Back to Journals » Cancer Management and Research » Volume 12
Elevated Platelet Count Predicts Poor Prognosis in Breast Cancer Patients with Supraclavicular Lymph Node Metastasis
Authors Liu S, Fang J , Jiao D, Liu Z
Received 10 April 2020
Accepted for publication 8 July 2020
Published 20 July 2020 Volume 2020:12 Pages 6069—6075
DOI https://doi.org/10.2147/CMAR.S257727
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Antonella D'Anneo
Shaoqing Liu, Jing Fang, Dechuang Jiao, Zhenzhen Liu
Department of Breast Surgery, Breast Cancer Center, Affiliated Cancer Hospital of Zhengzhou University, Zhengzhou, People’s Republic of China
Correspondence: Zhenzhen Liu
Department of Breast Surgery, Breast Cancer Center, Affiliated Cancer Hospital of Zhengzhou University, Zhengzhou, People’s Republic of China
Tel +86 13603862755
Email [email protected]
Background: More and more studies show that platelets are closely related to the occurrence and development of tumors. This study aims to explore the predictive value of peripheral blood platelet count on the prognosis of breast cancer patients with ipsilateral supraclavicular lymph node (ISLN) metastasis.
Methods: Eighty-five breast cancer patients with ISLN metastasis in the Affiliated Cancer Hospital of Zhengzhou University were collected retrospectively in this study. Chi-square test was used to analyze the correlation between clinical pathological data and platelet count. DFS rate was estimated by K-M curve and Log Rank test was performed. Univariate and multivariate Cox regression were used to determine the prognostic value of platelets. Time-dependent Cox regression was used to further analyze the correlation between peripheral blood platelets and prognosis to determine the stability of the results.
Results: The pathological complete response rate of ISLN after neoadjuvant chemotherapy (NAC) was 51.8%. Platelet count was correlated with PR status of breast cancer at first visit (P=0.01). After a median follow-up of 30 months, multivariate Cox analysis showed that high platelet count (HR=3.18, 95% CI=1.13– 8.93, P=0.028), premenopausal status (HR=0.40, 95% CI=0.17– 0.97, P=0.043), and ISLN pathological failure (HR=0.25 95%, CI=0.10– 0.62, P< 0.01) were associated with poor prognosis. K-M curve analysis showed that the prognosis of patients with a high platelet count was worse than that of patients with low platelet count (HR=5.32, 95% CI=2.41– 11.75, P< 0.01). To further verify the stability of this result, multivariate time-dependent Cox model also suggested that higher platelet level was related to poor prognosis (HR=1.009, 95% CI=1.003– 1.016, P< 0.01). Meanwhile, menopausal status (HR=0.32, 95% CI=0.14– 0.76, P=0.01) and sPCR (HR=0.29, 95% CI=0.12– 0.70, P=0.01) were also independent predictors of DFS.
Conclusion: Platelets have important predictive value for the prognosis of breast cancer patients with ISLN metastasis, which indicates that platelet count can be used to distinguish high-risk patients so as to obtain clinical benefits.
Keywords: platelet, neoadjuvant chemotherapy, prognosis, ipsilateral supraclavicular lymph nodes
Introduction
Breast cancer patients with ipsilateral supraclavicular lymph node (ISLN) metastasis without distant metastasis account for about 1–4.3% of all breast cancer patients.1,3 Metastasis of ISLN often indicates a poor prognosis, with a median survival time of 2–4 years.4,5 In the sixth edition of AJCC’s TNM staging of breast cancer, the ISLN metastasis is considered as N3C stage, and breast cancer with ISLN metastasis without distant metastasis is considered as locally advanced lesion, belonging to the IIIC stage.6 This indicates that such patients have changed from incurable to curable. Platelets are an important component of thrombosis and hemostasis. Studies have found that platelets interact with cancer cells. On the one hand, platelets promote tumor cell migration and invasion,7 participate in tumor cell metastasis,8 and protect tumor cells from immune elimination.9 On the other hand, cancer cells can activate platelets, thus rapidly changing their morphology.10 Cancer cells can also drive thrombocytosis and cause platelet aggregation, and thrombocytosis is a prognostic factor for inflammatory breast cancer.11,12 Previous studies have shown that increased platelet count is associated with poor disease-free survival (DFS) and overall survival of various cancers.13,15 However, the prognostic value of the platelet count for locally advanced breast cancer patients with ISLN metastasis is rarely reported. The purpose of this study is to explore the prognostic value of peripheral blood platelet count in breast cancer patients with ISLN metastasis.
Patients and Methods
Enrolled Patients
This study continuously and retrospectively collected 85 breast cancer patients with simultaneous ISLN metastasis from January 2012 to December 2019 in Affiliated Cancer Hospital of Zhengzhou University. Inclusion criteria: 1) diagnosis of unilateral primary invasive ductal breast cancer at first visit; 2) biopsy confirmation of ISLN metastasis; and 3) treatment measures include NAC, surgical resection, and radiotherapy. Among them, the NAC regimen is standard including EC-T8 (Epirubicin, Cyclophosphamide, Taxotere, eight cycles), TEC6 (Taxotere, Epirubicin, Cyclophosphamide, six cycles), and TCbH6 (Taxotere, Carboplatin, Herceptin, six cycles); 4) Complete peripheral blood platelet count at baseline and during treatment. Exclusion criteria: 1) Pathological biopsy confirmed distant metastasis at first visit; 2) accompanied by other tumors or with tumor history.
Clinical Data Collection
Clinical data were obtained by collecting information from patients’ electronic medical records. Clinical data include age at diagnosis, menopausal status, and clinical T staging. The state of ER, PR, and HER2 were obtained by immunohistochemistry of biopsy specimen; Chemotherapy scheme; Pathological response of tumor masses, axillary lymph nodes, and ISLN after NAC; Complete peripheral blood platelet count at baseline, after NAC (detected immediately after the last course of NAC), before surgery, before radiotherapy, and after radiotherapy (detected immediately after the last course of radiotherapy). Platelet count was obtained by routine laboratory examination of blood. The outpatient registration system was used for follow-up and DFS was determined. Pathological complete response (pCR) is defined as Miller-Payne grade 5 of tumor after NAC or only residual carcinoma in catheter.16 DFS is defined as: no local or regional recurrence, no distant recurrence, and no contralateral invasive breast cancer. This study was approved by the Ethics Committee of the Affiliated Cancer Hospital of Zhengzhou University.
Statistical Analysis
SPSS 23.0 and R software were used for statistical analysis in this study. The ROC curve is a curve drawn according to a series of different dichotomies with a true positive rate as the ordinate and false positive rate as the abscissa. It is mainly used for analysis and evaluation of dichotomies discrimination effect. Since there was no effective cutoff value, the best cutoff value for platelet diagnosis of long-term recurrence is obtained by using the ROC curve. The best cutoff value is the maximum value of the sum of sensitivity and specificity on the ROC curve. The correlation between clinical data and platelets was analyzed by bilateral Chi-square test. DFS rate under different platelet counts were estimated using K-M curves and Log Rank test was performed. Univariate Cox regression was used to analyze the relationship between clinicopathological factors and recurrence risk, and multivariate Cox regression was used to determine independent prognostic factors. In order to verify the stability of the results, our study further considered the peripheral blood platelet count as a time-dependent covariate and collected the complete peripheral blood platelet count at five time points including baseline, after NAC, before surgery, before radiotherapy, and after radiotherapy. Univariate and multivariate Cox models were used to determine the predictive value of platelet count for prognosis, and confounding factors were corrected. The risk ratio of covariates, 95% confidence interval, and Wald test P-value were reported. P-values less than 0.05 in bilateral tests are considered to have statistical significance.
Results
Patient Baseline Characteristics
According to the inclusion criteria, we identified 85 breast cancer patients with ISLN metastasis. Among them, the age distribution at first visit was from 19–64 (median=48), 45.9% (39) of the patients were postmenopausal, and 72.9% (62) of the patients were diagnosed with cT1–2. After NAC, the pCR rate of breast masses was 22.4% (19), axillary lymph node was 36.5% (31), and 51.8% (44) breast cancer patients reached supraclavicular lymph node pathological complete response (sPCR). Fifty-five, 49, and 25 breast cancer patients were ER, PR, and HER2 positive, respectively. Figure 1 showed platelet count at five different time points (mean±SD). In all patients, the mean value of peripheral blood platelet count at first visit was 261.08±67.61 (109/L). According to ROC curve analysis results, the best cut-off value for platelet diagnosis of long-term recurrence was 229.50.
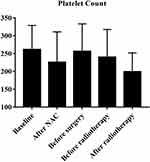 |
Figure 1 Platelet count at five different time points. |
Correlation Between Clinicopathological Data and Platelet Count
Table 1 shows the results of correlation analysis between clinical pathological data and platelet count. Among them, platelet count was correlated with PR status. Compared with the low platelet count group (Platelet<229.50), the PR positive rate in the high platelet count group (Platelet ≥229.50) was higher (P=0.01).
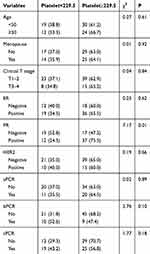 |
Table 1 Baseline Characteristics of Patients |
High Platelet Count Indicated Poor Prognosis
The outpatient registration system was used for follow-up, with a median follow-up time of 30 months (8–67 months), of which 29.4% (25) of patients had distant metastasis. First of all, we used univariate and multivariate Cox regression to analyze the prognostic value of clinicopathological data, of which platelet count at first visit was a binary variable (Table 2). Univariate analysis showed that platelet count (HR=2.94, 95% CI=1.07–8.10, P=0.037) and sPCR (HR=0.23, 95% CI=0.09–0.61, P<0.01) were correlated with prognosis. Multivariate analysis further showed that platelet count (HR=3.18, 95% CI=1.13–8.93, P=0.028), sPCR (HR=0.25, 95% CI=0.10–0.62, P<0.01), and menopausal status (HR=0.40, 95% CI=0.17–0.97, P=0.043) were independent predictors of prognosis. K-M curve analysis showed that the prognosis of patients with a high platelet count was worse than that of patients with a low platelet count (HR=5.32 95% CI=2.41–11.75, P<0.01; Figure 2). In order to verify the stability of the results, we further analyzed the correlation between clinicopathological data and prognosis using a time-dependent Cox model, and corrected other baseline characteristics, in which platelet count is a time-dependent covariate (Table 3). Data of peripheral blood platelet count at five time points including baseline, post-NAC, pre-surgery, pre-radiotherapy, and post-radiotherapy were included in the model. The multivariate time-dependent Cox model showed that high platelets were associated with poor prognosis (HR=1.009, 95% CI=1.003–1.016, P<0.01), menopausal status (HR=0.32, 95% CI=0.14–0.76, P=0.01), sPCR (HR=0.29, 95% CI=0.12–0.70, P=0.01), which were independent predictors of DFS.
 |
Table 2 Univariate and Multivariate Analysis for DFS |
 |
Table 3 Time-Dependent Cox Regression Analysis of Time-Varying Platelet Count |
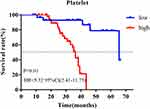 |
Figure 2 K-M analysis of different platelet counts. |
Discussion
Platelets played an important role in tumor cell migration, invasion, and metastasis. Platelets were prognostic factors for many tumors, and high platelets were associated with poor prognosis. ISLN metastasis was an important indicator of poor prognosis in breast cancer patients. In this study, the prognostic value of platelets in breast cancer patients with ISLN metastasis was analyzed in order to provide help for clinical decision-making of such patients.
At present, breast cancer patients with ISLN metastasis were considered as locally advanced breast cancer, namely IIIC stage. For these patients, NAC, surgical resection of the primary lesion, combined with local radiotherapy and other comprehensive treatments, were mostly used clinically, but the clinical benefits of supraclavicular lymph node dissection were still controversial.17,20 NAC can reduce tumor size and tumor stage, thus improving breast-conserving rate and increasing clinical survival benefits.21 Consistent with previous studies, the pCR rate of the axillary lymph node after NAC was higher than that of breast Pcr.22 In this study, the pCR rate of axillary lymph node was 36.5%, and the pCR rate of breast was 22.4%. Previous studies have shown that the pCR of breast and axillary lymph nodes can be regarded as a surrogate for the curative effect of NAC.23 The pCR of ISLN was significantly increased to 51.8%, suggesting that ISLN were more sensitive to NAC than breast and axillary lymph nodes. Then whether sPCR can be used as a surrogate for the curative effect of NAC like bPCR and aPCR and which was more accurate was worth further exploration.
After a 30-month median follow-up period, 29.4% (25) patients had distant metastasis. Previous studies have shown that pCR of ISLN after NAC was related to a local control rate and favorable DFS.24 In this study, the recurrence risk of the ISLN pCR group was 0.29 times that of the non-pCR group (HR=0.29, 95% CI=0.12–0.70, P=0.01). Previous studies have found that high platelet count before treatment was associated with poor prognosis of breast cancer.25 The results of this study showed that in breast cancer patients with ISLN metastasis, higher platelets was associated with poor DFS (HR=1.009, 95% CI=1.003–1.016, P<0.01), and the stability of this result was verified.
In addition, the study found that menopausal status was an independent prognostic factor for breast cancer patients with ISLN metastasis, and the recurrence risk of postmenopausal patients was 0.32-times that of premenopausal patients (HR=0.32, 95% CI=0.14–0.76, P=0.01). This result was inconsistent with our previous findings that menopausal status was not related to the prognosis of breast cancer patients with ISLN metastasis.26 We think the reasons were as follows: there were differences in the follow-up time between the two studies. The median follow-up time in this study was 30 months, while the median follow-up time in a previous study was 16.2 months. The median survival time of the breast cancer patients with ISLN metastasis was 24–48 months, which indicated that the follow-up time of the two studies was relatively short, and the short-term follow-up may cause the prognostic value of some clinicopathological indicators to fail to appear. In addition, there were differences in the chemotherapy regimens of the two studies. In this study, the NAC regimens were the standard regimens, so the influence of differences in NAC regimens on the prognosis was excluded, while the NAC regimens for patients in a previous study were different.
Previous studies have shown that high levels of platelets (Platelet ≥350) were associated with high expression of HER2 in breast cancer before treatment,25 while no correlation between platelets and HER2 expression had been found in this study. When the platelet level in this study was divided into high expression and low expression by the boundary of 350 (109/L), the platelet level and HER2 expression still had no correlation (results were not shown), which indicated that there were differences in the correlation between platelet level and HER2 expression in different types of breast cancer. In addition, some studies have found that when the level of platelet P-selectin was high, the hormone-treated T47D cell line showed larger membrane folds than the MCF-7 cell line.27 The T47D cell line has a lower expression of estrogen receptor and higher expression of progesterone receptor than MCF-7 cell line, thus showing higher invasiveness.28 This indicated that the difference in hormone receptors may result in different abilities of activating platelets. This study found that the PR positive rate of breast cancer was higher in the high platelet group (P=0.01). These clinical data and cell experiments showed that platelets were related to PR, but whether the ability of PR to activate platelets at cell level can directly result in a high PR positive rate of breast cancer in the high platelet group still needs further research.
There are some deficiencies in this study. First of all, this study is a retrospective clinical study. The evidence level is low and there may be bias. In addition, the research sample size is medium, and the follow-up time is relatively short, so the clinical research verification results of long-term follow-up of large samples are still needed.
In conclusion, as far as we know, this study is the first to collect breast cancer patients with ISLN metastasis and study the predictive value of peripheral blood platelet count on their prognosis. Our study shows that higher platelet levels are associated with poor DFS (HR=1.009, 95% CI=1.003–1.016, P<0.01), suggesting that platelets can be used as a clinical marker to predict the prognosis of breast cancer patients with ISLN metastasis.
Abbreviations
ISLN, ipsilateral supraclavicular lymph nodes; NAC, neoadjuvant chemotherapy; ROC, receiver operating characteristics; pCR, pathological complete response; DFS, disease-free survival; TCIPA, tumor cell-induced platelet aggregation; sPCR, supraclavicular lymph node pathological complete response; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of the Affiliated Cancer Hospital of Zhengzhou University and complied with the Declaration of Helsinki. The Ethics Committee of the Affiliated Cancer Hospital of Zhengzhou University did not require patients to agree to review their medical records (medical records were written by doctors and belonged to the medical records room. On the premise of not disclosing the privacy of patients, doctors can use it for clinical research).
Acknowledgments
We thank the Department of Pathology, Affiliated Cancer Hospital of Zhengzhou University for providing us with the data of tissue.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Galper S, Recht A, Silver B, et al. Factors associated with regional nodal failure in patients with early stage breast cancer with 0–3 positive axillary nodes following tangential irradiation alone. Int J Radiat Oncol Biol Phys. 1999;45(5):1157–1166. doi:10.1016/s0360-3016(99)00334-x
2. Recht A, Gray R, Davidson NE, et al. Locoregional failure 10 years after mastectomy and adjuvant chemotherapy with or without tamoxifen without irradiation: experience of the Eastern Cooperative Oncology Group. J Clin Oncol. 1999;17(6):1689–1700. doi:10.1200/JCO.1999.17.6.1689
3. Chen SC, Chen MF, Hwang TL, et al. Prediction of supraclavicular lymph node metastasis in breast carcinoma. Int J Radiat Oncol Biol Phys. 2002;52(3):614–619. doi:10.1016/s0360-3016(01)02680-3
4. Bonotto M, Gerratana L, Poletto E, et al. Measures of outcome in metastatic breast cancer: insights from a real-world scenario. Oncologist. 2014;19(6):608–615. doi:10.1634/theoncologist.2014-0002
5. Dawood S, Broglio K, Buzdar AU, Hortobagyi GN, Giordano SH. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol. 2010;28(1):92–98. doi:10.1200/JCO.2008.19.9844
6. Singletary SE, Allred C, Ashley P, et al. Revision of the American Joint Committee on Cancer staging system for breast cancer. J Clin Oncol. 2002;20(17):3628–3636. doi:10.1200/JCO.2002.02.026
7. Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20(5):576–590. doi:10.1016/j.ccr.2011.09.009
8. Foss A, Munoz-Sagredo L, Sleeman J, Thiele W. The contribution of platelets to intravascular arrest, extravasation, and outgrowth of disseminated tumor cells. Clin Exp Metastasis. 2020;37(1):47–67. doi:10.1007/s10585-019-10009-y
9. Nieswandt B, Hafner M, Echtenacher B, Mannel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999;59(6):1295–1300.
10. Augustine TN, van der Spuy WJ, Kaberry LL, Shayi M. Thrombin-mediated platelet activation of lysed whole blood and platelet-rich plasma: a comparison between platelet activation markers and ultrastructural alterations. Microsc Microanal. 2016;22(3):630–639. doi:10.1017/S1431927616000854
11. Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost. 2011;9(2):237–249. doi:10.1111/j.1538-7836.2010.04131.x
12. Harano K, Kogawa T, Wu J, et al. Thrombocytosis as a prognostic factor in inflammatory breast cancer. Breast Cancer Res Treat. 2017;166(3):819–832. doi:10.1007/s10549-017-4463-6
13. Sylman JL, Mitrugno A, Tormoen GW, Wagner TH, Mallick P, McCarty OJT. Platelet count as a predictor of metastasis and venous thromboembolism in patients with cancer. Converg Sci Phys Oncol. 2017;3:2. doi:10.1088/2057-1739/aa6c05
14. Bai YY, Du L, Jing L, et al. Clinicopathological and prognostic significance of pretreatment thrombocytosis in patients with endometrial cancer: a meta-analysis. Cancer Manag Res. 2019;11:4283–4295. doi:10.2147/CMAR.S186535
15. Zhang X, Lv Z, Yu H, Zhu J. The clinicopathological and prognostic role of thrombocytosis in patients with cancer: a meta-analysis. Oncol Lett. 2017;13(6):5002–5008. doi:10.3892/ol.2017.6054
16. Ogston KN, Miller ID, Payne S, et al. A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast. 2003;12(5):320–327. doi:10.1016/s0960-9776(03)00106-1
17. Grotenhuis BA, Klem TM, Vrijland WW. Treatment outcome in breast cancer patients with ipsilateral supraclavicular lymph node metastasis at time of diagnosis: a review of the literature. Eur J Surg Oncol. 2013;39(3):207–212. doi:10.1016/j.ejso.2012.11.002
18. Liu XH, Zhang L, Chen B. A meta-analysis of the prognosis in patients with breast cancer with ipsilateral supraclavicular lymph node metastasis versus patients with stage IIIb/c or IV breast cancer. Chronic Dis Transl Med. 2015;1(4):236–242. doi:10.1016/j.cdtm.2016.01.002
19. Jung J, Kim SS, Ahn SD, et al. Treatment outcome of breast cancer with pathologically proven synchronous ipsilateral supraclavicular lymph node metastases. J Breast Cancer. 2015;18(2):167–172. doi:10.4048/jbc.2015.18.2.167
20. Nikpayam M, Uzan C, Rivera S, et al. Impact of radical surgery on outcome in locally advanced breast cancer patients without metastasis at the time of diagnosis. Anticancer Res. 2015;35(3):1729–1734.
21. Berruti A, Amoroso V, Gallo F, et al. Pathologic complete response as a potential surrogate for the clinical outcome in patients with breast cancer after neoadjuvant therapy: a meta-regression of 29 randomized prospective studies. J Clin Oncol. 2014;32(34):3883–3891. doi:10.1200/JCO.2014.55.2836
22. Tadros AB, Yang WT, Krishnamurthy S, et al. Identification of patients with documented pathologic complete response in the breast after neoadjuvant chemotherapy for omission of axillary surgery. JAMA Surg. 2017;152(7):665–670. doi:10.1001/jamasurg.2017.0562
23. Prowell TM, Pazdur R. Pathological complete response and accelerated drug approval in early breast cancer. N Engl J Med. 2012;366(26):2438–2441. doi:10.1056/NEJMp1205737
24. Huang EH, Strom EA, Valero V, et al. Locoregional treatment outcomes for breast cancer patients with ipsilateral supraclavicular metastases at diagnosis. Int J Radiat Oncol Biol Phys. 2007;67(2):490–496. doi:10.1016/j.ijrobp.2006.08.040
25. Gu ML, Yuan CJ, Liu XM, et al. Pre-treatment elevated platelet count associates with HER2 overexpression and prognosis in patients with breast cancer. Asian Pac J Cancer Prev. 2015;16(13):5537–5540. doi:10.7314/apjcp.2015.16.13.5537
26. Zhu J, Jiao D, Guo X, et al. Predictive factors and prognostic value of pathologic complete response of ipsilateral supraclavicular lymph nodes in breast cancer after neoadjuvant chemotherapy. Ann Transl Med. 2019;7(22):666. doi:10.21037/atm.2019.10.22
27. Pather K, Dix-Peek T, Duarte R, Chetty N, Augustine TN. Breast cancer cell-induced platelet activation is compounded by tamoxifen and anastrozole in vitro. Thromb Res. 2019;177:51–58. doi:10.1016/j.thromres.2019.02.027
28. Ogba N, Manning NG, Bliesner BS, et al. Luminal breast cancer metastases and tumor arousal from dormancy are promoted by direct actions of estradiol and progesterone on the malignant cells. Breast Cancer Res. 2014;16(6):489. doi:10.1186/s13058-014-0489-4
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
