Back to Journals » Journal of Inflammation Research » Volume 15
Elevated Rheumatoid Factor Associates with Dry Eye in Patients with Common Autoimmune Diseases
Authors Zhao S, Xiao Y, Zhang S, Liu L , Chen K
Received 7 March 2022
Accepted for publication 28 April 2022
Published 3 May 2022 Volume 2022:15 Pages 2789—2794
DOI https://doi.org/10.2147/JIR.S365326
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Adam D Bachstetter
Shan Zhao,1 Yifan Xiao,1 Song Zhang,2 Lei Liu,3 Kang Chen4
1Department of Rheumatology and Immunology, The First Affiliated Hospital of China Medical University, Shenyang, People’s Republic of China; 2Department of Graduate School, China Medical University, Shenyang, People’s Republic of China; 3Department of Ophthalmology, Guangdong Eye Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, People’s Republic of China; 4Department of Ophthalmology, The First Affiliated Hospital of China Medical University, Shenyang, People’s Republic of China
Correspondence: Lei Liu; Kang Chen, Email [email protected]; [email protected]
Purpose: To investigate the association and application value of rheumatoid factor (RF) in diagnosis of dry eye among patients with common autoimmune diseases.
Patients and Methods: A retrospective case-control study was conducted from Jan 1st 2019 to Dec 31st 2020, and 117 patients with common autoimmune diseases were enrolled. The concentrations of serum RF were detected. The receiver operating characteristics (ROC) curve was plotted to evaluate the values of RF in diagnosis.
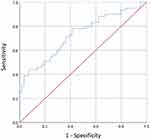
Results: Compared with no dry eye patients, patients with dry eye (n = 59, 50.43%) had higher levels of RF (P < 0.001). Moreover, Pearson’s correlation analysis showed that RF levels were negatively correlated with Schirmer-I tear test (P = 0.004) and invasive tear film break-up time (P = 0.002), respectively. The statistically significant threshold value (cut-off points) of the RF levels for dry eye was 21.4 (IU/mL), and the areas under the curve (AUC) of RF was 0.73 (95% confidence interval [CI]: 0.639– 0.821; P < 0.001) (sensitivity = 78%; specificity = 58.6%) for dry eye among patients with common autoimmune diseases.
Conclusion: Serum levels of RF may be an independent factor for dry eye among patients with common autoimmune diseases, and they may have a high significant value and clinical application value.
Keywords: rheumatoid factor, autoimmune diseases, dry eye, diagnosis, clinical application
Introduction
Autoimmune disorder is a series of chronic immune imbalance and an autoimmunity-mediated disease. Generally, rheumatoid arthritis (RA), systemic lupus erythematosus (SLE) and primary Sjogren’s syndrome (pSS) are common autoimmune diseases which can involve multiple systems and organs of the body, including joint, skin, lacrimal gland and ocular.1–3 Dry eye is thought to be one of the most common ocular disorders in patients of common autoimmune diseases. For example, the pooled prevalence of dry eye disease (DED) was 16% in patients of SLE, and its symptoms as well as abnormal Schirmer’s test were found in 26% and 24% of patients with SLE, respectively.4 Furthermore, the proportions of dry eye in other autoimmune diseases, such as RA and pSS, are 71.4%,3 and 35%,5 respectively. Thus, dry eye is one of the most common types of complications for autoimmune disease. Regarding symptoms and signs of autoimmune diseases, patients with dry eye have a more complex condition than cases without dry eye. Clinically, diagnosis of dry eye needs ophthalmic examinations such as Schirmer-I tear test and invasive tear film break-up time, which are difficult for physician to perform. Herein, this makes the early detection of dry eye among autoimmune disorders challenging, whereas there are few studies have explored the indicator of dry eye in autoimmune disorders.
The aim of this case-control study was to investigate the indicator for dry eye among clinical biochemical parameters administered concurrently for common autoimmune disorders using long-term clinical data and to suggest the detection edge for the diagnosis of dry eye in the real world.
Methods
Subjects
This retrospective case-control study was conducted at the Department of Rheumatology and Immunology of the First Affiliated Hospital of China Medical University between Jan 1st 2019 to Dec 31st 2020, following the tenets of the Declaration of Helsinki. The study was approved by the Ethics Committee of the First Affiliated Hospital of China Medical University (NO. 2019.216). Written informed consent was also obtained from each subject before study enrollment. Consecutive patients with dry eye who were combined with SLE, RA, or pSS were recruited for the current study. Age- and sex-matched controls with SLE, RA, or pSS but without any ocular surface disorder in the past 2 months were included as controls. Subjects who were less than 18-year old, wore contact lenses, had a meibomian gland dysfunction (MGD), any active ocular disease, or a history of ocular surgery were excluded. Subjects with systemic diseases involving ocular surface abnormalities including diabetes mellitus, and thyroid disease were also excluded from the study. SLE was diagnosed according to the 2012 Systemic Lupus International Collaborating Clinics (SLICC) classification criteria. RA was diagnosed according to the 2010 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) classification criteria. In addition, pSS was diagnosed according to the 2002 American and European Consensus Group (AECG) criteria.6
Dry Eye Symptoms
Participants were asked to describe any symptom regarding dry eye, such as burning, photophobia, blurred vision, and eye discomfort or pain.7
Schirmer`s I Test (STT-I)
A Schirmer strip of 5 mm × 30 mm (Jinming New Technological Development Co. Ltd., Tianjin, China) was inserted in the lower lid margin of each participant. Then the participants were instructed to close their eyes. The length of the wetting strip was recorded after five minutes.
Tear Film Break-Up Time (TBUT)
TBUT values were measured using fluorescein paper strips (Jinming New Technological Development Co. Ltd., Tianjin, China). Approximately a drop (20 μL) 0.9% sterile saline was instilled to the trip, and then the strip was touched with the inferior temporal bulbar conjunctiva. Participants were asked to blink naturally three times and then to cease blinking. The time between the last complete palpebral blink and the first dry spot on the tear film was observed with cobalt-blue light under a slit lamp.
Corneal Fluorescein Staining
After TBUT test, corneal fluorescein staining measurement was conducted using the same fluorescein staining strip under a yellow filter using the Oxford scale. A test score over 0 was regarded as positive.8
Diagnosis of Dry Eye
Dry eye was diagnosed according to previously reported criteria as following: (i) dry eye-related symptoms; (ii) positive corneal staining with fluorescein; and (iii) a Schirmer`s I test result less than 5 mm or TBUT less than 5 seconds.9 Patients who met these three criteria listed above were diagnosed as dry eye.
Data Extraction
The demographic (age and gender) and clinicopathological data (pathological type, biochemical, and immunologic laboratory tests) were retrieved from medical records. Blood routine tests including white blood cell (WBC), neutrophil (NE), lymphocyte (LY), hemoglobin (Hb), and platelet (PLT) counts were obtained. Laboratory biochemical tests were performed to examine the status of patients’ liver and renal function, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin (ALB), alkaline phosphatase (ALP), gamma glutamyl transferase (GGT) and creatinine (CR-S) levels. Then, immunity and inflammation indicators including erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), immunoglobulin A (IgA), immunoglobulin M (IgM), immunoglobulin G (IgG), C3, and C4 were also tested. Moreover, the levels of rheumatoid factor (RF) were measured by standard laboratory techniques.
Statistical Analysis
Statistical analysis was performed with SPSS software (version 25.0, IBM SPSS, IBM Corp, USA). The quantitative data were presented as the mean ± standard deviations (SD). Kolmogorov–Smirnov method was applied to perform normality test. The unpaired two-tailed Student’s test was used to analyze data with normal distribution. Mann–Whitney U-test was adopted to compare the nonparametric variables between individuals with or without dry eye. Pearson correlation coefficient was used to analyze the correlations between two variables. The receiver operating characteristic (ROC) curve was plotted, and the area under the curve (AUC) was calculated to assess the accuracy of the indicator. A P-value less than 0.05 was considered as statistically significant.
Results
Finally, 19 patients with SLE, 72 patients with RA, and 26 patients with pSS were included. There were 59 participants with dry eye and 58 participants without dry eye. The demographic, clinicopathologic and ophthalmological data of the dry eye and without dry eye groups are summarized in Table 1. Compared with no dry eye patients, individuals with dry eye had higher levels of RF (P < 0.001), but lower levels of STT-I tests (P < 0.001) and TBUT (P < 0.001). No statistically significant differences were found in the demographic and other clinicopathologic distributions between two groups.
 |
Table 1 Demographic and Clinical Characteristics of the Common Autoimmune Diseases Patients |
Table 2 shows outcomes by Pearson’s correlation analysis, which indicated a significant negative correlation between RF levels and STT-I tests (r = −0.26, P = 0.004), and similar association was found between RF levels and TBUT (r = −0.289, P = 0.002).
 |
Table 2 The Correlation Between RA and Dry Eye Index in Common Autoimmune Diseases Patients |
Moreover, the threshold value (cut-off points) of the RF levels for predicting dry eye was 21.4 (IU/mL) and the area under the curve (AUC) for the levels of RF detecting dry eye with common autoimmune diseases was 0.73 (95% confidence interval [CI]: 0.639–0.821, P < 0.001) with its sensitivity and specificity being 78% and 58.6%, respectively (Figure 1).
Discussion
Generally, SLE, RA, and pSS are common rheumatic disorders with the involvement of the immune system.10 They are different in prevalence, pathology, and clinical manifestations, but they all may evolve in a condition of chronicity, often causing a disability for exocrinopathy in lacrimal glands and inducing ocular surface discomfort. There is a well-known association of dry eye with rheumatic disease severity like pSS, RA, and SLE.11 Dry eye results in a significant increase in costs which falls on individual medication and health service and marks adverse financial consequences for economy.12 Likewise, dry eye has a devastating effect upon patients’ quality of life.13 Furthermore, dry eye in patients with underlying systemic autoimmune disease causes high risk of depression and anxiety.14 Herein, prompt detection of dry eye has a key role to perform the appropriate intervention that could increase quality of life most patients.
Currently, our single-center retrospective case-control study of autoimmune disease patients with or without dry eye demonstrated significant correlations between serum RF levels and STT-I tests as well as TBUT. Furthermore, RF also exhibited a good performance in predicting dry eye. To the best of our knowledge, this is the first study to investigate the potential predictive power of elevated RF levels for dry eye in patients with common autoimmune diseases. Notably, in current study, patients with elevated RF already developed dry eye, so these findings mostly showed correlation, but it remains to be determined for the predictive value of RF for future development of dry eye.
Contrary to our results, RF did not have significant correlation with Schirmer test, TBUT, and ocular staining score (OSS) in some cases. However, the duration of RA was significantly positively correlated with Schirmer`s test and OSS. That is to say, longstanding RA patients were more susceptible to develop dry eye.3 Moreover, Fujita et al concluded there was a correlation between RA activity, which was evaluated by Lansbury index LI, and Schirmer test in RA patients with pSS.15 Combined with our results, we believe that autoimmune disease patients with elevated RF levels can partially reveal a higher risk with eye dryness. Nevertheless, these findings have implications for rheumatologists in determining whether a patient has dry eye. For ophthalmologists, as RF is a very non-specific factor, it is very important to diagnose dry eye with ophthalmic examinations; therefore, use of RF as predictor can be interesting, but the value of RF for diagnosis would be limited in ophthalmology area.
We acknowledge the possibility that bacterial liposaccharides, viruses’ antigens, and immune complexes could have triggered RF production laying the stimulation of the immune system.16 RF can also change as an indicator in patients with RA and other rheumatic disorders, such as SLE and pSS,17,18 yet, it may emerge in healthy subjects as well. The RF constitutes a class of IgM or IgA, rarely IgG antibodies. In the present study, we evaluated the commonly mentioned RF, IgM RF.19 A recent report has showed an association between RF and prognostic marker in pSS patients. However, no significant beneficial effects of RF in diagnosis of pSS basically depended on no association between RF and serum concentrations of B-cell activation factor (BAFF), a proliferation-inducing ligand (APRIL), CRP, C3, and C4.20 In our study, we diagnosed dry eye depending on symptoms and its common parameters and analyzed the correlation between RF levels and independent parameters of dry eye. It is noteworthy that the mechanisms of RF in diagnosis of dry eye still limited; thus, further basic science studies are needed.
Notably, there were several limitations in this study, including this inherent to a single center case-control study, and relative small sample size to estimate the cause-effects. The correlation whether RF can serve as predictor for dry eye needs to be validated by longitudinal studies. Furthermore, laboratory data such as anti-Ro/SSA, anti-La/SSB, antinuclear antibodies, anti-mitochondrial antibodies, anti-centromere antibodies, and anti-CCP are insufficient. Combination of laboratory results may improve the accuracy of our findings. Further study is needed to determine whether combinations of these indexes are related to the identification of dry eye among autoimmunity diseases. Due to the limited technical test or machine, we did not divide the subtype of dry eye into aqueous tear deficient or evaporative in the current study. Last but not least, serum RF levels may be affected by various factors, such as anti-immunity or anti-inflammation medication use. Unfortunately, this study failed to consider these factors.
Conclusion
Serum RF level was negatively associated with STT-I, and TBUT outcomes, and that may be associated with the onset of dry eye, which had potentially relevant clinical implications in patients with common autoimmune diseases.
Data Sharing Statement
Datasets are available from the corresponding author on reasonable request.
Acknowledgments
We thank all patients for their participation.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest in this work.
References
1. Qi Y, Wan Y, Li T, et al. Comparison of the ocular microbiomes of dry eye patients with and without autoimmune disease. Front Cell Infect Microbiol. 2021;11:716867. Cited in: Pubmed; PMID 34631599. doi:10.3389/fcimb.2021.716867
2. Bunya VY, Maguire MG, Akpek EK, et al. A new screening questionnaire to identify patients with dry eye with a high likelihood of having Sjogren syndrome. Cornea. 2021;40(2):179–187. Cited in: Pubmed; PMID 33055548. doi: 10.1097/ICO.0000000000002515
3. Abd-Allah NM, Hassan AA, Omar G, et al. Dry eye in rheumatoid arthritis: relation to disease activity. Immunol Med. 2020;43(2):92–97. Cited in: Pubmed; PMID 32089102. doi:10.1080/25785826.2020.1729597
4. Wang L, Xie Y, Deng Y. Prevalence of dry eye in patients with systemic lupus erythematosus: a meta-analysis. BMJ Open. 2021;11(9):e047081. Cited in: Pubmed; PMID 34588240. doi:10.1136/bmjopen-2020-047081
5. Jacobsson LT, Axell TE, Hansen BU, et al. Dry eyes or mouth–an epidemiological study in Swedish adults, with special reference to primary Sjogren’s syndrome. J Autoimmun. 1989;2(4):521–527. Cited in: Pubmed; PMID 2789654. doi:10.1016/0896-8411(89)90185-6
6. Ciccacci C, Latini A, Perricone C, et al. TNFAIP3 gene polymorphisms in three common autoimmune diseases: systemic lupus erythematosus, rheumatoid arthritis, and primary Sjogren syndrome-association with disease susceptibility and clinical phenotypes in Italian patients. J Immunol Res. 2019;2019:6728694. Cited in: Pubmed; PMID 31534975. doi:10.1155/2019/6728694
7. Kojima T, Ishida R, Dogru M, et al. The effect of autologous serum eyedrops in the treatment of severe dry eye disease: a prospective randomized case-control study. Am J Ophthalmol. 2005;139(2):242–246. Cited in: Pubmed; PMID 15733983. doi:10.1016/j.ajo.2004.08.040
8. Toda I, Tsubota K. Practical double vital staining for ocular surface evaluation. Cornea. 1993;12(4):366–367. Cited in: Pubmed; PMID 7687944. doi:10.1097/00003226-199307000-00015
9. Wu M, Liu X, Han J, Shao T, Wang Y. Association between sleep quality, mood status, and ocular surface characteristics in patients with dry eye disease. Cornea. 2019;38(3):311–317. Cited in: Pubmed; PMID 30614900. doi:10.1097/ICO.0000000000001854
10. Padern G, Duflos C, Ferreira R, et al. Identification of a novel serum proteomic signature for primary Sjogren’s syndrome. Front Immunol. 2021;12:631539. Cited in: Pubmed; PMID 33708222. doi:10.3389/fimmu.2021.631539
11. Ziaragkali S, Kotsalidou A, Trakos N. Dry eye disease in routine rheumatology practice. Mediterr J Rheumatol. 2018;29(3):127–139. Cited in: Pubmed; PMID 32185314. doi:10.31138/mjr.29.3.127
12. Galor A, Zheng DD, Arheart KL, et al. Dry eye medication use and expenditures: data from the medical expenditure panel survey 2001 to 2006. Cornea. 2012;31(12):1403–1407. Cited in: Pubmed; PMID 22895158. doi:10.1097/ICO.0b013e31823cc0b7
13. Wu J, Wu X, Zhang H, et al. Dry eye disease among Mongolian and han older adults in Grasslands of Northern China: prevalence, associated factors, and vision-related quality of life. Front Med. 2021;8:788545. Cited in: Pubmed; PMID 34901096. doi:10.3389/fmed.2021.788545
14. Wan KH, Chen LJ, Young AL. Depression and anxiety in dry eye disease: a systematic review and meta-analysis. Eye. 2016;30(12):1558–1567. Cited in: Pubmed; PMID 27518547. doi:10.1038/eye.2016.186
15. Fujita M, Igarashi T, Kurai T, Sakane M, Yoshino S, Takahashi H. Correlation between dry eye and rheumatoid arthritis activity. Am J Ophthalmol. 2005;140(5):808–813. Cited in: Pubmed; PMID 16289424. doi:10.1016/j.ajo.2005.05.025
16. Corper AL, Sohi MK, Bonagura VR, et al. Structure of human IgM rheumatoid factor Fab bound to its autoantigen IgG Fc reveals a novel topology of antibody-antigen interaction. Nat Struct Biol. 1997;4(5):374–381. Cited in: Pubmed; PMID 9145108. doi:10.1038/nsb0597-374
17. Pope RM, Yoshinoya S, McDuffy SJ. Detection of immune complexes and their relationship to rheumatoid factor in a variety of autoimmune disorders. Clin Exp Immunol. 1981;46(2):259–267. Cited in: Pubmed; PMID 7337971.
18. Maslinska M, Manczak M, Kwiatkowska B, Ramsperger V, Shen L, Suresh L. IgA immunoglobulin isotype of rheumatoid factor in primary Sjogren’s syndrome. Rheumatol Int. 2021;41(3):643–649. Cited in: Pubmed; PMID 33496802. doi:10.1007/s00296-020-04782-3
19. Tiwari V, Jandu JS, Bergman MJ. Rheumatoid Factor. Treasure Island (FL): StatPearls; 2022. Eng.
20. Maslinska M, Manczak M, Kwiatkowska B. Usefulness of rheumatoid factor as an immunological and prognostic marker in PSS patients. Clin Rheumatol. 2019;38(5):1301–1307. Cited in: Pubmed; PMID 30810912. doi:10.1007/s10067-019-04438-z
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.