Back to Journals » OncoTargets and Therapy » Volume 13
Embelin Promotes Oncolytic Vaccinia Virus-Mediated Antitumor Immunity Through Disruption of IL-6/STAT3 Signaling in Lymphoma
Authors Wang P, Wu Y, Yang C, Zhao G, Liu Y, Cheng G, Wang S
Received 17 August 2019
Accepted for publication 29 December 2019
Published 17 February 2020 Volume 2020:13 Pages 1421—1429
DOI https://doi.org/10.2147/OTT.S209312
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Gaetano Romano
Peng Wang,1 Yi Wu,2 Chen Yang,3 Guanan Zhao,4 Yonghua Liu,5 Gang Cheng,6 Shibing Wang7,8
1Medical Laboratory Center, Lishui City People’s Hospital, Lishui, People’s Republic of China; 2Department of Hematology, Zhejiang Provincial People’s Hospital, People’s Hospital of Hangzhou Medical College, Hangzhou, People’s Republic of China; 3Department of Clinical Medicine, Qingdao University, Qingdao, People’s Republic of China; 4Department of General Surgery, Lishui City People’s Hospital, Lishui, People’s Republic of China; 5Department of Hematology, Lishui City People’s Hospital, Lishui, People’s Republic of China; 6Department of Stomatology, Zhejiang Provincial People’s Hospital, People’s Hospital of Hangzhou Medical College, Hangzhou, People’s Republic of China; 7Key Laboratory of Tumor Molecular Diagnosis and Individualized Medicine of Zhejiang Province, Zhejiang Provincial People’s Hospital, People’s Hospital of Hangzhou Medical College, Hangzhou, People’s Republic of China; 8Clinical Research Institute, Zhejiang Provincial People’s Hospital, People’s Hospital of Hangzhou Medical College, Hangzhou, People’s Republic of China
Correspondence: Gang Cheng
Department of Stomatology, Zhejiang Provincial People’s Hospital, People’s Hospital of Hangzhou Medical College, No. 158 Shangtang Road, Hangzhou 310014, People’s Republic of China
Email [email protected]
Shibing Wang
Clinical Research Institute, Zhejiang Provincial People’s Hospital People’s Hospital of Hangzhou Medical College, No. 158 Shangtang Road, Hangzhou 310014, People’s Republic of China
Tel/Fax +86-571-85893781
Email [email protected]
Objective: Oncolytic virotherapy is a promising alternative to conventional treatment, yet limited viral replication and immune-negative feedback are the major hurdles to effective viro-immunotherapy.
Methods: In this study, we found that use of an adjuvant of embelin, a small molecular inhibitor of XIAP, increased the replication of oncolytic vaccinia virus (OVV) by mitigating antiviral innate immunity. Moreover, embelin suppresses constitutive STAT3 phosphorylation and mitigates OVV-induced activation of STAT3 in lymphoma. In the subcutaneous lymphoma model, embelin significantly enhanced the therapeutic efficacy of OVV and prolonged the survival. In addition, embelin significantly increased the OVV-induced infiltration of T cells and NK cells and decreased the number of OVV-induced myeloid-derived suppressor cells (MDSCs) in the tumor microenvironment.
Results: Our results explored the ability of OVV and embelin in combination to enhance lymphoma cell lysis, revealing a beneficial combinatorial effect wherein both lymphoma cell lysis and OVV replication were enhanced both in vitro and in an in vivo murine model system.
Conclusion: Our findings indicate the utility of embelin as an adjuvant for oncolytic viro-immunotherapy.
Keywords: oncolytic vaccinia virus, embelin, IL-6/STAT3, immunotherapy, lymphoma
Introduction
A substantial volume of recent research has focused on a novel strategy of combating cancer through the use of oncolytic viruses, leading to ongoing clinical efforts to generate a range of viruses suited to specific therapeutic applications.1 Such oncolytic virotherapy functions via usage of viruses which are able to replicate within and destroy cancer cells.2–4 At present, OncoVEXGM-CSF, which is a genetically modified form of herpes simplex virus-1 (HSV-1) that has been engineered to express granulocyte-macrophage colony-stimulating factor (GM-CSF), has shown great promise as a therapeutic strategy.5,6 These oncolytic viral particles are capable of engaging a robust immune response in the tumor microenvironment (TME) that aids in cancer elimination through the activation of both type I interferon (IFN) signaling, which can drive lymphocyte infiltration of the tumor, and cell death, allowing for immune recognition of tumor antigens in the process.7,8 A number of different viruses have been tested in oncolytic applications, with many including vaccinia virus (VV) having shown promise as potentially viable therapeutic tools.9–11
VV is a poxvirus that offers several attractive features for cancer gene therapy applications, owing to its expression of a phosphotransferase gene (TK) that is essential for the replication of the virus. Disruption of this gene prevents VV from replicating in non-dividing cells. Cancer cells, in contrast, have high levels of the functional nucleotides needed for VV replication, thereby allowing for selective VV replication within the tumor and not in healthy tissues. In addition, with a relatively large 200 kb genome VV is amenable to the insertion of up to 25 kb of additional genomic material.12 It has been found to be highly safe,13 while readily being able to facilitate the expression of transgenes and to function in a hypoxic environment.14 Gene therapy efforts dependent upon VV have been successful in achieving effective disruption of tumor cell growth within causing significant death of healthy cells in models of myeloma,15 pancreatic carcinoma,16,17 hepatocellular carcinoma,18 and gastric carcinoma.19 However, the efficacy of oncolytic cancer treatment strategies can be severely constrained by both impaired viral replication and negative feedback responses within the TME.
Embelin (2,5-dihydroxy-3-undecyl-1,4-benzoquinone) is a compound often used in a traditional medicinal capacity which is isolated from the fruit of the Embelia ribes Burm plant. Previous work has suggested that embelin may possess anti-cancer activity,20 and may further be capable of reducing inflammation.21 Embelin can further serve to inhibit the X chromosome-linked inhibitor-of-apoptosis protein,22 promoting the apoptotic death of tumor cells via interfering with nuclear factor-kappa B (NF-κB) signaling,23 in addition to driving TRAIL-mediated apoptosis,24 inhibiting STAT3 activation,25 and blocking Akt/mTOR/S6K1 signaling.26
In the previous work, we have determined that embelin is capable of rendering acute myeloid leukemia (AML) cells more sensitive to trail via repressing NF-κB both in vitro and in vivo,27 which raises the possibility that embelin might be combined with oncolytic viruses in order to enhance their therapeutic utility as a means of treating this cancer. However, at present how embelin directly affects OVV replication and functionality remains uncertain. Owing to the previous work demonstrating its robust ability to modulate cellular signaling, thereby potentially helping to overcome detrimental immunosuppression, we sought to determine whether embelin and OVV could be combined to enhance viral replication while simultaneously favoring immune activation rather than suppression in the context of lymphoma.
Materials and Methods
Cell Cultures and Viruses
Human lymphoma cell lines (Raji and SU-DHL-4) and murine lymphoma cell line (EL4 and A20) were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China), retained in our laboratory, and cultured in RPMI-1640 supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin solution in a humidifying environment with 5% CO2. The oncolytic vaccinia virus (OVV) have been reported in our previous work.4,17
MTT Cell Viability Assay
Cells were seeded in 96-well plates and treated with embelin, OVV, or both at the indicated doses. Cell viability was determined after 48 hrs of incubation by adding 100 µL MTT solution (1 mg/mL). Following 4 hrs incubation at 37°C, the MTT solution was aspirated and 150 µL isopropanol were added to solubilize formazan, followed by shaking for 15 min. The absorbance from the plates was read at 595 nm with Cytation 3 Multi-Mode Reader (BioTek, Vermont, United States).
Trypan Blue Exclusion
Cells were harvested using trypsin-EDTA (0.25%) solution and stained with trypan blue solution; viability was determined by trypan blue exclusion using the Countstar Automated Cell Counter (Inno-Alliance Biotech, Wilmington, DE, USA). The cell death (%) was calculated as number of dead cells/total number of cells × 100%. The cell activity (%) was calculated as number of live cells/total number of cells × 100%.
Western Blot Analysis
For detection of proteins, embelin or OVV-treated whole cell extracts were lysed in a lysis buffer (20 mM Tris (pH 7.4), 250 mM NaCl, 2 mM EDTA (pH 8.0), 0.1% Triton X-100, 0.01 mg/mL aprotinin, 0.005 mg/mL leupeptin, 0.4 mM phenyl methane sulfonyl fluoride (PMSF), and 4 mM NaVO4). The lysates were then spun at 14,000 rpm for 10 min to remove insoluble material and resolved on a 10% SDS-PAGE. After electrophoresis, the proteins were electrotransferred to a nitrocellulose membrane, blocked with 5% nonfat milk, and probed with anti‑STAT3 (dilution 1:100; cat. no. ab119352; Abcam, China), anti‑pSTAT3 (dilution 1:100; cat. no. ab76315; Abcam, China) or anti-GAPDH antibodies (1:1000; cat. no. ab128915; Abcam, China) overnight at 4°C. The blots were washed, exposed to HRP-conjugated secondary antibodies for 2 hrs, and finally examined by chemiluminescence.
Quantitative RT-PCR
Total cellular RNA was extracted with Trizol (#15596-026; Thermo Fisher Scientific, Invitrogen) and was reverse-transcribed using PrimeScriptTM RT Master Mix (#DRR036A, TaKaRa, Shiga, Japan). Quantitative PCR was performed using FastStart Universal SYBR Green Master Mix (#04913914001; Roche) on a ViiA 7 Real-Time PCR System (Applied Biosystems, Foster, CA, USA). Gene expression was calculated by the comparative Ct method and normalized to that of GAPDH.
ELISA
Cells were seeded into 6-well plates and were starved overnight, then the cells were treated with the indicated concentrations of embelin or OVV in DMEM medium for 48 hrs, the cell culture supernatant was collected, and the concentration of IL-6 was assessed using the IL-6 ELISA Kit according to the manufacturer’s protocol.
Animal Experiments and Tumor Models
Six- to eight-week-old male C57/BL6 mice were purchased from the Laboratory Animal Center of Zhejiang Chinese Medical University (Hangzhou, China), and were acclimated for 7 days in the laboratory before experimentation. This study was carried out in Laboratory Animal Center of Zhejiang Chinese Medical University, and the project was approved by the ethics committee of Zhejiang Provincial People’s Hospital and all procedures were in according to the Guide for the Care and Use of Laboratory Animals (National Academies Press, Washington, DC).
A20 (5 × 106 cells in 100 μL of PBS) was subcutaneously injected into the right flanks of mice. On days 7, the mice received intratumoral (it.) injection of 5 × 107 pfu OVV per mouse and/or intraperitoneal (IP.) injection of 1 mg/mouse embelin. The mice received equal volume of PBS were used as untreated control. Tumor volume was monitored every 3 days by caliper measurement and calculated by length × width × width/2. Mice were sacrificed at 10 days post-injection according to ethical instructions by carbon dioxide. Tumors were separated and tumor cells were harvested for monocyte analysis, qPCR and determination of virus titer. For immunohistochemical analysis, tumors were separated, fixed using 4% paraformaldehyde, embedded in paraffin, finally cut into 4-µm sections for immunohistochemical analysis assay according to the manufacturers’ instructions. Slides were incubated with primary antibody anti‑STAT3 (dilution 1:100; cat. no. ab119352; Abcam, China) and anti‑pSTAT3 (dilution 1:100; cat. no. ab76315; Abcam, China) overnight at 4°C, and then incubated with biotinylated secondary antibody (dilution 1:1000; cat. no. B2763; Thermo Fisher Scientific, Inc.) and further visualized using a diaminobenzidine (DAB) kit (Thermo Scientific, Inc.).
Flow Cytometry
Apoptosis staining Kit containing AnnexinV-FITC/PI (MultiSciences, Hangzhou, China) was used to detect cell apoptosis according to the manufacture’s protocol. Cells were stained with 5 μL Annexin V-FITC and 5 μL PI after treatment of embelin, OVV or embelin plus OVV and then keep in dark at room temperature for 15 min. After that, these cells were analyzed by flow cytometer (Novocyte 3130, ACEA Biosciences, Santiago, CA).
For immune activation experiments in vivo, tumor cells were harvested and washed twice with PBS and incubated with antibodies. Samples were subjected to flow cytometry using a flow cytometer (Novo cyte 3130, ACEA Biosciences, Santiago, CA), and data were analyzed using FlowJo software (v. 7.6.5, Tree Star, Ashland, OR, USA).
Statistical Analysis
Statistical analysis was carried out using SPSS 17.0 (IBM Corp., Armonk, NY). All continuous data were presented as the mean ± standard deviation (SD). Comparisons of data in flow cytometry were performed using a two-tailed Student’s t-test (unpaired) or two-way analysis of variance (ANOVA) with Tukey multiple comparisons. Survival was analyzed by Kaplan–Meier curves, and statistical analysis was conducted using Prism (GraphPad Software, Inc., San Diego, CA). P-values <0.05 were considered statistically significant.
Results
Embelin Facilitates Enhanced OVV-Mediated Lymphoma Cell Lysis
We observed dose-dependent cytotoxicity when using embelin and OVV together to kill both mouse and human lymphoma cells (Figure 1A and B). The ability of OVV to kill these tumor cells was clearly enhanced by embelin (Figure 1C and D). The combination of embelin and OVV also led to an increase in the frequency of apoptotic (annexin-V positive) tumor cells (Figure S1), consistent with an embelin-mediated enhancement of tumor cell apoptosis. These results thus confirm an ability of embelin to enhance the oncolytic activity of OVV in the context of lymphoma.
We next explore OVV replication in this same experimental context, revealing a 3- to 9-fold increase in OVV replication in lymphoma cells after 24 hrs if cells were also treated with embelin (20 μM) (Figure 2A). With respect to the levels of the innate antiviral cytokines Ifnb (IFN-β) and Cxcl10 (C-X-C motif chemokine 10), embelin did not affect viral infection (Figure S2), whereas it did significantly reduce the levels of both Ifnb and Cxcl10 12 hrs post-infection (Figure 2B and C), indicating that embelin may have the ability to mitigate antiviral immunity, thus enhancing viral replication. These results therefore indicate that embelin can enhance OVV-mediated oncolysis, enhancing the ability of OVV to replicate in mouse and human lymphoma cell lines.
Embelin Suppresses Both Baseline and OVV-Induced STAT3 Phosphorylation
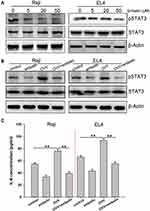
We next assessed the ability of embelin to alter the baseline phosphorylation status of STAT3 in lymphoma cells, given that this transcription factor is often constitutively active within these tumor cells. We observed a dose-dependent reduction in constitutive STAT3 phosphorylation in lymphoma cells treated with embelin, without any effect on overall STAT3 protein levels (Figure 3A).
STAT3 is a key regulator of inflammation in the context of cancer, driving immunosuppression in certain contexts. We found that OVV was able to induce STAT3 activation, whereas embelin markedly repressed this OVV-mediated STAT3 activation (Figure 3B). Furthermore, the pro-inflammatory cytokine IL-6, which is induced downstream of STAT3 and can enhance tumor growth, was induced by OVV, whereas embelin reduced its induction by 40–65% (Figure 3C). This thus indicates that embelin is capable of inhibiting inflammation induced by OVV at least in part via inhibiting STAT3 signaling within lymphoma cells.
Embelin Enhances OVV-Mediated Antitumor Immunity in a Xenograft Model of Lymphoma
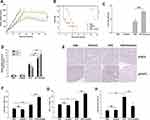
We next assessed the ability of embelin to enhance the ability of OVV to interfere with lymphoma in vivo using a xenograft model of subcutaneous lymphoma (Figure S3). In this model system, the combination of OVV and embelin was linked to significantly improved OVV efficacy and murine survival. We found that the combination of embelin and OVV treatment had the most prominent inhibitory effect on the tumor growth followed by embelin alone, and OVV (Figure 4A). Combined treatment has significantly prolonged survival (Figure 4B). We also explore OVV replication in the subcutaneous lymphoma tumor, revealing an obvious increase in OVV replication in lymphoma with combination therapy (Figure 4C). Consistently, Ifnb, Cxcl10, and Ifnb expression were significantly upregulated by OVV and further increased by embelin (Figure 4D). The tumor histopathological changes were further evaluated by immunohistochemistry (IHC). The combined treatment with OVV and embelin resulted in reduced expression of pSTAT3 in the tumor tissues was associated with the combined treatment as evidenced by IHC with anti‑pSTAT3, while there was no significant change in STAT3 expression (Figure 4E). Treatment of tumor-bearing mice with OVV led to increased TME infiltration by CD8+ T and NK1.1+ cells, and embelin further enhanced this effect (Figure 4F and G). This thus suggested that OVV efficacy is enhanced by embelin at least in part owing to improvements in tumor T and NK cell infiltration, in addition to modulations in innate antiviral cytokine expression.
LY6C+CD11b+LY6G− cells are a form of MDSC capable of significantly impairing immune responses in the context of antitumor immunity. We observed significant monocytic LY6C+CD11b+LY6G− MDSC polarization following OVV treatment, potentially interfering with antitumor immune responses (Figure 4H). When mice were also treated with embelin, the numbers of MDSCs decreased significantly. As MDSC differentiation is closely linked to the activity of STAT3, the increase STAT3 dephosphorylation induced by embelin may explain this difference in MDSC cell numbers.
Discussion
Herein we explored the ability of OVV and embelin in combination to enhance lymphoma cell lysis, revealing a beneficial combinatorial effect wherein both lymphoma cell lysis and OVV replication were enhanced both in vitro and in an in vivo murine model system. Together these findings provided evidence suggesting that embelin may be a valuable compound to use in combination with OVV therapy owing to its ability to synergistically enhance lymphoma clearance.
At present, efforts to clinically deploy oncolytic anti-tumor strategies have often been constrained by either negative regulation of the immune response and/or limited viral replication. In the present study, we determined that embelin was capable of both improving viral replication and oncolysis. Importantly, this compound was able to promote more rapid reductions in p-STAT3 levels, thereby helping to induce the infiltration of CD8+ and NK1.1+ cells into the TME, thus making this compound of particular promise in oncolytic therapeutic applications.
Oncolytic viruses must be able to efficiently replicate in cancer cells in order to achieve therapeutic success, and this replication is primarily governed by a combination of the tumor cell viability and innate antiviral immune responses. We determined embelin to be capable of enhancing OVV replication in lymphoma cells through an uncertain mechanism, without significantly impacting overall cell viability.
STAT3 activation can help mediate immunosuppression in the context of oncolytic therapy,28,29 with IFNs and viral infections being capable of inducing increased STAT3 activation in tumor cells and other cells in the TME,30 thus impairing oncolytic virotherapy outcomes. We found embelin to interfere IL-6/STAT3 signaling. Interestingly, the replication efficiency of oncolytic virus is closely related to the intensity of antiviral innate immune response in host cells. Our in vitro results showed that embelin can mitigate antiviral immunity to enhance the viral replication. On the other hand, IFNs and CXCL10 not only mediate antiviral immune response, but also play an important role in antitumor immune response. Our in vivo results showed that embelin enhanced OVV-mediated antitumor immunity. In short, embelin can promote OVV replication by inhibiting anti-virus immune factors IFN-beta and CXCL10 in vitro, while in vivo, embelin can enhance the anti-tumor immune effect of OVV by promoting virus replication, which is manifested by overexpression of IFNs and CXCL10. OVV is known to be capable of inducing local IFN production as well as immune cell infiltration and activation.31 When we locally injected OVV particles into lymphoma tissues, this mediated-local inflammation as well as CD8+ T and NK cell infiltration, and embelin enhanced these responses in a xenograft model system. NK cell activation can further enhance oncolytic virotherapy outcomes, and we observed superior NK cell activation following embelin treatment, which may have been a result of the higher levels of OVV replication observed.
In summary, OVV is capable of simultaneously inducing both anti-tumor immunity and negative feedback mechanisms within the TME through activation of STAT3. Embelin offers a means of increasing both OVV replication and STAT3 dephosphorylation while reducing MDSC infiltration, thus improving the survival of lymphoma model mice. Together these findings suggest embelin may be a viable adjuvant in the context of oncolytic virotherapy, although further validation of these findings is needed.
Acknowledgment
This article was supported by the National Science Foundation of China (No. 81602706), Funds of Science Technology Department of Zhejiang Province (No. 2018C37097), Funds of Science Technology Department of Lishui City (Nos. 2019ZDYF19, 2016RC23, 2016ZDXK03, 2015SJZC48), and State Administration of Traditional Chinese Medicine of Zhejiang (No. 2017ZB006).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Harrington K, Freeman DJ, Kelly B, Harper J, Soria JC. Optimizing oncolytic virotherapy in cancer treatment. Nat Rev Drug Discov. 2019;18:689–706. doi:10.1038/s41573-019-0029-0
2. Wang S-B, Tan Y, Lei W, et al. Complete eradication of xenograft hepatoma by oncolytic adenovirus ZD55 harboring TRAIL-IETD-Smac gene with broad antitumor effect. Hum Gene Ther. 2012;23:992–1002. doi:10.1089/hum.2011.159
3. Wang S, Shu J, Chen L, et al. Synergistic suppression effect on tumor growth of ovarian cancer by combining cisplatin with a manganese superoxide dismutase-armed oncolytic adenovirus. Onco Targets Ther. 2016;9:6381–6388. doi:10.2147/OTT
4. Peng J, Wang S, Fan W, et al. Synergistic suppression effect on tumor growth of acute myeloid leukemia by combining cytarabine with an engineered oncolytic vaccinia virus. Onco Targets Ther. 2018;11:6887–6900. doi:10.2147/OTT
5. Pol J, Kroemer G, Galluzzi L. First oncolytic virus approved for melanoma immunotherapy. Oncoimmunology. 2016;5:e1115641. doi:10.1080/2162402X.2015.1115641
6. Ribas A, Dummer R, Puzanov I, et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2017;170:1109–1119.e1110. doi:10.1016/j.cell.2017.08.027
7. Workenhe ST, Mossman KL. Oncolytic virotherapy and immunogenic cancer cell death: sharpening the sword for improved cancer treatment strategies. Mol Ther. 2014;22:251–256. doi:10.1038/mt.2013.220
8. Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov. 2015;14:642–662. doi:10.1038/nrd4663
9. Chon HJ, Lee WS, Yang H, et al. Tumor microenvironment remodeling by intratumoral oncolytic vaccinia virus enhances the efficacy of immune-checkpoint blockade. Clin Cancer Res. 2019;25:1612–1623. doi:10.1158/1078-0432.CCR-18-1932
10. Liu Z, Ravindranathan R, Kalinski P, Guo ZS, Bartlett DL. Rational combination of oncolytic vaccinia virus and PD-L1 blockade works synergistically to enhance therapeutic efficacy. Nat Commun. 2017;8:14754. doi:10.1038/ncomms14754
11. Feola S, Capasso C, Fusciello M, et al. Oncolytic vaccines increase the response to PD-L1 blockade in immunogenic and poorly immunogenic tumors. Oncoimmunology. 2018;7:e1457596. doi:10.1080/2162402X.2018.1457596
12. Worschech A, Haddad D, Stroncek DF, et al. The immunologic aspects of poxvirus oncolytic therapy. Cancer Immunol Immunother. 2009;58:1355–1362. doi:10.1007/s00262-009-0686-7
13. Poland GA, Grabenstein JD, Neff JM. The US smallpox vaccination program: a review of a large modern era smallpox vaccination implementation program. Vaccine. 2005;23:2078–2081. doi:10.1016/j.vaccine.2005.01.012
14. Hiley CT, Yuan M, Lemoine NR, Wang Y. Lister strain vaccinia virus, a potential therapeutic vector targeting hypoxic tumours. Gene Ther. 2010;17:281–287. doi:10.1038/gt.2009.132
15. Lei W, Wang S, Yang C, et al. Combined expression of miR-34a and Smac mediated by oncolytic vaccinia virus synergistically promote anti-tumor effects in multiple myeloma. Sci Rep. 2016;6:32174. doi:10.1038/srep32174
16. Chen W, Fan W, Ru G, et al. Gemcitabine combined with an engineered oncolytic vaccinia virus exhibits a synergistic suppressive effect on the tumor growth of pancreatic cancer. Oncol Rep. 2018. doi:10.3892/or.2018.6817
17. Wu Y, Mou X, Wang S, Liu XE, Sun X. ING4 expressing oncolytic vaccinia virus promotes anti-tumor efficiency and synergizes with gemcitabine in pancreatic cancer. Oncotarget. 2017;8:82728.
18. Pan Q, Huang Y, Chen L, Gu J, Zhou X. SMAC-armed vaccinia virus induces both apoptosis and necroptosis and synergizes the efficiency of vinblastine in HCC. Hum Cell. 2014;27:162–171. doi:10.1007/s13577-014-0093-z
19. Jun KH, Gholami S, Song TJ, et al. A novel oncolytic viral therapy and imaging technique for gastric cancer using a genetically engineered vaccinia virus carrying the human sodium iodide symporter. J Exp Clin Cancer Res. 2014;6:2. doi:10.1186/1756-9966-33-2
20. Allensworth JL, Aird KM, Aldrich AJ, Batinic-Haberle I, Devi GR. XIAP inhibition and generation of reactive oxygen species enhances TRAIL sensitivity in inflammatory breast cancer cells. Mol Cancer Ther. 2012;11:1518–1527. doi:10.1158/1535-7163.MCT-11-0787
21. Kalyan Kumar G, Dhamotharan R, Kulkarni NM, Mahat MY, Gunasekaran J, Ashfaque M. Embelin reduces cutaneous TNF-alpha level and ameliorates skin edema in acute and chronic model of skin inflammation in mice. Eur J Pharmacol. 2011;662:63–69. doi:10.1016/j.ejphar.2011.04.037
22. Nikolovska-Coleska Z, Xu L, Hu Z, et al. Discovery of embelin as a cell-permeable, small-molecular weight inhibitor of XIAP through structure-based computational screening of a traditional herbal medicine three-dimensional structure database. J Med Chem. 2430-2440;47:2430–2440.
23. Reuter S, Prasad S, Phromnoi K, et al. Embelin suppresses osteoclastogenesis induced by receptor activator of NF-κB ligand and tumor cells in vitro through inhibition of the NF-κB cell signaling pathway. Mol Cancer Res Mcr. 2010;8:1425. doi:10.1158/1541-7786.MCR-10-0141
24. Mori T, Doi R, Kida A, et al. Effect of the XIAP inhibitor embelin on TRAIL-induced apoptosis of pancreatic cancer cells. J Surg Res. 2007;142:281–286. doi:10.1016/j.jss.2007.03.068
25. Ji YH, Kim HJ, Kim S-M, et al. Embelin suppresses STAT3 signaling, proliferation, and survival of multiple myeloma via the protein tyrosine phosphatase PTEN. Cancer Lett. 2011;308:71–80. doi:10.1016/j.canlet.2011.04.015
26. Kim SW, Kim SM, Hang B, Nam D, Ahn KS. Embelin inhibits growth and induces apoptosis through the suppression of Akt/mTOR/S6K1 signaling cascades. Prostate. 2013;73:296–305. doi:10.1002/pros.22574
27. Yang T, Lan J, Huang Q, et al. Embelin sensitizes acute myeloid leukemia cells to TRAIL through XIAP inhibition and NF-κB inactivation. Cell Biochem Biophys. 2015;71:291–297. doi:10.1007/s12013-014-0197-9
28. Ludwig H, Nachbaur DM, Fritz E, Krainer M, Huber H. Interleukin-6 is a prognostic factor in multiple myeloma. Blood. 1991;77:2794–2795. doi:10.1182/blood.V77.12.2794.2794
29. Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nature Rev Cancer. 2009;9:798–809. doi:10.1038/nrc2734
30. Esposito CL, Nuzzo S, Catuogno S, et al. STAT3 gene silencing by aptamer-siRNA chimera as selective therapeutic for glioblastoma. Mol Ther Nucleic Acids. 2018;10:398–411. doi:10.1016/j.omtn.2017.12.021
31. Symons JA, Alcamí A, Smith GL. Vaccinia virus encodes a soluble type I interferon receptor of novel structure and broad species specificity. Cell. 1995;81:551–560. doi:10.1016/0092-8674(95)90076-4
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.