Back to Journals » Vascular Health and Risk Management » Volume 12
Elevated risk of venous thromboembolic events in patients with inflammatory myopathies
Authors Nowak M, Krolak-Nowak K, Sobolewska-Wlodarczyk A, Fichna J, Wlodarczyk M
Received 14 November 2015
Accepted for publication 6 April 2016
Published 3 June 2016 Volume 2016:12 Pages 233—238
DOI https://doi.org/10.2147/VHRM.S75308
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Daniel Duprez
Michał Nowak, Katarzyna Królak-Nowak, Aleksandra Sobolewska-Włodarczyk, Jakub Fichna, Marcin Włodarczyk
Department of Biochemistry, Faculty of Medicine, Medical University of Lodz, Lodz, Poland
Abstract: Venous thromboembolism (VTE) is a multifactorial disease manifesting as either deep vein thrombosis or pulmonary embolism. Its prevalence makes VTE a significant issue for both the individual – as a negative factor influencing the quality of life and prognosis – and the society due to economic burden. VTE is the third most common vascular disorder in Western countries, after myocardial infarction and stroke, making it a major cause of in-hospital mortality, responsible for 5%–10% of hospital deaths. Despite many studies conducted, only 50%–60% provoking factors have been identified, while the remaining 40%–50% have been classified as idiopathic or unprovoked. Chronic inflammatory disorders, with their underlying prothrombotic state, reveal an increased risk of VTE (six to eight times) compared with the general population. Among the inflammatory disorders, we can identify inflammatory myopathies – a group of rare, chronic diseases featuring weakness and inflammation of muscles with periods of exacerbation and remission; their main classes are polymyositis and dermatomyositis. The objective of this review is to emphasize the need of VTE prophylaxis in individuals with inflammatory myopathies in order to reduce morbidity and mortality rates among those patients and improve their quality of life and prognosis.
Keywords: deep vein thrombosis, pulmonary embolism, inflammation, polymyositis, dermatomyositis, prothrombotic state
Introduction
Inflammatory myopathies (IMMs) are a group of rare, chronic diseases featuring weakness and inflammation of muscles with periods of exacerbation and remission. High incidence rates are found at 5–15 and 50–60 years of age. The main classes of IMM are polymyositis (PM) and dermatomyositis (DM).1
Clinical manifestations of IMM include symmetric, painless, and rather proximal – than distal – limb paresis that proceeds within weeks or months. Dysphagia or paralysis of respiratory muscles may also occur. Physical examination does not reveal muscle atrophy until the late stages of disease. Moreover, typical purple heliotrope rash over upper eyelids and Gottron’s papules can be observed.1
The creatine kinase level in IMM patients is usually elevated. Electrocardiography and electroencephalography can also reveal abnormalities. Muscle biopsy depicts interstitial and perivascular inflammatory infiltration, muscle fiber atrophy, necrosis, regeneration, and characteristic ghost fibers.1
Treatment begins with prednisone at doses of 60–100 mg daily until paresis disappears (1–4 months); subsequently, the dose of the drug is gradually reduced. Approximately 50% of the patients respond to the treatment. Some respond positively to cyclosporine, azathioprine, methotrexate, intravenous immunoglobulin, and plasmapheresis treatment.1 However, drug-induced myopathies should also be taken into consideration particularly because such myopathies are potentially reversible if the causative drug is eliminated. Agents that may induce toxic myopathy are 1) statins and fibrates (most common);2–4 2) azathioprine, propylthiouracil, and cimetidine;5 3) antiretroviral drugs (including zidovudine and clevudine);6 4) high-dose fluorinated glucocorticoids (dexamethasone, betamethasone, triamcinolone) and some nonfluorinated steroids (prednisolone);7 5) colchicine;8 6) chloroquine and hydroxychloroquine; and 7) diuretics, laxatives, and amphotericin B.2
In addition, the aforementioned drugs can reveal a previously unrecognized neuromuscular disorder or induce an immune response to specific muscle antigens that may lead to a form of toxic IMM.9,10 Alcohol, opioids, and caffeine can exacerbate the drug-induced toxic muscular myopathy.3,7
Apart from ailments caused by the IMM itself, patients also suffer from venous thromboembolism (VTE) complications that greatly affect the patient’s quality of life and prognosis. The objective of this review is to discuss the current VTE epidemiology and its association with IMM to provide a perspective on the clinical management of VTE in IMMs.
Overview of the pathophysiology of venous thromboembolic events
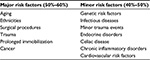
VTE is a multifactorial disease manifesting as either deep vein thrombosis or life-threatening pulmonary embolism (PE). VTE is the third most common vascular disorder in Western countries, after myocardial infarction and stroke.11,12 Moreover, PE is a major cause of in-hospital mortality, responsible for 5%–10% of hospital deaths.13,14 As a result of studies conducted by the German pathologist Virchow, the three main causes of venous thrombosis have now been recognized, which are venous stasis, hypercoagulability, and endothelial damage.15 However, despite many studies conducted, only in 50%–60% clinical cases, major provoking factors of VTE have been identified, while in the remaining 40%–50%, no trigger factors of VTE were observed, and these have been classified as idiopathic or unprovoked.16 The major provoking factors include aging,17–20 ethnicities,21–25 surgical procedures,26–27 trauma,28–30 prolonged immobilization,31 and cancer32–34.
Minor risk factors for VTE include infectious diseases,35 minor trauma events,36 endocrine disorders,37–38 celiac disease,39 chronic inflammatory disorders,40 as well as genetic41,42 and cardiovascular43 risk factors (Table 1). All these conditions have the potential to affect the coagulation system through different mechanisms. For instance, in patients with inflammatory bowel diseases (IBD), such as ulcerative colitis and Crohn’s disease, the risk of first VTE is two- to fourfold higher.40 The underlying mechanism in this case could be related to the activation of innate and acquired immunity, especially activated leukocytes.44
Association of inflammatory myopathies with elevated risk of VTE
There is still not enough data on the impact of IMMs on VTE, and the available data are mainly focused on atherosclerotic diseases.45,46 Carruthers et al47 investigated whether patients with PM and DM may have an increased risk of VTE. Acording to them, the increased risk of VTE may be explainded by influencing all components of the Virchow’s triad: venous stasis, increased blood coagulability, and endothelial damage.15,48 Inflammatory arthritic conditions may affect venous stasis by decreasing mobility. They may also increase blood coagulability through inflammation-associated mechanism.1 Both pathways are strongly linked by extensive cross talk.2 Inflammation affects thrombotic response by upregulating procoagulants such as tissue factors, downregulating natural anticoagulants, such as proteins C and S, and suppressing fibrynolysis leading to a hypercoagulable state.3 Moreover, inflammation may alter endothelial function in both arteries and veins leading to vessel wall damage.4 This is in line with other clinical-based studies, which reported an increased risk of VTE in patients with IMMs.49–52 Carruthers et al47 observed that the risk of VTE was substantially higher in individuals with PM and DM compared with the general population (six and eight times, respectively). The corresponding risk of DVT was six and nine times higher, respectively, in the PM and DM cohorts. The increased risk of PE was statistically significant only in the PM cohort (seven times higher). Moreover, the risk of VTE and PE was highest during the first year for PM (25 and 38 times, respectively) and progressively diminished with time. Likewise, cases with DM had the highest risk during the first 2 years after diagnosis.5 This distinction in risk can be explained by different pathogenesis of the two diseases. PM is a T-cell-mediated disorder with no or minimal involvement of the blood vessels, whereas DM is a humorally mediated microangiopathy where vascular damage plays a crucial role.6 The authors explain the fact of a higher risk of VTE in the initial years after diagnosis as a result of uncontrolled inflammatory activity before the full benefit of anti-inflammatory drugs such as corticosteroids.47
Recent European studies have also shown a relation between VTE and IMMs. Zoller et al7,49 found a threefold overall risk of PE in patients with PM/DM, with a 16-fold increased risk during the first year of follow-up. Ramagopalan et al50 found a threefold increased risk of VTE after hospital admission for PM/DM in the entire population of England.
The study conducted by Carruthers et al47 may have crucial implication for patients and clinicians. The authors suggest that thromboprophylaxis therapy could be considered in patients with PM/DM , particularly in the early phase after diagnosis due to an increased risk of VTE in patients with IMMs; however, they also underline that their findings need to be confirmed by further studies.47
VTE risk related with emerging and current therapies of inflammatory myopathies
IMM has a limited number of randomized controlled trials for most drugs used in its treatment; therefore, universally accepted guidelines for management of each subset of disease are still lacking.53 Treatment in patients with IMM can be divided into two categories – traditional therapy and novel therapeutic approaches.53
Traditional therapy encompasses corticosteroids, immunosuppressants, and intravenous immunoglobulin infusions.53 Introduction of corticosteroids was a milestone in the management of IMM, which significantly increased the survival rates. Before their usage, mortality was as high as 50%–61%.54 As stated earlier, the optimal corticosteroid dosage and treatment duration are not standardized; however, high doses of corticosteroids are recommended as initial dose (prednisone or its equivalent: 0.75–1 mg/kg/day), which should be maintained for 4–12 weeks. Dose tapering usually begins after 1 month and is ∼10%–20% of the daily dose every month until the lowest possible dosage that controls the disease is achieved (usually 5–15 mg/day).55 Immunosuppressants are another group of drugs used in IMM. They are often combined with corticosteroids in order to lower their dose and to reduce side effects.53 The most commonly used immunosuppresant in IMM patients is methotrexate (25 mg per week). Azathioprine and cyclosporine are also used in patients with IMM, usually at a dosage of 1–2 mg/kg/day, respectively.53 Finally, the intravenous immunoglobulin infusion is a treatment that is limited to patients refractory to immunosuppressive treatment.53
The group of novel therapeutic approaches includes anti-B-cell therapy, anti-T-cell therapy, and anticytokine therapy and is reserved to patients who fail to respond to traditional treatment.56
Anti-B-cell therapy is directed against B-cells, which play a critical role in the pathogenesis of IMM. Rituximab, a chimeric monoclonal antibody, aimed at CD20, a protein expressed on B-cell surface.53 Data from the national French registry57 showed that rituximab was well tolerated and effective in 53% of patients (16/30). Moreover, Spanish BIOGEAS registry58 reported good balance between efficacy and adverse events with a complete/partial response in 17 of 20 patients (85%). In their review, Nalotto et al59 reported an overall significant improvement in ∼80% of patients. However, long-term remission was reported in only 4.5% of cases.
In conclusion, authors underline the efficiency of rituximab in refractory diseases; however, the possibility of the occurrence of adverse events such as opportunistic infections should be kept in mind.
Anti-T-cell therapy, such as anti-B-cell therapy, is directed against autoantibodies. T-cells damage the muscles and induce chronic inflammation.53 Alemtuzumab, a monoclonal antibody which targets CD52, a protein which is expressed on CD28null T-cells, is able to deplete peripheral blood lymphocytes and may reduce endomysial inflammation.60 Nevertheless, these – even though encouraging – are still preliminary results and further investigations are needed to confirm their efficiency and safety.
Anticytokine therapy, another novel therapeutic approach, has a limited evidence of efficiency, since only few case reports are available. Cytokines, released by inflammatory cells, damage the muscles that may play a pathogenic role in IMM and can be potentially used both as disease biomarkers and as therapeutic target.53
VTE in patients with IMM
Apart from the primary disease, accompanying disorders such as VTE should also be treated. In the contrary case, they may not only lead to patient’s greater suffering but also have negative impact on disease prognosis.
Necessity of VTE prophylaxis may be illustrated by IBD and other inflammatory disorders. A recent study by Włodarczyk et al61 shows that despite considerable evidence of the association between IBD and VTE, there is still lack of recognition of thrombotic risk with dangerous consequences for patients. Authors present three case reports of patients hospitalized because of exacerbation of IBD complicated by VTE. They underline the need of identifying VTE risk factors and an adequate thrombotic prevention. Even though guidelines recommend VTE prevention by mechanical and pharmacological thromboprophylaxis in IMM patients, this method is still poorly implemented because of concerns about its safety in these patients.61–63 Besides risk factors related to the activity of the inflammatory process, associated with prolonged immobilization, indwelling catheters, hyperhomocysteinemia, vitamin deficiency, and use of oral contraceptives,64 the increased VTE risk is also observed in almost all therapies used in the IMM management. Steroid therapy was associated with both hypo- and hypercoagulating alternations.65–67 Furthermore, it was reported that infliximab induced a significant decrease in the number of circulating microparticles or led to hyperactivation of platelets by the CD40-CD40L pathway in IMM patients.64,68 Other therapies used in IMM patients, such as 5-aminosalicylic acid, sulfasalazine, and azathioprine, may also be responsible for an increased VTE risk by disturbing cytokine pathways and causing abnormalities of the endothelium, fibrinolysis, coagulation cascade, and platelet functions.65 Therefore, VTE prophylaxis in IMM should be considered on similar basis as in IBD. Overall, the IMM prophylaxis can be divided into two groups: nonpharmacological and pharmacological. The former includes proper hydration, correct vitamin levels, in particular B6 and B12, as well as effective control of inflammatory processes.62,65,69 The latter concerns prophylaxis with heparin in patients with severe IMM.63,70
The treatment of VTE in IMM patients is similar to non-IMM subjects. Low-molecular-weight heparin seems to be an ideal choice if there is no hemodynamically significant bleeding or indication of thrombolysis. Low-molecular-weight heparin may be typically switched to oral anticoagulants, that is, vitamin K antagonists (acenocumarol or warfarine).62 It is likely that such treatment would also be recommended in patients with myopathy with accompanying VTE.
Conclusion
VTE is a major issue in patients with IMM that is often – despite considerable evidence – ignored, leading to dangerous consequences due to high morbidity and mortality rates; it is also a high economic burden.71 Therefore, the recognition of and counteracting against VTE risk factors is crucial and requires further investigation.
Disclosure
The authors report no conflicts of interest in this work.
References
Osama O, Zaidat MD, Alan J, Lerner, MD. The Little Black Book of Neurology. 5th ed. Philadelphia: Saunders Elsevier; 2008. | ||
Baer A, Wortmann R. Noninflammatory myopathies. Rheum Dis Clin N Am. 2013;39:457–479. | ||
Mastaglia F, Needham M. Update on toxic myopathies. Curr Neurol Neurosci Rep. 2012;12:54–61. | ||
Padala S, Thompson P. Statins as a possible cause of inflammatory and necrotizing myopathies. Atherosclerosis. 2012;222:15–21. | ||
Guis S, Mattei J, Bendahan D. Toxic myopathies. Joint Bone Spine. 2013;80:231–233. | ||
Finsterer J, Segall L. Drugs interfering with mitochondrial disorders. Drug Chem Toxicol. 2010;33:138. | ||
Owczarek J, Jasinńska M, Orszulak-Michalak D. Drug-induced myopathies. An overview of the possible mechanisms. Pharmacol Rep. 2005;57:23–34. | ||
Teener J. Inflammatory and toxic myopathy. Semin Neurol. 2012;32:491–499. | ||
Mohassel P, Mammen A. The spectrum of statin myopathy. Curr Opin Rheumatol. 2013;25:747–752. | ||
Mohassel P, Mammen A. Statin-associated autoimmune myopathy and anti-HMGCR autoantibodies. Muscle Nerve. 2013;48:477–483. | ||
Abildstrom SZ, Rasmussen S, Rosen M, Madsen M. Trends in incidence and case fatality rates of acute myocardial infarction in Denmark and Sweden. Heart. 2003;89:507–511. | ||
Ellekjær H, Holmen J, Indredavik B, Terent A. Epidemiology of stroke in Innherred, Norway, 1994 to 1996. Incidence and 30-day case-fatality rate. Stroke. 1997;28:2180–2184. | ||
Dismuke SE, Wagner EH. Pulmonary embolism as a cause of death. The changing mortality in hospitalized patients. JAMA. 1986;255:2039–2042. | ||
Carson JL, Kelley MA, Duff A, et al. The clinical course of pulmonary embolism. N Engl J Med. 1992;326:1240–1245. | ||
Virchow R. Thrombose und embolie. Gefässentzündung und septische infektion. Gesammelte Abhandlungen zur wissenschaftlichen Medicin [Thrombosis and embolism. Vascular inflammation and septic infection. Collected Essays on Scientific Medicine]. Frankfurt am Main: Von Meidinger & Sohn; 1856:219–732. German. | ||
Naess IA, Christiansen SC, Romundstad P, Cannegieter SC, Rosendaal FR, Hammerstrøm J. Incidence and mortality of venous thrombosis: a populationbased study. J Thromb Haemost. 2007;5:692–699. | ||
Anderson FA Jr, Wheeler HB, Goldberg RJ, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism: the Worcester DVT Study. Arch Intern Med. 1991;151:933–938. | ||
Ageno W, Agnelli G, Imberti D, Moia M, Palareti G, Pistelli R, Verso M. Prevalence of risk factors for venous thromboembolism in the Italian population: results of a cross-sectional study from the MASTER registry. Intern Emerg Med. 2013;8:575–580. | ||
Rumley A, Emberson JR, Wannamethee SG, Lennon L, Whincup PH, Lowe GD. Effects of older age on fibrin D-dimer, C-reactive protein and other hemostatic and inflammatory variables in men aged 60–79 years. J Thromb Haemost. 2006;4:982–987. | ||
Mari D, Coppola R, Provenzano R. Hemostasis factors and aging. Exp Gerontol. 2008;43:66–73. | ||
White RH, Zhou H, Murin S, Harvey D. Effect of ethnicity and gender on the incidence on venous thromboembolism in a diverse population in California in 1996. Thromb Hemost. 2005;93:298–305. | ||
Cohen AT, Agnelli G, Anderson FA, et al. VTE Imapct Assessment Group in Europe (VITAE). Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb Haemost. 2007;98:756–764. | ||
Molina JA, Jiang ZG, Heng BH, Ong BK. Venous thromboembolism at the National Healthcare Group, Singapore. Ann Acad Med Singapore. 2009;38:470–478. | ||
Zakai NA, McClure LA, Judd SE, Safford MM, Folsom AR, Lutsey PL, Cushman M. Racial and regional differences in venous thromboembolism in the United States in 3 cohorts. Circulation. 2014;129:1502–1509. | ||
Zakai NA, McClure LA. Racial differences in venous thromboembolism. J Thromb Haemost. 2011;9:1877–1882. | ||
Mismetti P, Laporte S, Darmon JY, Buchmüller A, Decousus H. Metaanalysis of low molecular weight heparin in the prevention of venous thromboembolism in general surgery. Br J Surg. 2001;88:913930. | ||
Borgstroem S, Greitz T, Van der Linden W, Molin J, Rudics I. Anticoagulant prophylaxis of venous thrombosis in patients with fractured neck of the femur; a controlled clinical trial using venous phlebography. Acta Chir Scand. 1965;129:500–508. | ||
Geerts WH, Code KI, Jay RM, Chen E, Szalai JP. A prospective study of venous thromboembolism after major trauma. N Engl J Med. 1994;331:1601–1606. | ||
Acosta JA, Yang JC, Winchell RJ, Simons RK, Fortlage DA, Hollingsworth-Fridlund P, Hoyt DB. Lethal injuries and time to death in a level I trauma center. J Am Coll Surg. 1998;186:528–533. | ||
O’Malley KF, Ross SE. Pulmonary embolism in major trauma patients. J Trauma. 1990;30:748–750. | ||
Samama MM. An epidemiologic study of risk factors for deep vein thrombosis in medical outpatients. The Sirius Study. Arch Intern Med. 2000;160:3415–3420. | ||
Imberti D, Agnelli G, Ageno W, et al. Clinical characteristics and management of cancer-associated acute venous thromboembolism: findings from the MASTER Registry. Haematologica. 2008;93:273–278. | ||
Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293:715–722. | ||
Otten HM, Mathijssen J, ten Cate H, Soesan M, Inghels M, Richel DJ, Prins MH. Symptomatic venous thromboembolism in cancer patients treated with chemotherapy: an underestimated phenomenon. Arch Intern Med. 2004;164:190–194. | ||
Smeeth L, Cook C, Thomas S, Hall AJ, Hubbard R, Vallance P. Risk of deep vein thrombosis and pulmonary embolism after acute infection in a community setting. Lancet. 2006;367:1075–1079. | ||
van Stralen KJ, Rosendaal FR, Doggen CJ. Minor injuries as a risk factor for venous thrombosis. Arch Intern Med. 2008;168:21–26. | ||
Stuijver DJ, van Zaane B, Feelders RA, et al. Incidence of venous thromboembolism in patients with Cushing’s syndrome: a multicenter cohort study. J Clin Endocrinol Metab. 2011;96:3525–3532. | ||
Squizzato A, Romualdi E, Piantanida E, et al. Subclinical hypothyroidism and deep venous thrombosis. A pilot cross-sectional study. Thromb Haemost. 2007;97:803–806. | ||
Ludvigsson JF, Welander A, Lassila R, Ekbom A, Montgomery SM. Risk of thromboembolism in 14,000 individuals with coeliac disease. Br J Haematol. 2007;139:121–127. | ||
Tichelaar YI, Kluin-Nelemans HJ, Meijer K. Infections and inflammatory diseases as risk factors for venous thrombosis. A systematic review. Thromb Haemost. 2012;107:827–837. | ||
Margaglione M, Grandone E. Population genetics of venous thromboembolism. A narrative review. Thromb Haemost. 2011;105:221–231. | ||
Morange PE, Trégouët DA. Current knowledge on the genetics of incident venous thrombosis. J Thromb Haemost. 2013;11(Suppl 1):111–121. | ||
Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation. 2008;117:93–102. | ||
Reitsma PH, Versteeg HH, Middeldorp S. Mechanistic view of risk factors for venous thromboembolism. Arterioscler Thromb Vasc Biol. 2012;32:563–568. | ||
Lai YT, Dai YS, Yen MF, et al. Dermatomyositis is associated with an increased risk of cardiovascular and cerebrovascular events: a Taiwanese population-based longitudinal follow-up study. Br J Dermatol. 2013;168:1054–1059. | ||
Tisseverasinghe A, Bernatsky S, Pineau CA. Arterial events in persons with dermatomyositis and polymyositis. J Rheumatol. 2009;36:1943–1946. | ||
Carruthers EC, Choi HK, Sayre EC, et al. Risk of deep venous thrombosis and pulmonary embolism in individuals with polymyositis and dermatomyositis: a general population-based study. Ann Rheum Dis. 2016;75(1):110–116. | ||
Xu J, Lupu F, Esmon CT. Inflammation, innate immunity and blood coagulation. Hamostaseologie. 2010;30:5–6, 8–9. | ||
Zoller B, Li X, Sundquist J, et al. Risk of pulmonary embolism in patients with autoimmune disorders: a nationwide follow-up study from Sweden. Lancet. 2012;379:244–249. | ||
Ramagopalan SV, Wotton CJ, Handel AE, et al. Risk of venous thromboembolism in people admitted to hospital with selected immune-mediated diseases: record-linkage study. BMC Med. 2011;9:1. | ||
Johannesdottir SA, Schmidt M, Horvath-Puho E, et al. Autoimmune skin and connective tissue diseases and risk of venous thromboembolism: a population-based case-control study. J Thromb Haemost. 2012;10:815–21. | ||
Selva-O’Callaghan A, Fernandez-Luque A, Martinez-Gomez X, et al. Venous thromboembolism in patients with dermatomyositis and polymyositis. Clin Exp Rheumatol. 2011;29:846–849. | ||
Iaccarino L, Bartoloni E, Gerli R, et al. Drugs in induction and treatment of idiopathic inflammatory myopathies. Auto Immun Highlights. 2014;5:95–100. | ||
Marie I. Morbidity and mortality in adult polymyositis and dermatomyositis. Curr Rheumatol Rep. 2012;14:275–285. | ||
Oddis CV, Medsger TA Jr. Relationship between serum creatine kinase level and corticosteroid therapy in polymyositisdermatomyositis. J Rheumatol. 1988;15:807–811. | ||
Venalis P, Lundberg IE. Immune mechanisms in polymyositis and dermatomyositis and potential targets for therapy. Rheumatology (Oxford). 2014;53:397–405. | ||
Couderc M, Gottenberg JE, Mariette X, et al. Efficacy and safety of rituximab in the treatment of refractory inflammatory myopathies in adults: results from the AIR registry. Rheumatology (Oxford). 2011;50:2283–2289. | ||
Ramos-Casals M, García-Hernández FJ, de Ramón E, et al. Off-label use of rituximab in 196 patients with severe, refractory systemic autoimmune diseases. Clin Exp Rheumatol. 2010;28:468–476. | ||
Nalotto L, Iaccarino L, Zen M, et al. Rituximab in refractory idiopathic inflammatory myopathies and antisynthetase syndrome: personal experience and review of the literature. Immunol Res. 2013;56:362–370. | ||
Dalakas MC, Rakocevic G, Schmidt J, et al. Effect of alemtuzumab (CAMPATH1-H) in patients with inclusion-body myositis. Brain. 2009;132:1536–1544. | ||
Włodarczyk M, Sobolewska A, Fichna J, Wiśniewska-Jarosińska M. Prevention and therapeutic strategies of thromboembolic events in patients with inflammatory bowel diseases: a report of three cases. Curr Drug Targets. 2015;16(3):194–198. | ||
Papa A, Gerardi V, Marzo M, Felice C, Rapaccini GL, Gasbarrini A. Venous thromboembolism in patients with inflammatory bowel disease: focus on prevention and treatment. World J Gastroenterol. 2014;20:3173–3179. | ||
Fumery M, Xiaocang C, Dauchet L, Gower-Rousseau C, Peyrin-Biroulet L, Colombel JF. Thromboembolic events and cardiovascular mortality in inflammatory bowel diseases: a metaanalysis of observational studies. J Crohns Colitis. 2014;8:469–479. | ||
Danese S, Papa A, Saibeni S, Repici A, Malesci A, Vecchi M. Inflammation and coagulation in inflammatory bowel disease: the clot thickens. Am J Gastroenterol. 2007;102:174–186. | ||
Magro F, Soares JB, Fernandes D. Venous thrombosis and prothrombotic factors in inflammatory bowel disease. World J Gastroenterol. 2014;20:4857–4872. | ||
Frank RD, Altenwerth B, Brandenburg VM, Nolden-Koch M, Block F. Effect of intravenous high-dose methylprednisolone on coagulation and fibrinolysis markers. Thromb Haemost. 2005;94:467–468. | ||
Pandit HB, Spillert CR. Effect of methylprednisolone on coagulation. J Natl Med Assoc. 1999;91:453–456. | ||
Tan VP, Chung A, Yan BP, Gibson PR. Venous and arterial disease in inflammatory bowel disease. J Gastroenterol Hepatol. 2013;28:1095–1113. | ||
Sam JJ, Bernstein CN, Razik R, Thanabalan R, Nguyen GC. Physicians’ perceptions of risks and practices in venous thromboembolism prophylaxis in inflammatory bowel disease. Dig Dis Sci. 2013;58:46–52. | ||
Nguyen GC, Bernstein CN, Bitton A, et al. Consensus statements on the risk, prevention, and treatment of venous thromboembolism in inflammatory bowel disease: Canadian Association of Gastroenterology. Gastroenterology. 2014;146:835–848. | ||
Bernatsky S, Panopalis P, Pineau CA, et al. Healthcare costs of inflammatory myopathies. J Rheumatol. 2011;38:885–888. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.