Back to Journals » Journal of Inflammation Research » Volume 12
ELR positive CXCL chemokines are highly expressed in an animal model of ulcerative colitis
Authors Boshagh MA , Foroutan P, Moloudi MR , Fakhari S , Malakouti P, Nikkhoo B, Jalili A
Received 1 February 2019
Accepted for publication 16 May 2019
Published 25 June 2019 Volume 2019:12 Pages 167—174
DOI https://doi.org/10.2147/JIR.S203714
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Mohammad Amin Boshagh,1,2 Poorya Foroutan,1,2 Mohammad Raman Moloudi,3 Shohreh Fakhari,1 Parisa Malakouti,1 Bahram Nikkhoo,1 Ali Jalili1,2
1Cancer and Immunology Research Center, Kurdistan University of Medical Sciences, Sanandaj, Iran; 2Department of Immunology & Hematology, Faculty of Medicine, Kurdistan University of Medical Sciences, Sanandaj, Iran; 3Liver and Digestive Research Center, Kurdistan University of Medical Sciences, Sanandaj, Iran
Background: The presence of neutrophil-rich inflammation in colon tissues of patients with ulcerative colitis (UC) is one of the most important histological characteristics of this disease. However, the expression of CXCL chemokines governing the infiltration of neutrophils in UC has not been well elucidated.
Materials and methods: In this experimental study, the UC model was induced in Wistar rats by administration of 2 mL 4% acetic acid into the large colon through the rectum. Animals were anesthetized after 48 hrs; their colon tissue samples were isolated for macroscopic and histopathological examinations. The expression of CXCL family was assessed by reverse transcription polymerase chain reaction (qRT-PCR) technique.
Results: Heavy infiltration of neutrophils, coagulation necrosis, and ulcers were observed in H&E staining, which pathologically proved the UC model. qRT-PCR results showed that ELR+, CXC chemokines such as CXCL6 and CXCL3 had the highest expression in the UC group, which was 49 and 28 times higher than that of the control group, respectively. In addition, other chemokines of this group including CXCL1, CXCL2, and CXCL7 had a significant increase compared to the control group (P≤0.05). However, ELR−, CXC chemokines such as CXCL4, CXCL13, and CXCL16 showed a smaller upregulation, while CXCL14 chemokine showed a significant decrease compared to the control group (P≤0.05). However, the expression of CXCL9-12 and CXCL17 did not change.
Conclusion: The results showed that the ELR+, CXC chemokines, especially CXCL6 and CXCL3, many involved in the pathogenesis of UC; therefore, CXCL6 and CXCL3 chemokines can be used as therapeutic targets for UC, although more studies using human samples are required.
Keywords: ELR+, CXC chemokines, chemokine, CXCLs, ulcerative colitis
Introduction
Inflammatory bowel diseases (IBD) include Crohn’s disease (CD) and ulcerative colitis (UC), are associated with recurrent gastrointestinal inflammation.1 The inflammation causes acute gastrointestinal symptoms, such as diarrhea, vomiting, abdominal pain, and bleeding, which leads to impairment in the structure and function of the gastrointestinal tract.2 CD may occur in any part of the digestive tract and affects all layers of the intestinal. In contrast, UC only affects the colon and its inflammatory response is exclusively restricted to the mucosa and sometimes submucosa, and neutrophils are the dominant infiltrated cells in the affected areas.3–5 Excessive infiltration of inflammatory cells results in the production of various inflammatory mediators which in turn leads to infiltration of leukocytes into large intestine mucosa. As the result of chronic inflammation, collagen and components of the extracellular matrix are accumulated in the affected area, and the release of reactive oxygen species aggravates tissue degradation which ultimately leads to damage of mucosa and epithelial ducts in the patients.4,6
Chemokines are a group of proteins (8–12 KDs) responsible for transmitting signals for cell migration, inflammation regulation, and angiogenesis.7,8 Forty-five chemokines have been recognized in humans, which are divided into several families according to the order of their amino acids. The family of CXC ligands contains two cysteine molecules separated by another amino acid (X). The CXC family receptor is designated as CXCR and its ligand as CXCL.9,10 There are 17 ligands for CXC family chemokines in humans, which are named CXCL1 to CXCL17, respectively. CXC chemokines can be classified into two categories: ELR+ CXC family which structurally characterized by the presence of a Glu-Leu-Arg tripeptide motif in their N terminal and includes CXCL1-3, CXCL5, CXCL6, CXCL7, and CXCL8. However, ELR− CXC family lack this tripeptide motif and mainly include CXCL9-11.11–13 Singh et al have recently measured the levels of many chemokines and cytokines in the serum of patients with IBD, and reported that many of them such as CXCL5, CXCL13, CXCL10, CCL21, CCL25, CCL23, MCP1 as well as cytokines such as IL16, IFN-γ, and IL-1β were increased in the patients than the normal individuals.14 So far, the majority of studies have measured the levels of a few chemokines, but not all, in the serum of patients with IBD. Herein, we investigated expressions of CXCLs at mRNA levels in an animal model of UC and reported that all five ELR+ CXCL and only 3 of 9 ELR− CXCL chemokines are significantly upregulated in colon tissue of animal model of UC.
Materials and methods
Induction of UC
UC was induced in 6 adult male Wistar rats weighing 300–350 g and 6 rats with similar weights were used as the control. The colitis was induced three times (each time 2 rats) in 6 rats. All experiments were performed according to animal ethics guidelines and regulation of Kurdistan University of Medical Sciences. Animals were kept in proper laboratory conditions, standard light conditions and 12 hrs light/dark cycle, relative humidity, and continuous identical access to water and food. The UC model was induced as previously reported.15 All the rats were exposed to Nil per oscondition for 24 hrs. The rats were subject to mild inhaler anesthesia using chloroform. In the UC group, 2 mL of 4% acetic acid (pH=2.3) was injected into the large colon through the rectum by a plastic tube called NG tube (internal diameter of 2 mL, 8 cm of which enters the colon) and washed using 2 mL normal saline through the same NG tube. Control rats were only infused and washed with normal saline. Since the inflammation and tissue damage is at the highest level 48 hrs after the induction of UC,16 the animals were euthanized and appropriate samples were taken.
Sample preparation
A longitudinal incision was made using a surgical blade on the abdomen until perineum, and the 6-cm end of animal’s colon was separated at a distance of 1 cm from the anal sphincter. The ratio of colon weight to length (g/cm) was determined by dividing the weight of 6-cm isolated colon (g)/6 cm. The colon tissue was placed in an RNase free microtube and immediately stored at −70°C freezer for later use.
Histological analysis
The colon tissue was examined macroscopically for inflammation and ulcer. For microscopic analysis, the colon tissue was kept in formalin, and H&E staining was used for the detection of inflammation and verification of the model. In order to confirm UC, the microscopic analysis was performed to study WBC infiltration, ulcers, and tissue necrosis by our pathologist (BN) who was blind to each group.
RNA extraction and quantitative real time PCR
RNA of tissue samples was extracted using an RNA isolation kit (#740955.50; NucleoSpin RNA, Germany) and cDNA synthesis was performed for qRT-PCR using PrimeScriptTM RT reagent Kit (#RR037A; Takara, Japan). Real-time quantitative reverse transcription-PCR (real-time qRT-PCR) was performed by Corbett rotor gene 6,000 Real-Time PCR system (Corbett Research, Australia) and carried out using the SYBR Green dye detection protocol. The primers designed by Gene Runner and listed in Table 1. Amplification condition was as follows: initial denaturation at 94°C for 30 s followed by 40 cycles of denaturation at 95°C for 5 s, annealing temperature at 55°C for 30 s, extension temperature at 72°C for 30 s. Final extension temperature was at 72°C for 5 mins. To determine the differences between CXCLs expression (fold changes) in the UC and the control group, the expression of CXCLs genes was quantified in both groups as previously described.17 Briefly, the mean±SEM of each CXCL expression in each group was calculated using 2-ΔCT [2 - (CT CXCL – CT GAPDH)]. Then, the difference in the CXCLs expression in the UC group was compared to the control and fold changes were calculated using this formula: 2-ΔCT UC group/2-ΔCT control. GAPDH was used as the housekeeping gene.
 | Table 1 Primers used in this study |
Statistical analysis
In this study, SPSS version 20 (IBM Corporation, Armonk, NY, USA) and paired t-test were used to compare the results of gene expressions and colon weights of rats in UC and control groups (P≤0.05).
Results
Macroscopic examination of colon tissue
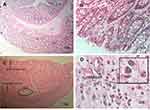
Our macroscopic examinations showed that colon structure including thickening of the colon, extensive hemorrhagic ulcers, bloody mucus, and sometimes widespread necrosis was present in the UC group. In contrast, there was no ulcer or inflammation in the control group (Figure 1A). Then, the weight to length ratio of colon tissue was evaluated in both groups and as shown in Figure 1B, this ratio was much higher in the UC group than the normal group.
Histopathological examination
Our histopathological examinations revealed that epithelial destruction, ulcerated colon mucosa with coagulation necrosis and infiltration of WBC, in particular, neutrophils were observed in rats with the UC. In the control group, the natural tissue of colon consisted of mucosal, submandibular, muscular, and serous layers were present and there was no signature of inflammation or necrosis (Figure 2).
ELR+ CXC chemokines are upregulated in colon tissues of rat with UC
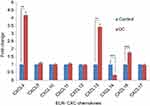
The expression levels ELR+ CXC chemokines at mRNA were detected by qRT-PCR. Our results showed increased expressions of all five members of the ELR+ CXC family in the colon samples of rats with the UC than the normal controls (Figure 3). The expression of CXCL6 and CXCL3 were remarkably upregulated, 49 and 28 folds, respectively. Moreover, the expression levels of the other three members (CXCL1, CXCL2, and CXCL7) of ELR+ CXCL family were significantly higher in the UC group than the control one.
Most of ELR− CXC chemokines were not upregulated in UC
Having shown that the expression levels of ELR+ CXC chemokines were significantly upregulated in UC, we next analyzed the levels of ELR− CXC chemokines at mRNA in this model. We observed that only 3 members of this family CXCL4 (4 times), CXCL13 (3.4 times), and CXCL16 (1.8 times) were significantly upregulated (Figure 4). In contrast, CXCL14 expression was significantly downregulated (0.32 times) in the UC model than the control group. In addition, there was no change in the expression of CXC9, CXCL10, CXCL11, CXCL12, and CXCL17 chemokines compared to the control group (Figure 4).
Discussion
Many studies have shown that production of a chemotactic gradient through the secretion of CXCL chemokines by intestinal epithelial cells may be one the mechanisms attracting leukocytes in particular neutrophils into the epithelial cell layer.18,19 Several ELR+ CXC chemokines are implicated in IBD; for example, CXCL1-2, CXCL5-7, and CXCL8 chemokines are significantly expressed in IBD-affected regions compared to normal tissues.20–22 Unlike ELR+ CXC chemokines, ELR− CXC chemokines have no chemotactic effect for neutrophils; however, they are highly reactive for memory T-cells and NK cells.23–25 Although a limited number of CXCL family have been shown to be involved in the process of immune cell migration into the inflamed tissue of patients with IBD, all of them have not been simultaneously investigated. In the current study using an animal model of UC, we showed that the expression levels of CXCL1-4, CXCL6-7, CXCL13, and CXCL16 chemokines were increased, the expressions of CXCL9, CXCL10, CXCL11, CXCL12, and CXCL17 chemokines had no change, and the expression of CXCL14 was decreased compared to the control group.
Egesten et al have demonstrated that CXCL1 expression was increased in patients with UC.26 However, another study showed that mice bearing a mutation in CXCL1 are more susceptible to UC and displayed deeper colitis, indicating that CXCL1 plays a protective role in recruiting neutrophils into the inflammatory sites.27 In contrast, in the absence of CXCL2, it has been shown that infection and inflammation of lungs are significantly reduced due to decreased neutrophil infiltration28 and conversely neutrophil invasion of lamina propria was significantly increased in CXCL2 transgenic mice.29 Accordingly, we observed in the current study that CXCL1 and CXCL2 expression were significantly upregulated in colon tissue of the rat with UC. So far, the role of CXCL3 in UC has not been yet studied and we are reporting it here, for the first time, that CXCL3 expression was 28 folds elevated in colon tissues of rats with UC. Although CXCL3 is an inflammatory ELR+ CXCL family and is known as one the chemoattractant factor of neutrophils, previous studies have also shown that CXCL3 is increased in human colon polyp and tumor compared to normal tissue.30 This is in agreement with our observation that CXCL3 is not only expressed in colon tissue of normal rat, but its expression upregulated after induction of UC, implying that CXCL3 may play a crucial role in the pathogenesis of inflammatory-based of UC.
Although CXCL4 or platelets factor 4 (PF4) is mainly secreted by alpha granules of platelets and involves in thrombosis, recent studies have reported that serum levels of CXCL4 are highly elevated in patients with IBD and CXCL4 levels show disease activity.31 In accordance with this previous observation, we demonstrated in the current study that CXCL4 expression was increased in colon tissue of rats with UC. Since patients with active IBD are at threefold risk for thrombosis than healthy people,32 we envision that induction of tissue necrosis and hemorrhage in colon tissue of rats with UC may result in upregulation of CXCL4. Moreover, CXCL6 has been shown to be expressed in 90% and 0–30% of epithelial cells from IBD patients and control group, respectively.33 In the line of the latter study, we observed that CXCL6 expression in colon tissues of rats with UC was 49 times more than the control highlighting the role of CXCL6 in the pathogenesis of UC. Moreover, in the early stages of bowel inflammation, pro-inflammatory cytokines such as IL-1 and TNF-α stimulate CXCL6 production, which is a potent chemotactic factor for neutrophils.34–36 In contrast, Alzoghaibi et al showed that serum CXCL6 is decreased in UC and CD patients than the normal individuals,37 indicating that CXCL6 levels in circulation do not represent its expression in colon epithelial cells.
Several studies have shown increasing expressions of CXCL9-12 chemokines in patients with UC and mice models of UC,38–44 while our results showed there are not any differences in the expression of these chemokines in the UC group compared with the control group. This inconsistency can be due to variations in the induced-model of UC and differences in the type and expression levels of the chemokines in humans and mice. In addition, neutrophils were dominant infiltrated cells in the colon tissues of our UC model. However, CXCL9-11 chemokines mainly recruit Th1 cells, monocytes, and NK cells.23 Carlsen et al showed that CXCL13 and CXCR5 not only expressed in GALT lymphoid follicles of the small and large intestine, but also they expressed in abnormal lymphoid aggregates of UC,45 which was in accordance with our study and suggested an increase in the expression of CXCL13 in the UC model. On the other hand, Legler et al reported the presence of CXCL13 in vermiform appendix and stomach but not in the colon or small intestine of humans.46 Yeung et al showed that in all cases of UC, lamina propria consisted of a number of basal aggregates including lymphocytes and follicular dendritic cells in comparison to healthy tissue.47 Therefore, this chemokine can be considered as one of the factors involved in inflammation and chemotaxis of immune cells in bowel inflammation.
CXCL14 is a homeostatic chemokine expressed by various types of epithelial cells, including basal keratinocytes and dermal fibroblasts. However, epithelial cells in the colon do not express this chemokine, which is exclusively expressed by LP cells and has chemotactic activity for monocytes but not neutrophil.48 In our study, the expression of CXCL14 in UC group was reduced compared to the control group, which could be due to the anti-inflammatory effects of this chemokine as the analog of C-terminal α-helix of CXCL14 possess anti-inflammatory property.49 It should be noted that there is insufficient information showing the role of CXCL14 in IBD and other inflammatory diseases which needs to be elucidated in the future. Although CXCL17 has been shown to be expressed in the gastrointestinal tract and lung,50 we observed that there is no difference in the expression levels of this chemokine in the rats with UC and the control.
In conclusion, the results of the current study showed that the ELR+ CXC chemokines, especially CXCL6 and CXCL3, may involve in the pathogenesis of UC; therefore, CXCL6 and CXCL3 chemokines could be used as therapeutic targets for UC, although more studies are required.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Disclosure
A grant from Kurdistan University of Medical Sciences was offered to AJ to purchase the materials used for this study. The grant was for supporting of MAB’s thesis as a master student in Medical Immunology. The authors report no other conflicts of interest in this work.
References
1. Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis. 2006;12(suppl_1):S3–S9. doi:10.1097/01.mib.0000195385.19268.68
2. Bernstein CN, Blanchard JF, Rawsthorne P, Wajda A. Epidemiology of Crohn’s disease and ulcerative colitis in a central Canadian province: a population-based study. Am J Epidemiol. 1999;149(10):916–924. doi:10.1093/oxfordjournals.aje.a009735
3. Monteleone G, Caruso R, Pallone F. Targets for new immunomodulation strategies in inflammatory bowel disease. Autoimmun Rev. 2014;13(1):11–14. doi:10.1016/j.autrev.2013.06.003
4. Bamias G, Pizarro TT, Cominelli F. Pathway-based approaches to the treatment of inflammatory bowel disease. Transl Res. 2016;167(1):104–115. doi:10.1016/j.trsl.2015.09.002
5. Mas-Moya J, Singhi AD. The gross pathology of inflammatory bowel disease. Diagn Histopathol. 2015;21(7):261–266. doi:10.1016/j.mpdhp.2015.07.001
6. Moura FA, de Andrade KQ, Dos Santos JCF, Araújo ORP, Goulart MOF. Antioxidant therapy for treatment of inflammatory bowel disease: does it work? Redox Biol. 2015;6:617–639. doi:10.1016/j.redox.2015.10.006
7. Sallusto F, Baggiolini M. Chemokines and leukocyte traffic. Nat Immunol. 2008;9(9):949. doi:10.1038/ni.f.214
8. Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392(6676):565. doi:10.1038/33340
9. Scholten D, Canals M, Maussang D, et al. Pharmacological modulation of chemokine receptor function. Br J Pharmacol. 2012;165(6):1617–1643. doi:10.1111/j.1476-5381.2011.01551.x
10. Chemokine C. Chemokine/chemokine receptor nomenclature. J Interferon Cytokine Res. 2002;22:1067–1068. doi:10.1089/107999002760624305
11. Clark-Lewis I, Dewald B, Geiser T, Moser B, Baggiolini M. Platelet factor 4 binds to interleukin 8 receptors and activates neutrophils when its N terminus is modified with Glu-Leu-Arg. Proc Natl Acad Sci. 1993;90(8):3574–3577. doi:10.1073/pnas.90.8.3574
12. Cotton J, Platnich J, Muruve D, Jijon H, Buret A, Beck P. Interleukin-8 in gastrointestinal inflammation and malignancy: induction and clinical consequences. Int J Interferon Cytokine Mediat Res. 2016;8:13–34.
13. Strieter RM, Polverini PJ, Kunkel SL, et al. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem. 1995;270(45):27348–27357. doi:10.1074/jbc.270.45.27348
14. Singh UP, Singh NP, Murphy EA, et al. Chemokine and cytokine levels in inflammatory bowel disease patients. Cytokine. 2016;77:44–49. doi:10.1016/j.cyto.2015.10.008
15. Mascolo N, Izzo AA, Autore G, Maiello FM, Di Carlo G, Capasso F. Acetic acid-induced colitis in normal and essential fatty acid deficient rats. J Pharmacol Exp Ther. 1995;272(1):469–475.
16. Randhawa PK, Singh K, Singh N, Jaggi AS. A review on chemical-induced inflammatory bowel disease models in rodents. Korean J Physiol Pharmacol. 2014;18(4):279–288. doi:10.4196/kjpp.2014.18.4.279
17. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C T method. Nat Protoc. 2008;3(6):1101. doi:10.1038/nprot.2008.73
18. Chin AC, Parkos CA. Pathobiology of neutrophil transepithelial migration: implications in mediating epithelial injury. Annu Rev Pathol Mech Dis. 2007;2:111–143. doi:10.1146/annurev.pathol.2.010506.091944
19. Kucharzik T, Walsh SV, Chen J, Parkos CA, Nusrat A. Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. Am J Pathol. 2001;159(6). doi:10.1016/S0002-9440(10)63051-9
20. Banks C, Bateman A, Payne R, Johnson P, Sheron N. Chemokine expression in IBD. Mucosal chemokine expression is unselectively increased in both ulcerative colitis and Crohn’s disease. J Pathol. 2003;199(1):28–35. doi:10.1002/(ISSN)1096-9896
21. Autschbach F, Giese T, Gassler N, et al. Cytokine/chemokine messenger-RNA expression profiles in ulcerative colitis and Crohn’s disease. Virchows Archiv. 2002;441(5):500–513. doi:10.1007/s00428-002-0684-z
22. Gijsbers K, Van Assche G, Joossens S, et al. CXCR1‐binding chemokines in inflammatory bowel diseases: down‐regulated IL‐8/CXCL8 production by leukocytes in Crohn’s disease and selective GCP‐2/CXCL6 expression in inflamed intestinal tissue. Eur J Immunol. 2004;34(7):1992–2000. doi:10.1002/eji.200324807
23. Cole KE, Strick CA, Paradis TJ, et al. Interferon–inducible T cell alpha chemoattractant (I-TAC): a novel Non-ELR CXC Chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J Exp Med. 1998;187(12):2009–2021. doi:10.1084/jem.187.12.2009
24. Cole AM, Ganz T, Liese AM, Burdick MD, Liu L, Strieter RM. Cutting edge: IFN-inducible ELR− CXC chemokines display defensin-like antimicrobial activity. J Immunol. 2001;167(2):623–627.
25. Song F, Ito K, Denning TL, et al. Expression of the neutrophil chemokine KC in the colon of mice with enterocolitis and by intestinal epithelial cell lines: effects of flora and proinflammatory cytokines. J Immunol. 1999;162(4):2275–2280.
26. Egesten A, Eliasson M, Olin AI, et al. The proinflammatory CXC-chemokines GRO-α/CXCL1 and MIG/CXCL9 are concomitantly expressed in ulcerative colitis and decrease during treatment with topical corticosteroids. Int J Colorectal Dis. 2007;22(12):1421–1427. doi:10.1007/s00384-007-0370-3
27. Shea-Donohue T, Thomas K, Cody MJ, et al. Mice deficient in the CXCR2 ligand, CXCL1 (KC/GRO-α), exhibit increased susceptibility to dextran sodium sulfate (DSS)-induced colitis. Innate Immun. 2008;14(2):117–124. doi:10.1177/1753425908088724
28. Standiford TJ, Kunkel SL, Lukacs NW, et al. Macrophage inflammatory protein-1 alpha mediates lung leukocyte recruitment, lung capillary leak, and early mortality in murine endotoxemia. J Immunol. 1995;155(3):1515–1524.
29. Ohtsuka Y, Lee J, Stamm D, Sanderson I. MIP-2 secreted by epithelial cells increases neutrophil and lymphocyte recruitment in the mouse intestine. Gut. 2001;49(4):526–533. doi:10.1136/gut.49.4.526
30. Drew JE, Mayer C-D, Farquharson AJ, Young P, Barrera LN. Custom design of a GeXP multiplexed assay used to assess expression profiles of inflammatory gene targets in normal colon, polyp, and tumor tissue. J Mol Diagn. 2011;13(2):233–242. doi:10.1016/j.jmoldx.2010.10.001
31. Ye L, Zhang Y-P, Yu N, Jia Y-X, Wan S-J, Wang F-Y. Serum platelet factor 4 is a reliable activity parameter in adult patients with inflammatory bowel disease: a pilot study. Medicine. 2017;96(11):e6323.
32. Grainge MJ, West J, Card TR. Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet. 2010;375(9715):657–663. doi:10.1016/S0140-6736(09)61963-2
33. Z’Graggen K, Walz A, Mazzucchelli L, Strieter RM, Mueller C. The CXC chemokine ENA-78 is preferentially expressed in intestinal epithelium in inflammatory bowel disease. Gastroenterology. 1997;113(3):808–816.
34. Lukacs N, Kunkel S, Allen R, et al. Stimulus and cell-specific expression of CXC and CC chemokines by pulmonary stromal cell populations. Am J Physiol Lung Cell Mol Physiol. 1995;268(5):L856–L861. doi:10.1152/ajplung.1995.268.5.L856
35. Walz A, Burgener R, Car B, Baggiolini M, Kunkel SL, Strieter RM. Structure and neutrophil-activating properties of a novel inflammatory peptide (ENA-78) with homology to interleukin 8. J Exp Med. 1991;174(6):1355–1362. doi:10.1084/jem.174.6.1355
36. Strieter R, Kunkel S, Burdick M, Lincoln P, Walz A. The detection of a novel neutrophil-activating peptide (ENA-78) using a sensitive ELISA. Immunol Invest. 1992;21(6):589–596.
37. Alzoghaibi MA, Al‐Mofleh IA, Al‐Jebreen AM. Neutrophil chemokines GCP‐2 and GRO‐α in patients with inflammatory bowel disease. J Dig Dis. 2008;9(3):144–148.
38. Østvik AE, Granlund A, Bugge M, et al. Enhanced expression of CXCL10 in inflammatory bowel disease: potential role of mucosal Toll-like receptor 3 stimulation. Inflamm Bowel Dis. 2012;19(2):265–274. doi:10.1002/ibd.23034
39. Uguccioni M, Gionchetti P, Robbiani DF, et al. Increased expression of IP-10, IL-8, MCP-1, and MCP-3 in ulcerative colitis. Am J Pathol. 1999;155(2):331–336. doi:10.1016/S0002-9440(10)65128-0
40. Lacher M, Kappler R, Berkholz S, Baurecht H, von Schweinitz D, Koletzko S. Association of a CXCL9 polymorphism with pediatric Crohn’s disease. Biochem Biophys Res Commun. 2007;363(3):701–707. doi:10.1016/j.bbrc.2007.09.020
41. Liu Z, Chen X, Wang X, et al. Chemokine CXCL11 links microbial stimuli to intestinal inflammation. Clin Exp Immunol. 2011;164(3):396–406. doi:10.1111/j.1365-2249.2011.04382.x
42. Dwinell MB, Lügering N, Eckmann L, Kagnoff MF. Regulated production of interferon-inducible T-cell chemoattractants by human intestinal epithelial cells. Gastroenterology. 2001;120(1):49–59.
43. Mikami S, Nakase H, Yamamoto S, et al. Blockade of CXCL12/CXCR4 axis ameliorates murine experimental colitis. J Pharmacol Exp Ther. 2008;327(2):383–392. doi:10.1124/jpet.108.141085
44. Dotan I, Werner L, Vigodman S, et al. CXCL12 is a constitutive and inflammatory chemokine in the intestinal immune system. Inflamm Bowel Dis. 2009;16(4):583–592. doi:10.1002/ibd.21106
45. Carlsen H, Baekkevold E, Johansen F, Haraldsen G, Brandtzaeg P. B cell attracting chemokine 1 (CXCL13) and its receptor CXCR5 are expressed in normal and aberrant gut associated lymphoid tissue. Gut. 2002;51(3):364–371. doi:10.1136/gut.51.3.364
46. Legler DF, Loetscher M, Roos RS, Clark-Lewis I, Baggiolini M, Moser B. B cell–attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5. J Exp Med. 1998;187(4):655–660. doi:10.1084/jem.187.4.655
47. Yeung MM, Melgar S, Baranov V, et al. Characterisation of mucosal lymphoid aggregates in ulcerative colitis: immune cell phenotype and TcR-γδ expression. Gut. 2000;47(2):215–227. doi:10.1136/gut.47.2.215
48. Kurth I, Willimann K, Schaerli P, Hunziker T, Clark-Lewis I, Moser B. Monocyte selectivity and tissue localization suggests a role for breast and kidney–expressed chemokine (BRAK) in macrophage development. J Exp Med. 2001;194(6):855–862. doi:10.1084/jem.194.6.855
49. Rajasekaran G, Kumar SD, Nam J, et al. Antimicrobial and anti-inflammatory activities of chemokine CXCL14-derived antimicrobial peptide and its analogs. Biochimica Et Biophysica Acta (Bba)-Biomembranes. 2019;1861(1):256–267.
50. Maravillas-Montero JL, Burkhardt AM, Hevezi PA, Carnevale CD, Smit MJ, Zlotnik A. Cutting edge: GPR35/CXCR8 is the receptor of the mucosal chemokine CXCL17. J Immunol. 2015;194(1):29–33. doi:10.4049/jimmunol.1401704
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.