healthy controls (P=0.0188 and 0.0090, respectively). Haplotype analysis showed that CAV1 rs3807987/rs7804372 haplotypes carrying GG/TT, AG/TT, and AA/TT variants have a significantly high risk of UTUC compared with the GG/AT and GG/AA haplotypes (OR =1.61, 1.50 and 2.67, 95% CI =1.05–2.47, 1.18–1.90, and 1.37–5.18, respectively).78,79 In addition, the cell cycle regulator, cyclin D1 (CCND1) G870A and CCND1 C1722G polymorphisms were related with development and prediction of UTUC.80,81 With regard to genomic biomarkers of urine, detection of promoter mutation in telomerase reverse transcriptase (TERT) could distinguish patients with UTUC from healthy controls and TERT promoter mutations were significantly correlated with distant metastasis in patients with UTUC.82 As epigenetic biomarkers, GDF15, TMEFF2, and VIM promoter methylations were also investigated as diagnostic biomarkers for UTUC. The detection of GDF15, TMEFF2, and VIM promoter methylations in urine at the same time can lead to accurate diagnosis of patients with UTUC (sensitivity 91% and specificity 100%) and patients with low level of VIM promotor methylation were at a high risk of cancer-specific mortality.83 The presence of CDH1, HSPA2, RASSF1A, TMEFF2, VIM, and GDF15 promoter methylations simultaneously identified the cause of gross hematuria as UTUC (sensitivity 82% and specificity 68%).84
Previous studies demonstrated that a subset of UTUC tumors harbors FGFR3 mutation and this mutation is associated with favorable clinical outcome as well as BCa.85,86 A case report by Silverberg indicates that the FGFR3 urine assay, which was originally developed to diagnose BCa (CertNDx™ Bladder Cancer Assay), could be a useful detection tool for UTUC.87
Circulating biomarkers
In a study regarding comprehensive genomic profiling of circulating tumor DNA, 75 blood samples of metastatic UTUC were evaluated by cell-free circulating DNA next-generation sequencing, and genetic alterations, including single-nucleotide variants, indels, fusions, and copy number amplifications, were identified in 71 patients (95%). Among these, TP53 (51%), PIK3CA (23%), ARID1A (20%), TERT (17%), EGFR (14%), BRCA1 (11%), ERBB2 (11%), FGFR3 (11%), NF1 (11%), and MET (10%) were the ten most frequent alterations. In patients with metastatic UTUC, the frequency of genetic alterations significantly differed between circulating tumor DNA next-generation sequencing and historical tumor tissue studies for TP53 and FGFR3. Gene alterations in TP53 and FGFR3 were significantly decreased and increased, respectively, in circulating tumor DNA compared to tumor tissue.88 FGFR3 and HRAS alterations are more common in UTUC, whereas TP53 and RB1 alterations are more common in BCa.89 Accumulation of evidence could lead to a novel convenient method for diagnosis and monitoring of UTUC.
Limitations of urine-based biomarkers
Miyake et al have shown that BTA stat/BTA TRAK is a surrogate for hematuria and NMP-22 test, which detects nuclear mitotic apparatus-associated protein, identifies status of cellular proliferation.90,91 The target protein of BTA is complement factor H-related protein, which is abundant in blood. Therefore, positive BTA or NMP-22 test results can be obtained easily in benign urologic conditions such as benign prostatic hyperplasia, stones, endourologic stents, or urinary tract infections.
Fantony et al suggested that no advancement in urine- and blood-based noninvasive testing has occurred in BCa during recent years and no significant change in the current monitoring scheme (cystoscopy and urine cytology) has occurred.92 In addition, they pointed out the poor performance, marginal clinical utility, and potential harm of the currently available urine-based tests, which make them inadequate for regular clinical use. One of the biggest limitations of both cytology and FISH is spectrum bias. In biostatistics, spectrum bias is defined as the phenomenon in which the performance of a diagnostic test varies in different clinical settings because each setting has a different mix of patients. For example, age, sex, and smoking history can result in a change in diagnostic test accuracy. Urine tests for BCa have different sensitivities and specificities depending greatly on the patient background, which would halt the wide use of a diagnostic test to real-world populations being screened for BCa. Another concern of urine-based tests for cancer is the potential harm. A positive or equivocal result of urine-based tests accompanied with a negative result of cystoscopy leads to patients’ anxiety and pressure for further examinations, such as bladder biopsies, dynamic contrast-enhanced imaging, retrograde pyelography, or ureteroscopy. Some of these procedures require preoperative counseling, medical optimization, and anesthesia. Moreover, these are time-consuming, expensive, and put the patient at risk for iatrogenic complications including hematuria, ureteral injury, and urinary tract infections, sometimes associated with multidrug-resistant bacteria and leading to septic status. A false-positive urine test result can raise significant risks caused by overtesting, overestimation, and overtreatment that should be avoided and prevented.
Future perspective
This review does not mention about biomarkers on treating UC. A recent publication, “Comprehensive molecular characterization of the muscle-invasive bladder cancer”, by Robertson et al refers to the new classification of BCa, specifying how biomarkers can play a role in selecting the patients most likely to respond to treatment with different agents, including immunotherapeutic agents.93 Our prospective view is whether blood/urine-based biomarkers shown in this review would be applicable to the therapeutic approach, such as predictive markers for drug efficacy/resistance and disease markers reflecting clinical status of the advanced disease.
Conclusion
This review highlights that there is considerable interest in the use of urinary/blood biomarkers for the clinical management of BCa and UTUC. Unfortunately, many potential biomarkers are still under evaluation. The most frequent indication for these malignancies is hematuria. Urine-based biomarkers apply to the initial screening test of hematuria patients as well as postoperative surveillance of patients with treated NMIBC. Cystoscopy has great sensitivity (<95%) for detecting BCa, and computed tomography urography has sensitivity and specificity of ~95% for detecting UTUC.31,94
The requirement for cystoscopy and computed tomography urography represents a significant cost to health care services in diagnosing UC. Traditional noninvasive imaging modalities with high-cost performance do not have the satisfactory diagnostic accuracy to replace cystoscopy for the detection of BCa and computed tomography for the detection of UTUC. A highly sensitive and specific urinary/blood assay will revolutionize both the screening method for hematuria and surveillance pathway for NMIBC and UTUC. Although emerging biomarkers are continuously developed and reported in medical journals, there is a significant lack on following external validation using different cohorts. Positive results need to be confirmed by more studies with large-scale cohorts and long follow-up periods to prove the true value of novel biomarkers, followed by their adoption in clinical practice.
Acknowledgment
The authors thank Dr Charles Rosser and Dr Hideki Furuya (University of Hawaii Cancer Center, Clinical and Translational Research Program) for their advice and assistance.
Disclosure
The authors report no conflicts of interest in this work.
References
Miyazaki J, Nishiyama H. Epidemiology of urothelial carcinoma. Int J Urol. 2017;24(10):730–734. | ||
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. | ||
National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology: bladder cancer, version 5; 2017. Available from: NCCN.org. Accessed December 13, 2017. | ||
Pommer W, Bronder E, Klimpel A, Helmert U, Greiser E, Molzahn M. Urothelial cancer at different tumour sites: role of smoking and habitual intake of analgesics and laxatives. Results of the Berlin Urothelial Cancer Study. Nephrol Dial Transplant. 1999;14(12):2892–2897. | ||
McLaughlin JK, Silverman DT, Hsing AW, et al. Cigarette smoking and cancers of the renal pelvis and ureter. Cancer Res. 1992;52(2):254–257. | ||
Reulen RC, Kellen E, Buntinx F, Brinkman M, Zeegers MP. A meta-analysis on the association between bladder cancer and occupation. Scand J Urol Nephrol. 2008;42(218):64–78. | ||
Mostafa MH, Sheweita SA, O’Connor PJ. Relationship between schistosomiasis and bladder cancer. Clin Microbiol Rev. 1999;12(1):97–111. | ||
Yan H, Xie H, Ying Y, et al. Pioglitazone use in patients with diabetes and risk of bladder cancer: a systematic review and meta-analysis. Cancer Manag Res. 2018;10:1627–1638. | ||
Turner RM, Kwok CS, Chen-Turner C, Maduakor CA, Singh S, Loke YK. Thiazolidinediones and associated risk of bladder cancer: a systematic review and meta-analysis. Br J Clin Pharmacol. 2014;78(2):258–273. | ||
Babjuk M, Burger M, Zigeuner R, et al; European Association of Urology. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol. 2013;64(4):639–653. | ||
Miyake M, Fujimoto K, Hirao Y. Active surveillance for nonmuscle invasive bladder cancer. Investig Clin Urol. 2016;57(Suppl 1):S4–S13. | ||
Miyake M, Gotoh D, Shimada K, et al. Exploration of risk factors predicting outcomes for primary T1 high-grade bladder cancer and validation of the Spanish Urological Club for Oncological Treatment scoring model: long-term follow-up experience at a single institute. Int J Urol. 2015;22(6):541–547. | ||
Sugano K, Kakizoe T. Genetic alterations in bladder cancer and their clinical applications in molecular tumor staging. Nat Clin Pract Urol. 2006;3(12):642–652. | ||
Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49(3):466–477. | ||
Fernandez-Gomez J, Solsona E, Unda M, et al; Club Urológico Español de Tratamiento Oncológico (CUETO). Prognostic factors in patients with non-muscle-invasive bladder cancer treated with bacillus Calmette-Guérin: multivariate analysis of data from four randomized CUETO trials. Eur Urol. 2008;53(5):992–1002. | ||
Miyake M, Morizawa Y, Hori S, et al. Clinical impact of postoperative loss in psoas major muscle and nutrition index after radical cystectomy for patients with urothelial carcinoma of the bladder. BMC Cancer. 2017;17(1):237. | ||
Miyake M, Morizawa Y, Hori S, et al. Integrative assessment of pretreatment inflammation-, nutrition-, and muscle-based prognostic markers in patients with muscle-invasive bladder cancer undergoing radical cystectomy. Oncology. 2017;93(4):259–269. | ||
Morizawa Y, Miyake M, Shimada K, et al. Neutrophil-to-lymphocyte ratio as a detection marker of tumor recurrence in patients with muscle-invasive bladder cancer after radical cystectomy. Urol Oncol. 2016;34(6):257.e11–7. | ||
Lee DJ, Chang SS. Cost considerations in the management of bladder cancer. Urol Times. Available from: http://www.urologytimes.com/modern-medicine-feature-articles/cost-considerationsmanagement- bladder-cancer. Accessed October 16, 2017. | ||
Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103(2):117–128. | ||
Huben RP, Mounzer AM, Murphy GP. Tumor grade and stage as prognostic variables in upper tract urothelial tumors. Cancer. 1988;62(9):2016–2020. | ||
Catto JW, Hartmann A, Stoehr R, et al. Multifocal urothelial cancers with the mutator phenotype are of monoclonal origin and require panurothelial treatment for tumor clearance. J Urol. 2006;175(6):2323–2330. | ||
Catto JW, Yates DR, Rehman I, et al. Behavior of urothelial carcinoma with respect to anatomical location. J Urol. 2007;177(5):1715–1720. | ||
Raman JD, Messer J, Sielatycki JA, Hollenbeak CS. Incidence and survival of patients with carcinoma of the ureter and renal pelvis in the USA, 1973-2005. BJU Int. 2011;107(7):1059–1064. | ||
Rouprêt M, Babjuk M, Compérat E, et al. European association of urology guidelines on upper urinary tract urothelial cell carcinoma: 2015 update. Eur Urol. 2015;68(5):868–879. | ||
Miyake M, Tatsumi Y, Fujimoto K, et al; Nishinipon Uro-Oncology Collaborative Group. Changes in oncological outcomes after radical nephroureterectomy in patients with upper urinary tract urothelial carcinoma treated in the last two decades: a retrospective analysis based on a multicenter collaborative study. Jpn J Clin Oncol. 2016;46(12):1148–1155. | ||
Rink M, Ehdaie B, Cha EK, et al; Bladder Cancer Research Consortium (BCRC); Upper Tract Urothelial Carcinoma Collaboration (UTUCC). Stage-specific impact of tumor location on oncologic outcomes in patients with upper and lower tract urothelial carcinoma following radical surgery. Eur Urol. 2012;62(4):677–684. | ||
Margulis V, Shariat SF, Matin SF, et al; Upper Tract Urothelial Carcinoma CollaborationThe Upper Tract Urothelial Carcinoma Collaboration. Outcomes of radical nephroureterectomy: a series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer. 2009;115(6):1224–1233. | ||
Li CC, Chang TH, Wu WJ, et al. Significant predictive factors for prognosis of primary upper urinary tract cancer after radical nephroureterectomy in Taiwanese patients. Eur Urol. 2008;54(5):1127–1134. | ||
Novara G, de Marco V, Gottardo F, et al. Independent predictors of cancer-specific survival in transitional cell carcinoma of the upper urinary tract: multi-institutional dataset from 3 European centers. Cancer. 2007;110(8):1715–1722. | ||
Blick CG, Nazir SA, Mallett S, et al. Evaluation of diagnostic strategies for bladder cancer using computed tomography (CT) urography, flexible cystoscopy and voided urine cytology: results for 778 patients from a hospital haematuria clinic. BJU Int. 2012;110(1):84–94. | ||
Yafi FA, Brimo F, Steinberg J, Aprikian AG, Tanguay S, Kassouf W. Prospective analysis of sensitivity and specificity of urinary cytology and other urinary biomarkers for bladder cancer. Urol Oncol. 2015;33(2):66.e25–31. | ||
McCroskey Z, Pambuccian SE, Kleitherms S, et al. Accuracy and interobserver variability of the cytologic diagnosis of low-grade urothelial carcinoma in instrumented urinary tract cytology specimens. Am J Clin Pathol. 2015;144(6):902–908. | ||
Miyake M, Morizawa Y, Hori S, et al. Diagnostic and prognostic role of urinary collagens in primary human bladder cancer. Cancer Sci. 2017;108(11):2221–2228. | ||
He H, Han C, Hao L, Zang G. ImmunoCyt test compared to cytology in the diagnosis of bladder cancer: a meta-analysis. Oncol Lett. 2016;12(1):83–88. | ||
Hajdinjak T. UroVysion FISH test for detecting urothelial cancers: meta-analysis of diagnostic accuracy and comparison with urinary cytology testing. Urol Oncol. 2008;26(6):646–651. | ||
Galván AB, Salido M, Espinet B, et al. A multicolor fluorescence in situ hybridization assay: a monitoring tool in the surveillance of patients with a history of non-muscle-invasive urothelial cell carcinoma: a prospective study. Cancer Cytopathol. 2011;119(6):395–403. | ||
Kojima T, Nishiyama H, Ozono S, et al. Clinical evaluation of two consecutive UroVysion fluorescence in situ hybridization tests to detect intravesical recurrence of bladder cancer: a prospective blinded comparative study in Japan. Int J Clin Oncol. 2018;23(6):1140–1147. | ||
Kim PH, Sukhu R, Cordon BH, et al. Reflex fluorescence in situ hybridization assay for suspicious urinary cytology in patients with bladder cancer with negative surveillance cystoscopy. BJU Int. 2014;114(3):354–359. | ||
Kipp BR, Karnes RJ, Brankley SM, et al. Monitoring intravesical therapy for superficial bladder cancer using fluorescence in situ hybridization. J Urol. 2005;173(2):401–404. | ||
Savic S, Zlobec I, Thalmann GN, et al. The prognostic value of cytology and fluorescence in situ hybridization in the follow-up of nonmuscle-invasive bladder cancer after intravesical bacillus Calmette-Guérin therapy. Int J Cancer. 2009;124(12):2899–2904. | ||
Schubert T, Rausch S, Fahmy O, Gakis G, Stenzl A. Optical improvements in the diagnosis of bladder cancer: implications for clinical practice. Ther Adv Urol. 2017;9(11):251–260. | ||
Miyake M, Nakai Y, Anai S, et al. Diagnostic approach for cancer cells in urine sediments by 5-aminolevulinic acid-based photodynamic detection in bladder cancer. Cancer Sci. 2014;105(5):616–622. | ||
Nakai Y, Ozawa T, Mizuno F, et al. Spectrophotometric photodynamic detection involving extracorporeal treatment with hexaminolevulinate for bladder cancer cells in voided urine. J Cancer Res Clin Oncol. 2017;143(11):2309–2316. | ||
Hatzichristodoulou G, Kübler H, Schwaibold H, et al. Nuclear matrix protein 22 for bladder cancer detection: comparative analysis of the BladderChek® and ELISA. Anticancer Res. 2012;32(11):5093–5097. | ||
Mowatt G, Zhu S, Kilonzo M, et al. Systematic review of the clinical effectiveness and cost-effectiveness of photodynamic diagnosis and urine biomarkers (FISH, ImmunoCyt, NMP22) and cytology for the detection and follow-up of bladder cancer. Health Technol Assess. 2010;14(4):1–331. | ||
Sarosdy MF, Hudson MA, Ellis WJ, et al. Improved detection of recurrent bladder cancer using the Bard BTA stat Test. Urology. 1997;50(3):349–353. | ||
Gutiérrez Baños JL, Martín García B, Hernández Rodríguez R, et al. [Usefulness of BTA Stat test (Bard) in the diagnosis of bladder cancer. Preliminary results and comparison with cytology and cystoscopy]. Arch Esp Urol. 1998;51(8):778–782. | ||
D’Costa JJ, Goldsmith JC, Wilson JS, Bryan RT, Ward DG. A systematic review of the diagnostic and prognostic value of urinary protein biomarkers in urothelial bladder cancer. Bladder Cancer. 2016;2(3):301–317. | ||
Miyake M, Morizawa Y, Hori S, et al. Diagnostic and prognostic role of urinary collagens in primary human bladder cancer. Cancer Sci. 2017;108(11):2221–2228. | ||
Rosser CJ, Chang M, Dai Y, et al. Urinary protein biomarker panel for the detection of recurrent bladder cancer. Cancer Epidemiol Biomarkers Prev. 2014;23(7):1340–1345. | ||
Shimizu Y, Furuya H, Bryant Greenwood P, et al. A multiplex immunoassay for the non-invasive detection of bladder cancer. J Transl Med. 2016;14(1):31. | ||
Miyake M, Sugano K, Sugino H, et al. Fibroblast growth factor receptor 3 mutation in voided urine is a useful diagnostic marker and significant indicator of tumor recurrence in non-muscle invasive bladder cancer. Cancer Sci. 2010;101(1):250–258. | ||
Kandimalla R, Masius R, Beukers W, et al. A 3-plex methylation assay combined with the FGFR3 mutation assay sensitively detects recurrent bladder cancer in voided urine. Clin Cancer Res. 2013;19(17):4760–4769. | ||
Guancial EA, Werner L, Bellmunt J, et al. FGFR3 expression in primary and metastatic urothelial carcinoma of the bladder. Cancer Med. 2014;3(4):835–844. | ||
Ross JS, Wang K, Al-Rohil RN, et al. Advanced urothelial carcinoma: next-generation sequencing reveals diverse genomic alterations and targets of therapy. Mod Pathol. 2014;27(2):271–280. | ||
Tomlinson DC, Baldo O, Harnden P, Knowles MA. FGFR3 protein expression and its relationship to mutation status and prognostic variables in bladder cancer. J Pathol. 2007;213(1):91–98. | ||
Zuiverloon TC, van der Aa MN, van der Kwast TH, et al. Fibroblast growth factor receptor 3 mutation analysis on voided urine for surveillance of patients with low-grade non-muscle-invasive bladder cancer. Clin Cancer Res. 2010;16(11):3011–3018. | ||
Fernandez CA, Millholland JM, Zwarthoff EC, Feldman AS, Karnes RJ, Shuber AP. A noninvasive multi-analyte diagnostic assay: combining protein and DNA markers to stratify bladder cancer patients. Res Rep Urol. 2012;4:17–26. | ||
Roperch JP, Grandchamp B, Desgrandchamps F, et al. Promoter hypermethylation of HS3ST2, SEPTIN9 and SLIT2 combined with FGFR3 mutations as a sensitive/specific urinary assay for diagnosis and surveillance in patients with low or high-risk non-muscle-invasive bladder cancer. BMC Cancer. 2016;16(1):704. | ||
O’Sullivan P, Sharples K, Dalphin M, et al. A multigene urine test for the detection and stratification of bladder cancer in patients presenting with hematuria. J Urol. 2012;188(3):741–747. | ||
Lotan Y, OʼSullivan P, Raman JD, et al. Clinical comparison of noninvasive urine tests for ruling out recurrent urothelial carcinoma. Urol Oncol. 2017;35(8):531.e15–531.e22. | ||
Kavalieris L, O’Sullivan P, Frampton C, et al. Performance characteristics of a multigene urine biomarker test for monitoring for recurrent urothelial carcinoma in a multicenter study. J Urol. 2017;197(6):1419–1426. | ||
van Valenberg FJP, Bridge JA, Mayne D, et al. Validation of a mRNA-based urine test for bladder cancer detection in patients with hematuria. Eur Urol Suppl. 2017;16(3):e190–e191. | ||
Pichler R, Fritz J, Tulchiner G, et al. Increased accuracy of a novel mRNA-based urine test for bladder cancer surveillance. BJU Int. 2018;121(1):29–37. | ||
Gazzaniga P, Gradilone A, de Berardinis E, et al. Prognostic value of circulating tumor cells in nonmuscle invasive bladder cancer: a CellSearch analysis. Ann Oncol. 2012;23(9):2352–2356. | ||
Rink M, Chun FK, Minner S, et al. Detection of circulating tumour cells in peripheral blood of patients with advanced non-metastatic bladder cancer. BJU Int. 2011;107(10):1668–1675. | ||
Msaouel P, Koutsilieris M. Diagnostic value of circulating tumor cell detection in bladder and urothelial cancer: systematic review and meta-analysis. BMC Cancer. 2011;11(1):336. | ||
Lodde M, Mian C, Wiener H, Haitel A, Pycha A, Marberger M. Detection of upper urinary tract transitional cell carcinoma with ImmunoCyt: a preliminary report. Urology. 2001;58(3):362–366. | ||
Chen GL, El-Gabry EA, Bagley DH. Surveillance of upper urinary tract transitional cell carcinoma: the role of ureteroscopy, retrograde pyelography, cytology and urinalysis. J Urol. 2000;164(6):1901–1904. | ||
Bier S, Hennenlotter J, Esser M, et al. Performance of urinary markers for detection of upper tract urothelial carcinoma: is upper tract urine more accurate than urine from the bladder? Dis Markers. 2018;2018(4):5823870. | ||
Marín-Aguilera M, Mengual L, Ribal MJ, et al. Utility of fluorescence in situ hybridization as a non-invasive technique in the diagnosis of upper urinary tract urothelial carcinoma. Eur Urol. 2007;51(2):409–415. | ||
Coskuner E, Cevik I, Ozkan A, Dillioglugil O, Akdas A. In the cystoscopic follow-up of non-muscle-invasive transitional cell carcinoma, NMP-22 works for high grades, but unreliable in low grades and upper urinary tract tumors. Int Urol Nephrol. 2012;44(3):793–798. | ||
Walsh IK, Keane PF, Ishak LM, Flessland KA. The BTA stat test: a tumor marker for the detection of upper tract transitional cell carcinoma. Urology. 2001;58(4):532–535. | ||
Tao J, Yang X, Li P, et al. Identification of circulating microRNA signatures for upper tract urothelial carcinoma detection. Mol Med Rep. 2015;12(5):6752–6760. | ||
Kriebel S, Schmidt D, Holdenrieder S, et al. Analysis of tissue and serum microRNA expression in patients with upper urinary tract urothelial cancer. PLoS One. 2015;10(1):e0117284. | ||
Montalbo R, Izquierdo L, Ingelmo-Torres M, et al. Prognostic value of circulating microRNAs in upper tract urinary carcinoma. Oncotarget. 2018;9(24):16691–16700. | ||
Chang WS, Liao CH, Hsu CM, et al. Significant association of cyclo-oxygenase 2 genotypes with upper tract urothelial cancer. Anticancer Res. 2015;35(5):2725–2730. | ||
Chang WS, Lin SS, Li FJ, et al. Significant association of caveolin-1 (CAV1) genotypes with upper urothelial tract cancer. Anticancer Res. 2013;33(11):4907–4912. | ||
Lin HH, Ke HL, Hsiao KH, et al. Potential role of CCND1 G870A genotype as a predictor for urothelial carcinoma susceptibility and muscle-invasiveness in Taiwan. Chin J Physiol. 2011;54(3):196–202. | ||
Lin HH, Hl K, Hsiao KH, et al. CCND1 1722 polymorphism and potential relevance to upper tract urothelial cancer. Anticancer Res. 2011;31(3):1043–1047. | ||
Wang K, Liu T, Ge N, et al. TERT promoter mutations are associated with distant metastases in upper tract urothelial carcinomas and serve as urinary biomarkers detected by a sensitive castPCR. Oncotarget. 2014;5(23):12428–12439. | ||
Monteiro-Reis S, Leça L, Almeida M, et al. Accurate detection of upper tract urothelial carcinoma in tissue and urine by means of quantitative GDF15, TMEFF2 and VIM promoter methylation. Eur J Cancer. 2014;50(1):226–233. | ||
Guo RQ, Xiong GY, Yang KW, et al. Detection of urothelial carcinoma, upper tract urothelial carcinoma, bladder carcinoma, and urothelial carcinoma with gross hematuria using selected urine-DNA methylation biomarkers: a prospective, single-center study. Urol Oncol. 2018;36(7):342.e15–342.e23. | ||
Bagrodia A, Cha EK, Sfakianos JP, et al; Collaborators. Genomic biomarkers for the prediction of stage and prognosis of upper tract urothelial carcinoma. J Urol. 2016;195(6):1684–1689. | ||
Lyle SR, Hsieh CC, Fernandez CA, Shuber AP. Molecular grading of tumors of the upper urothelial tract using FGFR3 mutation status identifies patients with favorable prognosis. Res Rep Urol. 2012;4:65–69. | ||
Silverberg DM. Urothelial carcinoma of the upper urinary tract diagnosed via FGFR3 mutation detection in urine: a case report. BMC Urol. 2012;12(1):20. | ||
Agarwal N, Pal SK, Hahn AW, et al. Characterization of metastatic urothelial carcinoma via comprehensive genomic profiling of circulating tumor DNA. Cancer. 2018;124(10):2115–2124. | ||
Sfakianos JP, Cha EK, Iyer G, et al. Genomic characterization of upper tract urothelial carcinoma. Eur Urol. 2015;68(6):970–977. | ||
Miyake M, Goodison S, Rizwani W, Ross S, Bart Grossman H, Rosser CJ. Urinary BTA: indicator of bladder cancer or of hematuria. World J Urol. 2012;30(6):869–873. | ||
Miyake M, Goodison S, Giacoia EG, Rizwani W, Ross S, Rosser CJ. Influencing factors on the NMP-22 urine assay: an experimental model. BMC Urol. 2012;12(1):23. | ||
Fantony JJ, Inman BA. It may be time to abandon urine tests for bladder cancer. J Natl Compr Canc Netw. 2015;13(9):1163–1166. | ||
Robertson AG, Kim J, Al-Ahmadie H, et al. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell. 2018;174(4):1033. | ||
Cowan NC, Turney BW, Taylor NJ, McCarthy CL, Crew JP. Multidetector computed tomography urography for diagnosing upper urinary tract urothelial tumour. BJU Int. 2007;99(6):1363–1370. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.