Back to Journals » OncoTargets and Therapy » Volume 14
Elevated RGMA Expression Predicts Poor Prognosis in Patients with Glioblastoma
Authors Phan TL, Kim HJ, Lee SJ, Choi MC, Kim SH
Received 23 April 2021
Accepted for publication 10 September 2021
Published 21 September 2021 Volume 2021:14 Pages 4867—4878
DOI https://doi.org/10.2147/OTT.S317285
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr William C. Cho
Thi Le Phan,1,* Hyun-Jin Kim,1,* Suk Jun Lee,2 Moon-Chang Choi,3 Sung-Hak Kim1
1Department of Animal Science, College of Agriculture and Life Sciences, Chonnam National University, Gwangju, 61186, Republic of Korea; 2Department of Biomedical Laboratory Science, College of Health & Medical Sciences, Cheongju University, Chungbuk, 28503, Republic of Korea; 3Department of Biomedical Science, Chosun University, Gwangju, 61452, Republic of Korea
*These authors contributed equally to this work
Correspondence: Moon-Chang Choi
Department of Biomedical Science, Chosun University, 309 Pilmundaero, Gwangju, 61452, Republic of Korea
Tel +82 62 230 6758
Email [email protected]
Sung-Hak Kim
Department of Animal Science, College of Agriculture and Life Sciences, Chonnam National University, 77 Yongbongro, Gwangju, 61186, Republic of Korea
Tel +82 62 530 2115
Email [email protected]
Background: Glioblastoma (GBM) is the most aggressive type of human brain tumor with a poor prognosis and a low survival rate. Secreted proteins from tumors are recently considered as important modulators to promote tumorigenesis by communicating with microenvironments. Repulsive guidance molecule A (RGMA) was initially characterized as an axon guidance molecule after secretion in the brain during embryogenesis but has not been studied in GBM. In this study, we investigated secreted gene expression patterns and the correlation between RGMA expression and prognosis in GBM using in silico analysis.
Methods: RGMA mRNA levels in normal human astrocyte (NHA), human glioma cells, and GBM patient-derived glioma stem cells (GSCs) were assessed by qRT‐PCR. Patient survival analysis was performed with the Kaplan–Meier curve and univariate and multivariate analyses using publicly available datasets. The predictive roles of RGMA in progressive malignancy were evaluated using Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG), and Gene Set Enrichment Analysis (GSEA).
Results: RGMA mRNA expression was elevated in glioma cells and GSCs compared with NHA and correlated with unfavorable prognosis in glioma patients. Thus, RGMA could serve as an independent predictive factor for GBM. Furthermore, the increased levels of RGMA expression and its putative receptor, neogenin (NEO1), were associated with poor patient survival rates in GBM.
Conclusion: We identified RGMA as an independent prognostic biomarker for progressive malignancy in glioblastoma and address the possibilities to develop novel therapeutic strategies against glioblastoma.
Keywords: bioinformatics, glioblastoma, glioma, glioma stem cell, RGMA
Graphical Abstract:
Introduction
Glioblastoma (GBM) ranks among the most fatal and recalcitrant brain tumors in the human central nervous system (CNS).1 Despite extensive therapies such as surgical resection, radiotherapy, and chemotherapy, the median survival rate of GBM patients is approximately 15 months after diagnosis.1,2 Glioma stem cells (GSCs) have recently emerged as a small subset of cells in GBM that may self-renew and proliferate while retaining normal stem cell features.3–5 GSCs can initiate tumor formation and become resistant to conventional treatments by secreting molecules to communicate with their microenvironment.6,7 Thus, identifying secreted molecules to regulate GSCs is urgently needed.
The process of tumor progression is affected by either genetic/epigenetic alterations in GBM cells or communications between GBM cells and the tumor microenvironment.3,8 The reciprocal intracellular interaction between glial cells and tumor microenvironment can institute a complicated heterogeneous tumor microenvironment.8–10 As the most dominant glial cells in the tumor microenvironment, activated astrocytes can surround and penetrate glioma cells and protect them from the cytotoxic effects of various chemotherapies.9,11,12 Gap junctions existing between glioma cells and nearby astrocytes can protect glioma cells by regulating various critical survival genes of astrocytes.9 Astrocytes could also increase the release of several cytokines (IL-6, IGF1, and TGF-β) to the tumor microenvironment and stimulate the migration and invasion of gliomas, providing evidence that the microenvironment can influence the behavior of tumor cells.9,13 A previous study has demonstrated that glioma resection-induced injury can lead to reactive astrocyte changes in the peritumoral microenvironment and that astrocyte injury can cause alterations in transcriptome and secretome to promote glioma progression.12
The repulsive guidance molecule (RGM) family encodes glycosylphosphatidylinositol-anchored glycoprotein and includes RGMA, RGMB (Dragon), and RGMC (Hemojuvelin), which are critical for nervous system development.14 Among them, RGMA is known as a regulator in cephalic neural tube closure by inhibiting neurite outgrowth and modulating inflammation and neurodegeneration in autoimmune encephalomyelitis disease.15–17 RGMA initially has been reported as an axon guidance molecule in the brain,18 but also as a potential tumor suppressor in some cancers,19–21 and a tumor prognostic marker in triple-negative breast cancer.22 However, it is less known about the explicit function of RGMA in GBM tumors. RGMA can directly bind to the cell surface receptor neogenin (NEO1) during axonal guidance, causing growth cones to collapse and axons to develop along with specific directions.23–25 Recent studies have shown that NEO1 is abundantly expressed in brain astrocytes, neurons, neural stem cells, and oligodendrocytes, and it has a function in astrocytic differentiation in the mouse neocortex.26 Zhang et al27 have reported that RGMA can regulate reactive astrogliosis and glial scar formation through TGF-β1/Smad2/3 signaling in stroke patients. Also, reactive astrocytes in the tumor microenvironment can augment GBM malignancy by causing abnormal cell proliferation13 and support immunosuppressive response in the GBM microenvironment.28 Here, we hypothesized that RGMA could regulate tumorigenesis through interaction with the NEO1 receptor in the tumor microenvironment.
In this study, we examined mRNA expression levels of secreted proteins predicted from the Human Protein Atlas (HPA) secretome during gliomagenesis and found that RGMA could be used as a prognostic marker for poor glioma patient survival. Moreover, RGMA expression was positively correlated with NEO1 expression in GBM tissues. Their concomitant high expression resulted in poor GBM patients’ clinical outcomes. Taken together, RGMA might serve as a predictive factor for the occurrence of GBM.
Materials and Methods
Cell Culture
The human glioma cell lines (A172, A1207, U87MG, and LN229, ATCC, USA) were cultured in Dulbecco’s modified Eagle’s medium (DMEM/F12; Welgene, South Korea) supplemented with 10% fetal bovine serum (FBS) (HyClone, USA) and 1% penicillin-streptomycin solution (100X; Welgene, South Korea). The normal human astrocytes (NHA, Lonza, Switzerland) were maintained in astrocyte media (ScienCell, USA) supplemented with 10% FBS (HyClone, USA) and 1% penicillin-streptomycin solution (100X, Welgene, South Korea). The GBM patient-derived glioma stem cell lines (GSC11, GSC20, GSC23, and GSC267) were obtained from the University of Texas MD Anderson Cancer Center and cultured with DMEM/F12 medium (Welgene, South Korea) supplemented with 2% B27 (Gibco, USA), epidermal growth factor (EGF) (20 ng/mL; R&D Systems, USA), basic fibroblast growth factor (bFGF) (20 ng/mL; R&D Systems, USA), and 1% penicillin-streptomycin solution (100X; Welgene, South Korea). Cells were cultured in a humidified chamber at 37 °C containing 5% CO2 and 20% O2.
Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted from cell lines using the RiboEx reagent (GeneAll, South Korea) and purified using a Hybrid‐R kit (GeneAll, South Korea) followed each manufacturer’s instructions. Then complementary DNA (cDNA) from 1 µg of total RNA was synthesized using Thermo Scientific RevertAid First‐Strand cDNA Synthesis Kit (Thermo Fisher Scientific, USA). The levels of RGMA and 18S (reference gene) were measured by a CFX96 real‐time polymerase chain reaction detection system (Bio‐Rad Laboratories, USA) using SYBR Premix Ex Taq (Takara Bio, Japan). Results of qRT‐PCR were evaluated as cycle threshold (Ct) values and calculated using the 2−ΔΔCt method.29 Primer sequences used for qRT‐PCR amplification were as follows: RGMA forward (F) 5ʹ-CAACACGCCTGTGCTGCCCG-3ʹ and RGMA reverse (R) 5ʹ-CCACCGTTCTTAGAGCCATCCA-3ʹ; 18S forward (F) 5ʹ-CAGCCACCCGAGATTGAGCA-3ʹ and 18S reverse (R) 5ʹ-TAGTAGCGACGGGCGGTGTG-3ʹ.
Dataset Preparation for RGMA Expression and Analysis
Data from Gene Expression Omnibus (GSE4536) were used to determine RGMA mRNA expression levels in different GSC culture mediums. Gene expression profile in the Gravendeel dataset was used for RGMA expression and patients’ survival analyses. The Ivy Glioblastoma Atlas Project RNAseq was used for evaluating RGMA expression in different regions of GBM. Public datasets such as Gravendeel, CGGA, TCGA GBM, and Rembrandt were obtained from the Gliovis website (http://gliovis.bioinfo.cnio.es/) to determine correlations of RGMA and NEO1 gene expression and their concurrent expression with GBM patient survival outcome.
Gene Set Enrichment Analysis (GSEA)
Gene signatures analyzed were obtained from C2 chemical and genetic perturbation collection of the Molecular Signatures Database (http://software.broadinstitute.org/gsea/msigdb). The raw expression data profile of glioma patients was extracted from the Gravendeel dataset (n = 276). In this GSEA method, the continuous phenotype was applied to find gene sets that correlated with RGMA (gene neighbors). Pearson’s correlation was applied to score and rank genes. Default options were used with 1000 repetitions of gene set permutations. Significant enrichments were considered when the false discovery rate (FDR) was less than 0.01 and the p-value was less than 0.05.
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Analysis
GO and KEGG analyses of genes that exhibited correlation /R/ ≥ 0.4 with RGMA in the Gravendeel dataset were performed using the Database for Annotation, Visualization, and Integrated Discovery (DAVID) bioinformatics resources (version 6.8, https://david.ncifcrf.gov/). Gene sets were then displayed by using the dot plot, in which significant pathways with gene counts of ≥ 2 and p < 0.05 were set as the threshold.
Statistical Analysis
Microsoft Excel, SPSS 20, GraphPad Prism version 9, R version 4.0.3, and Python version 3.8.3 were used for statistical analyses. In univariate and multivariate Cox regression analyses, p < 0.05 indicated statistical significance. Heat maps were created using Java TreeView and R language. Significant quantitative differences between and among groups were determined by an unpaired Student’s t-test (two-tailed) and one-way ANOVA, respectively, followed by Tukey’s multiple comparison test. Bivariate correlation analysis (Pearson’s R test) was applied to examine the correlation of two variables in human specimens. Kaplan-Meier survival analysis was performed to estimate survival distribution, followed by a Log rank test.
Results
RGMA is Identified as a Gene Elevated Under GSCs Culture Condition
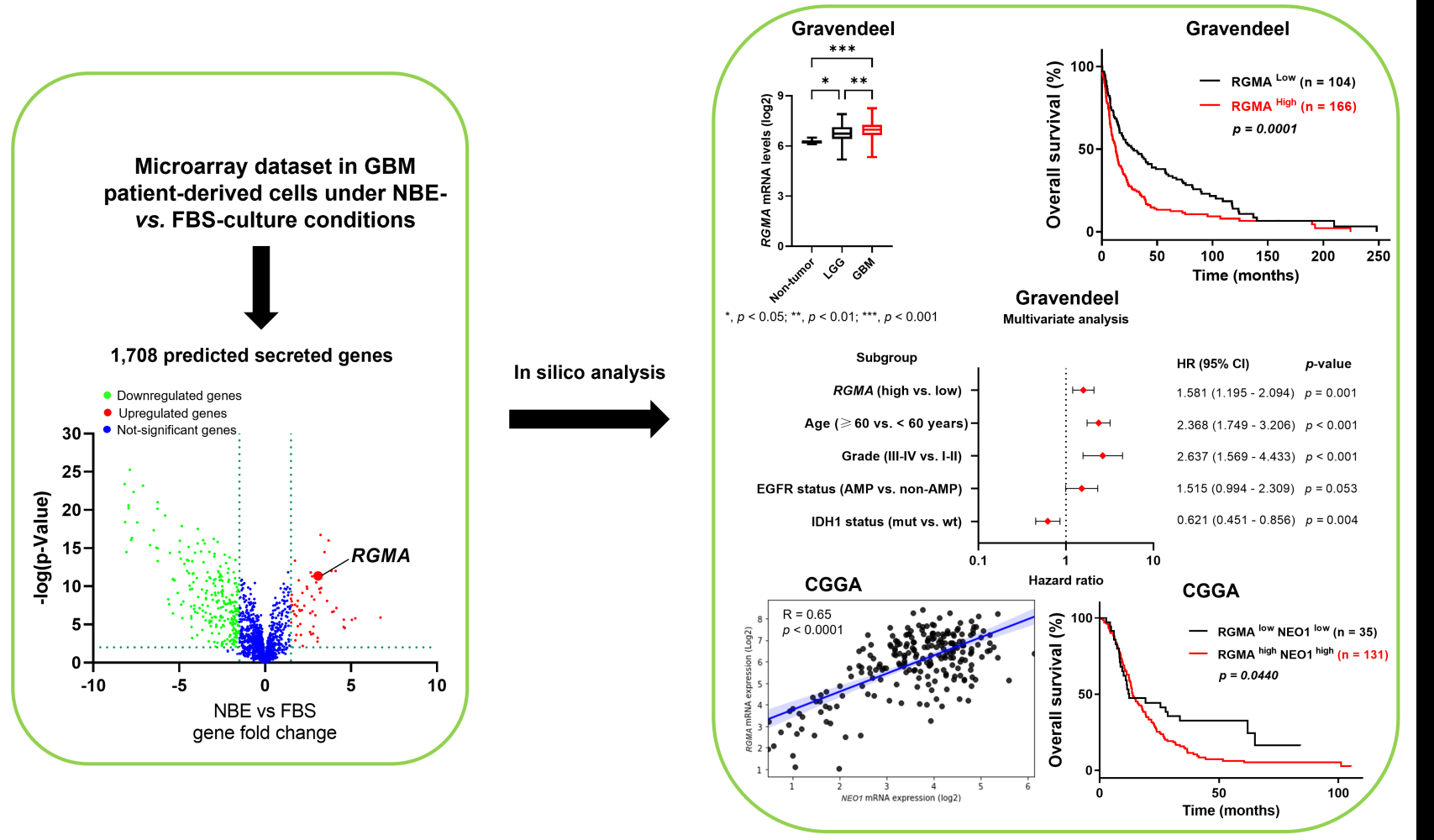
To analyze secreted gene expression patterns to regulate the stem cell character of GSCs, we generated a database of mRNA expression profiles from the public dataset GSC4536.30 We used 1708 putative secreted genes listed from the Human Protein Atlas secretome (https://www.proteinatlas.org/search/protein_class%3APredicted+secreted+proteins) (Figure 1A–C), and candidate genes with a fold change > 1.5 and p < 0.05 were selected. A total of 49 and 42 genes were selected from GSC0308 and GSC1228 cell lines, respectively. Among them, 24 genes (ADAMTS3, ADAMTS9, AZGP1, B3GAT1, BCAN, BMP7, C21orf62, CX3CL1, DHRS13, FAM131A, FGFBP3, LPL, MDK, NCAN, NXPH1, PTN, PTPRZ1, RGMA, SCG2, SCG3, SORL1, SPARCL1, TIMP4, and VGF) were found to be overlapped in both cell lines (Figure 1D) and re-displayed in heatmaps (Figure 1E and F). Based on its high basal expression and clinical relevance in GBM, the RGMA gene was selected to further investigate whether this gene is indeed involved in gliomagenesis. We found that RGMA mRNA expression level was significantly increased more than three-fold in GSC0308 and two-fold in GSC1228 under NBE-culture condition compared to that under FBS-culture condition (Figure 1G and H). We next performed qRT‐PCR analysis to measure RGMA mRNA levels in publicly available glioma cell lines (A172, A1207, LN229, and U87MG), GBM patient-derived GSC cell lines (GSC23, GSC11, GSC 20, and GSC267), and NHA. As expected, RGMA expression was significantly upregulated in glioma cell lines and GSCs, as compared to that in NHA (Figure 1I). These results indicate that RGMA is highly expressed in glioma.
RGMA is Preferentially Expressed in GBM Tissues and Correlated with Poor Prognosis
Next, we investigated the relationship between RGMA expression and glioma patient prognosis. The Gravendeel dataset showed that RGMA mRNA was highly expressed in GBM samples than in low-grade glioma and non-tumor samples (Figure 2A). Furthermore, glioma patient survival analysis revealed that high RGMA expression was correlated with short patient survival using the Kaplan-Meier curve (Figure 2B). Immunohistochemistry analysis from human protein atlas data revealed RGMA was presented in the peripheral region in the glioma tissues (Figure 2C). Collectively, these data suggest that RGMA expression is associated with glioma malignancy and tumor microenvironments.
RGMA Expression is an Independent Risk Factor Associated with Glioma Patient Survival
We further performed Cox regression analysis with the survival data from the Gravendeel dataset to analyze the relationship of the survival time of glioma patients with RGMA expression level, age, gender, tumor grade, IDH1, and EGFR status. A forest map was adopted to perform univariate and multivariate Cox regression analysis (Figure 3). Results of the univariate analysis indicated significant correlations of overall patient survival with RGMA expression level, age, grade, EGFR status, and IDH1 status (all p < 0.001) (Figure 3A and B). Furthermore, multivariate analysis confirmed that high RGMA expression (p = 0.001), age (p < 0.001), grade (p < 0.001), and IDH1 status (p = 0.004) were covariates influencing the survival of glioma patients (Figure 3C and D). Taken together, these results indicate that RGMA might be a prognostic factor that can influence the survival time of glioma patients.
RGMA, a Predictive Gene for GBM Malignancy, is Enriched in GBM Classical Subtype
To examine the preferential expression of RGMA in GBM subtypes, we used three previously defined subtypes of GBM.31,32 RGMA expression was significantly higher in classical subtype than in mesenchymal and proneural subtypes based on the Gravendeel dataset (Figure 4A). Furthermore, there was a remarkable difference between EGFR non-amplification and EGFR amplification (Figure 4B), in which EGFR vIII amplification was known as a genetic alteration of the classical subtype.31,33 In the Gravendeel dataset, RGMA mRNA levels were positively correlated with classical GSC markers (FGFR3, HEY1, NOTCH1, PDGFA, NES, and EGFR), mesenchymal GSC markers (CD44, STAT3, RELB, CHI3L1, and VIM), and proneural GSC markers (BCAN, OLIG2, and DLL3) (Figure 4C). Gene set enrichment analysis also revealed that Verhaak_Glioblastoma_Classical phenotypes were positively correlated with RGMA expression in the Gravendeel dataset (Figure 4D). We next employed the Ivy Glioblastoma Atlas dataset to understand the association of individual histologic features of an anatomic human glioblastoma with RGMA expression patterns. Results revealed that RGMA expression was significantly upregulated in the infiltration tumor (IT) and cellular tumor (CT), suggesting a possible role of RGMA during the invasion of GBM (Figure 4E and F). Collectively, these findings indicate that RGMA expression is associated with GBM’s classical signature and that it is present in the IT and CT regions of the tumor.
Identification of Co-Expressed Genes with RGMA in GBM Samples and Their Enrichment Analysis
To understand the related signaling pathways of RGMA, we summarized the down- and upregulated genes with RGMA expression from the dataset. Pearson’s correlation coefficient was used to identify co-expressed genes positively correlated with RGMA (R ≥ 0.4) in the Gravendeel dataset, and 300 co-expression genes were shown in the heatmap (Figure 5A). These genes were subjected to analyses of GO biological processes and KEGG pathways using the DAVID database. The first enriched categories were ranked based on their significant p-values and then they were visualized by utilizing the “ggplot2” R package. Regarding its biological processes, co-expressed genes were involved in cell adhesion, intracellular signal transduction, extracellular matrix organization, positive regulation of ERK1 and ERK2 cascade, positive regulation of cell division, and activation of MAPKK activity (Figure 5B). Furthermore, the co-expressed genes in KEGG categories were enriched in the regulation of actin cytoskeleton, Hippo signaling pathway, Rap1 signaling pathway, signaling pathways of regulation of pluripotency of stem cells, and TGF-β signaling pathway (Figure 5C). A previous study has reported that the Rap1 signaling pathway is involved in tumor cell proliferation and invasion,34 specifically protein Rap1A can promote GBM growth in vivo.35 Muramatsu et al36 have shown that RGMA can activate Rap1 and regulate CD4+ T cell adhesion in autoimmune encephalomyelitis disease, implying the possible role of RGMA in GBM development through Rap1 pathway activation. Collectively, the elevated expression of RGMA may be associated with dysregulated growth signaling and immune response in the microenvironments of GBM.
RGMA Expression is Positively Correlated with Its NEO1 Receptor in GBM Samples
NEO1 is known as a receptor of RGMA and their interaction has been shown in neural development.24,37 We then hypothesized that RGMA might also be associated with NEO1 in GBM. Results revealed that RGMA expression was significantly correlated with NEO1 in Gravendeel (R = 0.26), CGGA (R = 0.65), TCGA GBM (R = 0.41), and Rembrandt (R = 0.32) datasets (Figure 6A). Importantly, high expression levels of both RGMA and NEO1 in GBM tissues were correlated with poor patient survival (Figure 6B). This result suggests that co-activation of RGMA and NEO1 might be an important signaling event that occurs during GBM tumorigenesis.
In conclusion, we found that RGMA is overexpressed in GBM samples and that RGMA expression is linked to poor glioma patient survival. As a result, RGMA could be a potential target for GBM treatment.
Discussion
High-grade glioma is featured by striking heterogeneous cellularity, encompassing GSCs and non-neoplastic tumor cells which consist of endothelial cells, vascular pericytes, astrocytes, tumor-associated macrophages, and other immune cells.4,38 Despite the best available treatment, this tumor can still recur and become resistant to treatment.39 Lee et al30 have reported that NBE cultured cells show more aggressiveness in tumor growth and mimic the phenotype and biology of primary tumors than FBS cultured cells. By performing bioinformatics analysis, we tried to find a biomarker to predict GBM tumorigenesis. Finally, we found that RGMA, a secreted protein-encoding gene, was highly expressed under the NBE culture condition. RGMA expression was remarkably increased in glioma and GSC cell lines. This finding indicated that RGMA might give rise to tumorigenesis and support the self-renewal of GSCs. RGMA expression was also increased in GBM tissues than in either low-grade tumors or non-tumor tissues and such high expression of RGMA was correlated with devastating outcomes of glioma patients. Taken together, these results postulate that RGMA is a potential biomarker for predicting glioma patient survival.
Identifying multiple subtypes within GBM has revealed distinct molecular alteration and tumoral heterogeneity which reflect tumor histology and are related to the clinical outcome of the disease.33 By performing classification of GBM subtypes,31,32 the present study indicated that RGMA expression was preferentially expressed in the classical subtype with high EGFR amplification. Hence, further experiments with EGFR inhibitors are needed to interpret RGMA-related pathways that correlate with a worse prognosis of GBM patients. Meanwhile, genes co-expressed with RGMA in the Gravendeel dataset indicated that they were mainly enriched in cell adhesion, intracellular signal, extracellular matrix organization, positive regulation of ERK1 and ERK2 cascade, positive regulation of cell division, activation of MAPKK activity, regulation of actin cytoskeleton, Hippo or Rap1 signaling pathway, regulation of pluripotency of stem cells, and TGF-β signaling pathways. Recent studies have shown that TGF-β signaling pathways are dysregulated in cancer and play a role in the initiation and advancement of glioma cells.40 On the other hand, ERK1 and ERK2 were found to activate matrix metalloproteinase molecules and engage many cancer hallmarks to promote tumor invasion and preserve GSCs.41 Also, earlier reports have shown that active MAPKK can stimulate the MAPK pathway, which then activates transcription factors such Elk1, c-MYC, STAT1/3, and PPARγ, resulting in cell transformation and glioma cell apoptosis.42 Additionally, Hippo pathway-mediated pro-tumoral immunosuppression was found to be associated with poor GBM outcomes.43,44 Lastly, Rap1 signaling is upregulated in GBMs, and this increase is linked to a higher tumor grade by influencing GSC migration.35 Thus, we can infer that RGMA and its co-expressed genes are involved in a complex network that controls GBM development.
Meanwhile, previous studies have also shown that when RGMA binds to its NEO1 receptor, it activates the RhoA-ROCK1/Rho-kinase signaling cascade, inhibiting neurite outgrowth.25 The Rho/ROCK or RhoA/Rho kinase activation can influence GBM cell growth and migration by interacting with different pathways.45 NEO1 also has been identified as a netrin family receptor. In GBM, the overexpression of either netrin-1 or netrin-4 can mediate the enhanced invasiveness of GBM cells and predict poor patient survival.46,47 Meanwhile, NEO1 is abundantly expressed in astrocytes of the brain and it is involved in regulating cortical blood vessel homeostasis and function.26
In brain tumors, the tumor microenvironment holds a critical role in cancer development, propagation, and therapy response.9,38 As one of the major stromal cells in the brain, astrocytes account for nearly 50% of brain cells. They urge cell-cell communications, reinforce the neural network, and maintain neural circuits in the human central nervous system.38 In particular, tumor-associated astrocytes contribute to tumor malignancy by engendering cell proliferation, eventually affecting anti-inflammatory responses.11,13,28 GBM tumors can also alter the local microenvironment, increasing astrocyte cytokine production and encouraging astrocyte migration. Also, GBM cells may cooperate with their neighboring astrocyte to enhance tumor recurrence.38,48 In this study, RGMA was shown to be positively correlated with the expression of NEO1 in public datasets. We assume RGMA is released into the tumor microenvironment by glioma cells to interact with NEO1 in astrocytes, which are ubiquitously tiled surrounding GBM cells. This binding might contribute to the role of tumor-associated astrocytes in forming reactive astrocytes and facilitating tumor proliferation and migration.11,28 Moreover, high concurrent expression of RGMA and NEO1 in GBM patients is associated with poor survival outcomes. Nevertheless, the interaction between RGMA and NEO1 entails future investigations to understand their synergistic effects on GBM tumor malignancy and aggressiveness.
Conclusion
By applying bioinformatics analysis, we profiled the clinical value of RGMA in glioma. Our results demonstrate that RGMA is a promising biomarker for GBM occurrence. Hence, it could open opportunities to develop more relevant therapies to treat GBM.
Acknowledgments
This research was supported by a grant (no. 2019R1I1A3A01059211) of the National Research Foundation (NRF) funded by the Ministry of Science and ICT (MSIT), Republic of Korea.
Disclosure
The authors have no conflicts of interest relevant to this study to disclose.
References
1. Lapointe S, Perry A, Butowski NA. Primary brain tumours in adults. Lancet. 2018;392(10145):432–446. doi:10.1016/S0140-6736(18)30990-5
2. Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy–temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. doi:10.1056/NEJMoa1308345
3. Prager BC, Bhargava S, Mahadev V, Hubert CG, Rich JN. Glioblastoma stem cells: driving resilience through chaos. Trends Cancer. 2020;6(3):223–235. doi:10.1016/j.trecan.2020.01.009
4. Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355(12):1253–1261. doi:10.1056/NEJMra061808
5. Bradshaw A, Wickremesekera A, Brasch HD, et al. Cancer stem cells in glioblastoma. Genes Dev. 2015;29(12):1203–1217. doi:10.1101/gad.261982.115
6. Tao W, Chu C, Zhou W, et al. Dual role of WISP1 in maintaining glioma stem cells and tumor-supportive macrophages in glioblastoma. Nat Commun. 2020;11(1). doi:10.1038/s41467-020-16827-z
7. Yan K, Wu Q, Yan DH, et al. Glioma cancer stem cells secrete Gremlin1 to promote their maintenance within the tumor hierarchy. Genes Dev. 2014;28(10):1085–1100. doi:10.1101/gad.235515.113
8. Baghban R, Roshangar L, Jahanban-esfahlan R, Seidi K, Ebrahimi-kalan A. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun Signal. 2020;18(1):1–19. doi:10.1186/s12964-020-0530-4
9. Li G, Qin Z, Chen Z, Xie L, Wang R, Zhao H. Tumor microenvironment in treatment of glioma. Open Med. 2017;12(1):247–251. doi:10.1515/med-2017-0035
10. Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21(3):309–322. doi:10.1016/j.ccr.2012.02.022
11. Zhang H, Zhou Y, Cui B, Liu Z, Shen H. Novel insights into astrocyte-mediated signaling of proliferation, invasion, and tumor immune microenvironment in glioblastoma. Biomed Pharmacother. 2020;126:110086. doi:10.1016/j.biopha.2020.110086
12. Okolie O, Bago JR, Schmid RS, et al. Reactive astrocytes potentiate tumor aggressiveness in a murine glioma resection and recurrence model. Neuro Oncol. 2016;18(12):1622–1633. doi:10.1093/neuonc/now117
13. Guan X, Hasan MN, Maniar S, Jia W, Sun D. Reactive astrocytes in glioblastoma multiforme. Mol Neurobiol. 2018;55(8):6927–6938. doi:10.1007/s12035-018-0880-8
14. Wu Q, Sun CC, Lin HY, Babitt JL. Repulsive guidance molecule (RGM) family proteins exhibit differential binding kinetics for bone morphogenetic proteins (BMPs). PLoS One. 2012;7(9):3–10.
15. Corradini E, Babitt JL, Lin HY. The RGM/DRAGON family of BMP co-receptors. Cytokine Growth Factor Rev. 2009;20(5–6):389–398. doi:10.1016/j.cytogfr.2009.10.008
16. Matsunaga E, Nakamura H, Che A, Curie-paris PM. Repulsive guidance molecule plays multiple roles in neuronal differentiation and axon guidance. J Neurosci. 2006;26(22):6082–6088. doi:10.1523/JNEUROSCI.4556-05.2006
17. Metzger M, Conrad S, Skutella T, Just L. RGMa inhibits neurite outgrowth of neuronal progenitors from murine enteric nervous system via the neogenin receptor in vitro. J Neurochem. 2007;103(6):2665–2678.
18. Siebold C, Yamashita T, Monnier PP, Mueller BK, Pasterkamp RJ. RGMs: structural insights, molecular regulation, and downstream signaling. Trends Cell Biol. 2017;27(5):365–378. doi:10.1016/j.tcb.2016.11.009
19. Zhao ZW, Lian WJ, Chen GQ, et al. Decreased expression of repulsive guidance molecule member A by DNA methylation in colorectal cancer is related to tumor progression. Oncol Rep. 2012;27(5):1653–1659.
20. Li J, Ye L, Kynaston HG, Jiang WG. Repulsive guidance molecules, novel bone morphogenetic protein co-receptors, are key regulators of the growth and aggressiveness of prostate cancer cells. Int J Oncol. 2012;40(2):544–550.
21. Li VSW, Yuen ST, Chan TL, et al. Frequent inactivation of axon guidance molecule RGMA in human colon cancer through genetic and epigenetic mechanisms. Gastroenterology. 2009;137(1):176–187. doi:10.1053/j.gastro.2009.03.005
22. Bao C, Lu Y, Chen J, et al. Exploring specific prognostic biomarkers in triple-negative breast cancer. Cell Death Dis. 2019;10:11. doi:10.1038/s41419-019-2043-x
23. Bell CH, Healey E, Van Erp S, et al. Structure of the repulsive guidance molecule (RGM)-neogenin signaling hub. Science. 2013;341(6141):77–80. doi:10.1126/science.1232322
24. Key B, Lah GJ. Repulsive guidance molecule A (RGMa). Cell Adh Migr. 2012;6(2):85–90. doi:10.4161/cam.20167
25. Conrad S, Genth H, Hofmann F, Just I, Skutella T. Neogenin-RGMa signaling at the growth cone is bone morphogenetic protein-independent and involves RhoA, ROCK, and PKC. J Biol Chem. 2007;282(22):16423–16433. doi:10.1074/jbc.M610901200
26. Yao LL, Hu JX, Li Q, et al. Astrocytic neogenin/netrin-1 pathway promotes blood vessel homeostasis and function in mouse cortex. J Clin Invest. 2020;130(12):6490–6509. doi:10.1172/JCI132372
27. Zhang R, Wu Y, Xie F, et al. RGMa mediates reactive astrogliosis and glial scar formation through TGFβ1/Smad2/3 signaling after stroke. Cell Death Differ. 2018;25(8):1503–1516. doi:10.1038/s41418-018-0058-y
28. Heiland H, Ravi D, Behringer VM, et al. Tumor-associated reactive astrocytes aid the evolution of immunosuppressive environment in glioblastoma. Nat Commun. 2019;10(1):1–12.
29. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25(4):402–408. doi:10.1006/meth.2001.1262
30. Lee J, Kotliarova S, Kotliarov Y, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9(5):391–403. doi:10.1016/j.ccr.2006.03.030
31. Verhaak RGW, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. doi:10.1016/j.ccr.2009.12.020
32. Wang Q, Hu B, Hu X, et al. Tumor evolution of glioma-intrinsic gene expression subtypes associates with immunological changes in the microenvironment. Cancer Cell. 2017;32(1):42–56. doi:10.1016/j.ccell.2017.06.003
33. Dunn GP, Rinne ML, Wykosky J, et al. Emerging insights into the molecular and cellular basis of glioblastoma. Genes Dev. 2012;26:756–784. doi:10.1101/gad.187922.112
34. Zhang Y, Wang R, Cheng K, Ring BZ, Su L. Roles of Rap1 signaling in tumor cell migration and invasion. Cancer Biol Med. 2017;14(1):90–99. doi:10.20892/j.issn.2095-3941.2016.0086
35. Sayyah J, Bartakova A, Nogal N, Quilliam LA, Stupack DG, Brown JH. The Ras-related protein, Rap1A, mediates thrombin-stimulated, integrin-dependent glioblastoma cell proliferation and tumor growth. J Biol Chem. 2014;289(25):17689–17698. doi:10.1074/jbc.M113.536227
36. Muramatsu R, Kubo T, Mori M, et al. RGMa modulates T cell responses and is involved in autoimmune encephalomyelitis. Nat Med. 2011;17(4):488–494. doi:10.1038/nm.2321
37. Yamashita T, Mueller BK, Hata K. Neogenin and repulsive guidance molecule signaling in the central nervous system. Curr Opin Neurobiol. 2007;17(1):29–34. doi:10.1016/j.conb.2006.12.001
38. Quail DF, Joyce JA. The microenvironmental landscape of brain tumors. Cancer Cell. 2017;31(3):326–341. doi:10.1016/j.ccell.2017.02.009
39. Norden AD, Drappatz J, Wen PY. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. doi:10.1056/NEJMra0708126
40. Han J, Alvarez-Breckenridge CA, Wang QE, Yu J. TGF-β signaling and its targeting for glioma treatment. Am J Cancer Res. 2015;5(3):945–955.
41. Hannen R, Hauswald M, Bartsch JW. A rationale for targeting extracellular regulated kinases ERK1 and ERK2 in glioblastoma. J Neuropathol Exp Neurol. 2017;76(10):838–847. doi:10.1093/jnen/nlx076
42. Nakada M, Kita D, Watanabe T, et al. Aberrant signaling pathways in glioma. Cancers. 2011;3(3):3242–3278. doi:10.3390/cancers3033242
43. Masliantsev K, Karayan-tapon L, Guichet P. Hippo signaling pathway in gliomas. Cells. 2021;10(1):1–14. doi:10.3390/cells10010184
44. Kim EH, Sohn BH, Eun Y, et al. Silence of hippo pathway associates with pro-tumoral immunosuppression: potential therapeutic target of glioblastomas. Cells. 2020;9(8):1–16. doi:10.3390/cells9081761
45. Zohrabian VM, Forzani B, Chau Z. Rho/ROCK and MAPK signaling pathways are involved in glioblastoma cell migration and proliferation. Anticancer Res. 2009;29(1):119–123.
46. Hu Y, Ylivinkka I, Chen P, et al. Netrin-4 promotes glioblastoma cell proliferation through integrin β4 signaling. Neoplasia. 2012;14(3):219–227. doi:10.1593/neo.111396
47. Ylivinkka I, Hu Y, Chen P, Rantanen V, Hautaniemi S, Nyman TA. Netrin-1-induced activation of Notch signaling mediates glioblastoma cell invasion. J Cell Sci. 2013;126:2459–2469.
48. Brandao M, Simon T, Critchley G, Giamas G. Astrocytes, the rising stars of the glioblastoma microenvironment. Glia. 2019;67(5):779–790. doi:10.1002/glia.23520
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.