Back to Journals » Cancer Management and Research » Volume 11
Elevated levels of pre-treatment lactate dehydrogenase are an unfavorable predictor factor in patients with EML4-ALK rearrangement non-small cell lung cancer treated with crizotinib
Authors Liang H , Ma D, Xu Y , Zhao J, Chen M , Liu X, Zhong W, Li J, Wang M
Received 26 April 2019
Accepted for publication 23 August 2019
Published 5 September 2019 Volume 2019:11 Pages 8191—8200
DOI https://doi.org/10.2147/CMAR.S213572
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Hongge Liang1, Di Ma2, Yan Xu1, Jing Zhao1, Minjiang Chen1, Xiaoyan Liu1, Wei Zhong1, Junling Li2, Mengzhao Wang1
1Department of Respiratory and Critical Care Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing, People’s Republic of China; 2Department of Oncology, Chinese Academy of Medical Sciences Cancer Institute and Hospital, Beijing, People’s Republic of China
Correspondence: Wei Zhong
Department of Respiratory and Critical Care Medicine, Peking Union Medical College Hospital, No. 1 Shuanfuyuan Wangfujing Dongcheng District, Beijing 100730, People’s Republic of China
Tel +86 0 106 915 5039
Email [email protected]
Mengzhao Wang
Department of Respiratory and Critical Care Medicine, Peking Union Medical College Hospital, No. 1 Shuanfuyuan Wangfujing Dongcheng District, Beijing 100730, People’s Republic of China
Tel +86 0 106 915 5154
Email [email protected]
Background: Targeted therapy is an important treatment for advanced non-small cell lung cancer (NSCLC) patients with specific genetic mutations, crizotinib can prolong survival in advanced NSCLC patients with echinoderm microtubule-associated protein-like 4–anaplastic lymphoma kinase (EML4-ALK) rearrangement. We performed a retrospective analysis to investigate the association between the lactate dehydrogenase (LDH) levels and progression-free survival (PFS) in patients with EML4-ALK rearrangement NSCLC receiving treatment with crizotinib.
Methods: Advanced (stage IIIb–IV) NSCLC patients with EML4-ALK rearrangement receiving treatment with crizotinib were enrolled between January 2007 and January 2016 at Peking Union Medical College and Cancer Hospital Chinese Academy of Medical Sciences.
Results: Overall, 212 patients were enrolled. Kaplan–Meier univariate analysis showed that elevated pre-treatment LDH level (7.9 vs 14.1 months, HR =1.251, CI: 1.008–1.553, P=0.004) was significantly associated with shorter PFS, while the post-treatment mean-LDH level (13.3 vs 14.3 months, HR=1.439, 95% CI: 0.994–2.082, P=0.970) was not significantly associated with PFS. Cox proportional hazards model also identified that pre-treatment LDH level (HR=2.085, 95% CI: 1.150–3.781, P=0.016) was associated with the PFS. Logistic regression analysis showed that post-treatment LDH level was associated with creatine kinase (OR=6.712, 95% CI 3.395–13.273, P<0.01), creatine kinase isoenzyme (OR=6.297, 95% CI 2.953–13.427, P<0.01), and hemoglobin (OR=4.163, 1.741–9.956, P<0.001).
Conclusion: An elevated pre-treatment serum LDH level (>250 U/L) was significantly associated with shorter PFS in patients with EML4-ALK rearrangement NSCLC. Post-treatment elevated serum LDH level was not significantly associated with PFS, which related to adverse events including muscle damage and anemia.
Keywords: non-small cell lung cancer, lactate dehydrogenase, crizotinib, echinoderm microtubule-associated protein-like 4–anaplastic lymphoma kinase, progression-free survival
Introduction
Lung cancer is a leading cause of cancer-related mortality worldwide and non-small cell lung cancer (NSCLC) accounts for 80% of all lung cancer.1 Targeted therapy is an important treatment for advanced NSCLC patients with specific genetic mutations, which can significantly improve their outcomes. echinoderm microtubule-associated protein-like 4–anaplastic lymphoma kinase (EML4-ALK) rearrangement was one of the known therapeutic targets. The incidence rate of EML4-ALK rearrangement in NSCLC patients is 3.3–6.1%.2 A small molecule tyrosine kinase inhibitor, crizotinib can prolong survival in EML4-ALK rearrangement advanced NSCLC patients.3–6 Lactate dehydrogenase (LDH) is a glycolytic enzyme that can convert pyruvate into lactic acid in an anaerobic environment, contribute to anaerobic glycolysis, and produce adenosine triphosphate for cells. A previous study reported that there was a significant association between the pre-treatment serum LDH level and poor survival in NSCLC patients receiving treatment with EGFR-tyrosine kinase inhibitor (EGFR-TKI), PD-1/PD-L1 inhibitors, or standard chemotherapy,7–12 which may be related to that LDH can provide energy for tumor cells, enhance tumor invasion, and angiogenesis.10,13–17 However, little is known about the association between LDH level and progression-free survival (PFS) in NSCLC patients with EML4-ALK rearrangement receiving treatment with crizotinib. We also found that many patients with EML4-ALK rearrangement developed elevated LDH levels after treatment with crizotinib. The reason for the elevated LDH level and its influence on prognosis in patients remains unclear. Recently, major therapeutic advances have occurred in the management of advanced EML4-ALK rearrangement NSCLC patients. Several second- and third-generation ALK inhibitors have shown clinical benefits in these patients, and have been shown to dramatically prolong overall survival (OS) in this group.18 Thus, it has become even more important to identify prognostic factors and create appropriate follow-up schedules for advanced ALK+ NSCLC patients. We thus performed a retrospective analysis to investigate the association between the pre-treatment and post-treatment serum LDH levels and PFS in patients with EML4-ALK rearrangement receiving treatment with crizotinib.
Patients and methods
Study design and patients
The present study retrospectively enrolled 212 patients with advanced EML4-ALK rearranged NSCLC who received treatment with crizotinib from January 2007 to January 2018 at Peking Union Medical College Hospital and Chinese Academy of Medical Sciences Cancer Hospital. The patients’ clinical data were based on CAPTRA-Lung (NCT03334864) database. The study was approved by the Institutional Review Board (IRB) (Approval Number: JS‐1410), and it was conducted in accordance with the Declaration of Helsinki. All the patients provided written informed consent for the collection of their clinical data. The inclusion criteria were as follows: 1) age ≥18 years, 2) histologically or cytologically confirmed NSCLC, 3) stage IIIb (do not meet surgery or radical chemoradiotherapy criteria) or stage IV, 4) histology positive for EML4-ALK rearrangement, and the evaluation method for ALK fusion should be fluorescence in situ hybridization (FISH), next-generation sequence (NGS), or Ventana immunohistochemistry (IHC with D5F3 antibody), 5) patients receiving treatment with crizotinib, 6) LDH level was detected before or after treatment. Exclusion criteria were as follows: 1) only sputum pathology specimens available, 2) genetic results from sputum or blood samples, and 3) ALK evaluation methods that did not fulfill the inclusion criteria.
Methods
The treatment regimen was crizotinib 250 mg/time, twice daily orally until disease progression or intolerable adverse events occurred. All patients were evaluated regularly, and were carried out every 2–3 months by imaging examinations, including chest and abdominal computed tomography, enhanced head magnetic resonance imaging and scintigraphy. PFS was calculated from the beginning of crizotinib administration until the date of disease progression (according to RECIST 1.1 criteria) or the date of mortality done. All patients were followed up regularly in outpatient department.
The normal value of LDH level was according to the analyzer standard of Peking Union Medical College Hospital and Cancer Hospital. LDH levels were evaluated using the Siemens ADVIA2400 unit and its corollary reagent, and LDH >250 U/L is defined as elevated LDH level. Pre-treatment LDH level was divided into the LDH-elevated group and the LDH-normal group. Post-treatment LDH levels were evaluated after the initiation of treatment with crizotinib until the disease progression or mortality, and multiple values were thus obtained. The post-treatment mean-LDH level was defined as the mean value of multiple LDH detecting levels in one patient who have received crizotinib, which was calculated and divided into the ≤250U/L group (normal post-treatment mean-LDH group) and >250 U/L group (elevated post-treatment mean-LDH group).
Data collection
In this retrospective study, the following patient data were collected: gender, age, smoking history, pathological types, disease stage, sites of metastasis, EGFR mutation status, number of treatment regimens received previously and LDH levels.
Statistical analyses
Statistical analyses were performed by IBM SPSS software (version 21.0; IBM Corp., Armonk, NY, USA). Continuous variables were expressed as mean ± SD. PFS curves were drawn according to the Kaplan–Meier method. The univariate analysis of PFS was performed by the Kaplan–Meier method and the log-rank test. The multivariate analysis was performed by the Cox proportional hazard model and calculation of HRs using the 95% CI. The correlation between LDH serum levels and clinicopathological parameters was performed using Fisher’s exact test and logistic regression analysis. All tests were two-sided, and P≤0.05 was considered statistically significant.
Results
Patients' characteristics
Between January 2007 and January 2018, a total of 212 patients fulfilled the criteria and were included in the analysis at Peking Union Medical College Hospital and Chinese Academy of Medical Sciences Cancer Hospital. Among these patients, the most common pathological type was adenocarcinoma (203 cases), followed by unclassified carcinomas (3 cases), squamous cell carcinoma (2 cases), adenosquamous carcinoma (2 cases), mucoepidermoid carcinoma (1 case), and carcinosarcoma (1 case). There were 12 patients with EGFR-active mutations, 190 with wild-type EGFR, and 10 patients with unknown EGFR mutations due to insufficient pathological specimens. The ALK fusion detection methods were FISH method in 124 cases, IHC targeting D5F3 method in 103 cases, and NGS method in 10 cases. Table 1 summarizes the other clinical pathological characteristics of 212 NSCLC patients.
 |
Table 1 Log-rank test analysis of clinical pathological characteristics and PFS in 212 ALK-positive NSCLC patients |
LDH results
Among all patients, pre-treatment LDH level was evaluated in 129 patients, with normal LDH levels in 100 cases and elevated LDH levels in 29 cases. Pre-treatment LDH levels were not associated with any patients’ clinical pathological feathers (Table 2).
 |
Table 2 Chi-square test analysis of LDH level and clinical pathological characteristics |
Post-treatment LDH level was evaluated in 193 patients, with normal post-treatment mean-LDH levels in 64 cases and elevated post-treatment mean-LDH levels in 129 patients. With normal post-treatment LDH levels in 28 cases and elevated post-treatment LDH levels in 165 patients. Post-treatment LDH levels were not associated with any patients’ clinical pathological feathers (Table 2). Among patients with elevated post-treatment LDH level, 41.2% (68/165) patients had LDH elevated for the first time in the first month after crizotinib treatment (Table 3) (Figure S1). The mean value of LDH levels evaluated in each month among all patients who have received crizotinib fluctuated between 248 and 281 U/L (Table 3).
 |
Table 3 Evaluation of post-treatment LDH levels in every month |
To further analyze the reason of elevated post-treatment LDH levels, we performed analysis of association between LDH levels and concurrent liver function indicators (alanine aminotransferase/aspartate aminotransferase), renal function indicators (creatinine), muscle enzymes (creatine kinase/creatine kinase isoenzyme), and hemoglobin levels (hemoglobin). Logistic regression analysis showed that elevated post-treatment LDH levels were significantly consistent with creatine kinase (OR=6.712, 95% CI 3.395–13.273, P<0.01), creatine kinase isoenzyme (OR=6.297, 95% CI 2.953–13.427, P<0.01), and hemoglobin (OR=4.163, 1.741–9.956, P<0.001) (Table 4).
 |
Table 4 Logistic regression analysis of elevated post-treatment LDH levels |
PFS
The efficacy was evaluated in 212 patients: 128 (60.4%) with partial response, 51 (24.1%) with stable disease, 5 (2.4%) with progression disease, 28 (13.2%) with uncertain, and with an objective response rate of 60.4% and a disease control rate of 84.5%. At the end of follow-up, the median follow-up time was 15.9 months, and the median PFS was 13.4 months (95% CI 10.6–16.3 months). Of all patients, only 21.1% (45/212) were followed up to death, and it was immature for analysis of OS.
In the Kaplan-Meier univariate analysis, PFS in patients with elevated pre-treatment LDH level was shorter than that in patients with normal pre-treatment LDH level (7.9 vs 14.1 months, HR=1.251, 95% CI: 1.008–1.553, P=0.004) (Figure 1). PFS was not significantly related to post-treatment mean-LDH level (13.3 vs 14.3 months, HR=1.439, 95% CI: 0.994–2.082, P=0.970) (Figure 2). In addition, Kaplan-Meier univariate suggested liver metastasis (10.9 vs 15.0 months, P=0.032) and adrenal metastasis (5.0 vs 14.3 months, P=0.001) were also associated with shorter PFS (Table 1). All clinical pathological factors were included in the Cox regression multivariate analysis. The results showed that only pre-treatment LDH levels (HR=2.085, 95% CI 1.150–3.781, P=0.016) and adrenal metastasis (HR=3.827, 95% CI 1.307–11.205, P=0.014) were associated with shorter PFS (Table S1).
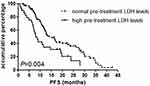 |
Figure 1 Kaplan-Meier progression-free survival curve according to pre-treatment LDH levels. |
 |
Figure 2 Kaplan-Meier progression-free survival curve according to post-treatment mean-LDH levels. |
Discussion
There are several previous studies on the relationship between LDH levels and chemotherapy, PD-1/PD-L1 inhibitors or EGFR-TKI treatment. To our knowledge, this is the first study of the relationship between LDH levels and crizotinib treatment. The present study revealed the existence of a significant association between the pre-treatment serum LDH level and PFS in patients with EML4-ALK rearranged NSCLC receiving treatment with crizotinib, while post-treatment mean-LDH levels were not associated with PFS. Furthermore, elevated post-treatment LDH level was associated with multiple factors including muscle damage, and anemia.
Previous studies showed that elevated pre-treatment serum LDH level had a shorter PFS in inoperable NSCLC patients.17,19–28 Zhu et al analyzed the prognostic factors in 105 patients with advanced NSCLC receiving first-line chemotherapy. The results showed that patients with high LDH levels before treatment had shorter PFS (3.6 vs 6.6 months, P=0.005) and shorter OS (10.8 vs 17.0 months, P=0.014) than those with lower LDH levels. Multivariate analysis also suggested that patients with elevated LDH levels had shorter PFS (P=0.019) and OS (P=0.006).8 Minehiko Inomata et al analyzed the prognostic factors affecting patients with EGFR mutation-positive NSCLC treated with gefitinib or erlotinib. A total of 65 patients were included in the study. The results showed that patients with high LDH levels before treatment had shorter PFS (6.2 vs 13.2 months, P<0.01) and OS (10.5 vs 36.1 months, P<0.01) than those with lower LDH levels. Cox regression multivariate also suggested that patients with higher LDH levels had shorter PFS (P=0.05) and shorter OS (P<0.01).9,12 Mezquita et al analyzed the prognostic impact of pre-treatment LDH levels on advanced NSCLC receiving immunotherapy (a PD-1/PD-L1 inhibitor). A total of 466 NSCLC patients were classified as high LDH levels group (n=179) and normal LDH level group (n=287). The results showed that patients with higher LDH level had a shorter OS time (P<0.01) than the normal LDH level group.7
Unlike previous studies, this present study showed that elevated serum LDH levels before treatment with crizotinib were significantly associated with shorter PFS in patients with NSCLC harboring ALK-rearrangement. The reasons may be as follows: 1) NSCLC relies on the anaerobic metabolism of glucose and has a phenotype closely related to the clinical invasion behavior of tumor cells.13,29 LDH is a glycolytic enzyme that catalyzes the conversion of pyruvate to lactic acid in anoxic environment, producing adenosine triphosphate and nicotinamide adenine dinucleotide, which is beneficial to the growth of tumor cells and increases tumor burden in vivo;14,30 2) LDH can increase the invasive ability and angiogenic ability of tumor cells.10,16,17
Our study also suggested that post-treatment LDH levels were not associated with PFS. The elevated post-treatment LDH level was significantly associated with creatine kinase, creatine kinase isoenzyme, and hemoglobin levels. Therefore, elevated post-treatment LDH levels maybe mainly caused by muscle damage, and anemia, and therefore do not reflect the real ability of growth, infiltration, and invasion of tumor cells. In addition, activation of the receptor tyrosine kinase, c-Met, by hepatocyte growth factor (HGF) leads to increased cell motility, proliferation, invasion, and metastasis. Crizotinib is also a c-mesenchymal-epithelial transition (c-MET) inhibitor. It is reported that an LDH inhibitor can reduce c-Met activation and HGF-induced cell motility, indicating a potential connection between LDH activity and the c-Met signaling axis.31 Thus, we assume that crizotinib can inhibit the c-MET/HGF axis, which can stimulate an increase in LDH levels to re-active c-MET signaling. Similarly, NSCLC patients have 13 EML4-ALK fusion variants, which contain exons 20–29 of ALK and eight different EML4 exons.32 This variants induce the phosphorylation of one or more of the juxtamembrane tyrosine residues and then active the downstream signaling, including the Ras/Raf/MEK/ERK1/2 pathway, the Janus activated kinase (JAK)/STAT pathway, and the phosphatidylinositol 3-kinase/Akt (PKB) pathway.33 Activation of these pathways can mediate tumor cell proliferation and survival. While LDH can contribute to anaerobic metabolism of glucose in tumor cells and increase their invasive ability and angiogenic ability. Thus, we assume that the survival environment of tumor cells are poor after crizotinib inhibits the EML4-ALK procedure, which, in turn, stimulates the production of LDH and contributes to the survival of tumor cells.
Brain metastasis (BM) is a common complication of NSCLC. Because of its poor ability to penetrate the blood-brain barrier (0.0026), crizotinib has a lower efficacy against BMs.34,35 The incidence of BM increases with increasing duration of the disease, with up to 60% of patients receiving crizotinib developing BMs for the first time during treatment.36 In recent studies, second- or third-generation ALK inhibitors have shown favorable intracranial activities in patients with ALK-rearranged NSCLC and BMs.37–41 However, in our study, BMs were not found to be associated with PFS in ALK+ NSCLC patients treated with crizotinib. We suspect the following reasons: First, local therapies have been the primary method for treatment of patients with BMs, including surgery, whole-brain radiation therapy, and stereotactic radiosurgery. Most patients with baseline BMs received local therapies, which effectively controlled intracranial symptoms and inhibited intracranial progression to some extent. Second, only 43 patients had baseline BMs, and the sample size needs to be increased to further study the association between baseline BM and PFS.
Our research has several limitations. First, this was a retrospective research, and the result should be confirmed in further studies. Second, we only tested serum total LDH levels, but did not detect LDH subtypes. Third, there are many factors affecting the increase of LDH levels. We only analyzed the four most common influencing factors: liver dysfunction, renal dysfunction, muscle damage, and anemia.
Conclusion
In conclusion, the present study indicates the existence of an association between pretreatment serum LDH levels and PFS in NSCLC patients with EML4-ALK rearrangement receiving treatment with crizotinib, while post-treatment LDH levels were found to be mainly associated with adverse effects and not with PFS.
Data sharing statement
No additional unpublished data are available.
Acknowledgment
The abstract of this paper was presented at the American Society of Clinical Oncology as online publication with interim findings. The abstract was published in “Poster Abstracts” in the Journal of Clinical Oncology: https://ascopubs.org/doi/abs/10.1200/JCO.2019.37.15_suppl.e20513.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi:10.3322/caac.21254
2. Li Y, Li YW, Yang T, et al. Clinical significance of EML4-ALK fusion gene and association with EGFR and KRAS gene mutations in 208 Chinese patients with non-small cell lung cancer. PLoS One. 2013;8:1.
3. Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;13(10):1011–1019. doi:10.1016/S1470-2045(12)70344-3
4. Crino L, Kim D, Riely GJ, et al. Initial phase II results with crizotinib in advanced ALK-positive non-small cell lung cancer (NSCLC): PROFILE 1005. J Clin Oncol. 2011;29:15. doi:10.1200/jco.2011.29.15_suppl.7514
5. Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. New Engl J Med. 2013;368(25):2385–2394. doi:10.1056/NEJMoa1214886
6. Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. New Engl J Med. 2014;371(23):2167–2177. doi:10.1056/NEJMoa1408440
7. Mezquita L, Auclin E, Ferrara R, et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol. 2018;4(3):351–357. doi:10.1001/jamaoncol.2017.4771
8. Zhu LZ, Li XF, Shen YW, et al. A new prognostic score based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Oncotargets Ther. 2016;9:4879–4886. doi:10.2147/OTT.S107279
9. Inomata M, Hayashi R, Tanaka H, et al. Elevated levels of plasma lactate dehydrogenase is an unfavorable prognostic factor in patients with epidermal growth factor receptor mutation-positive non-small cell lung cancer, receiving treatment with gefitinib or erlotinib. Mol Clin Oncol. 2016;4(5):774–778. doi:10.3892/mco.2016.779
10. Ulas A, Turkoz FP, Silay K, et al. A laboratory prognostic index model for patients with advanced non-small cell lung cancer. PLoS One. 2014;9(12):e114471. doi:10.1371/journal.pone.0114471
11. Ramalingam S, Goss G, Rosell R, et al. A randomized phase II study of ganetespib, a heat shock protein 90 inhibitor, in combination with docetaxel in second-line therapy of advanced non-small cell lung cancer (GALAXY-1). Ann Oncol. 2015;26(8):1741–1748. doi:10.1093/annonc/mdv220
12. Inomata M, Hayashi R, Yamamoto A, et al. Plasma neuron-specific enolase level as a prognostic marker in patients with non-small cell lung cancer receiving gefitinib. Mol Clin Oncol. 2015;3(4):802–806. doi:10.3892/mco.2015.568
13. Heiden MGV, Cantley LC, Thompson CB. Understanding the warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi:10.1126/science.1160809
14. Gallo M, Sapio L, Spina A, Naviglio D, Calogero A, Naviglio S. Lactic dehydrogenase and cancer: an overview. Front Biosci-Landmrk. 2015;20:1234–1249. doi:10.2741/4368
15. Xie H, Hanai JI, Ren JG, et al. Targeting lactate dehydrogenase-A inhibits tumorigenesis and tumor progression in mouse models of lung cancer and impacts tumor-initiating cells. Cell Metab. 2014;19(5):795–809. doi:10.1016/j.cmet.2014.03.003
16. Koukourakis MI, Giatromanolaki A, Sivridis E, et al. Lactate dehydrogenase-5 (LDH-5) overexpression in non-small-cell lung cancer tissues is linked to tumour hypoxia, angiogenic factor production and poor prognosis. Brit J Cancer. 2003;89(5):877–885. doi:10.1038/sj.bjc.6601205
17. Danner BC, Didilis VN, Wiemeyer S, et al. Long-term survival is linked to serum LDH and partly to tumour LDH-5 in NSCLC. Anticancer Res. 2010;30(4):1347–1351.
18. Sgambato A, Casaluce F, Maione P, Gridelli C. Targeted therapies in non-small cell lung cancer: a focus on ALK/ROS1 tyrosine kinase inhibitors. Expert Rev Anticanc. 2018;18(1):71–80. doi:10.1080/14737140.2018.1412260
19. Soussi G, Ben Alaya N, Chaouch N, Racil H. Development and validation of a prognostic index for survival in non-small cell lung cancer: results from a Tunisian cohort study. Cancer Epidemiol. 2018;53:111–118. doi:10.1016/j.canep.2018.01.018
20. O’Connell JP, Kris MG, Gralla RJ, et al. Frequency and prognostic importance of pretreatment clinical characteristics in patients with advanced non small-cell lung-cancer treated with combination chemotherapy. J Clin Oncol. 1986;4(11):1604–1614. doi:10.1200/JCO.1986.4.11.1604
21. Kim ST, Lee J, Sun JM, et al. Prognostic model to predict outcomes in non-small cell lung cancer patients with erlotinib as salvage treatment. Oncology-Basel. 2010;79(1–2):78–84. doi:10.1159/000320190
22. Albain KS, Crowley JJ, Leblanc M, Livingston RB. Survival determinants in extensive-stage non-small-cell lung-cancer – the southwest-oncology-group experience. J Clin Oncol. 1991;9(9):1618–1626. doi:10.1200/JCO.1991.9.9.1618
23. Espinosa E, Feliu J, Zamora P, et al. Serum-albumin and other prognostic factors related to response and survival in patients with advanced nonsmall cell lung-cancer. Lung Cancer. 1995;12(1–2):67–76.
24. Hespanhol V, Queiroga H, Magalhaes A, Santos AR, Coelho M, Marques A. Survival predictors in advanced non-small cell lung cancer. Lung Cancer. 1995;13(3):253–267.
25. Liu L, He Y, Ge G, et al. Lactate dehydrogenase and creatine kinase as poor prognostic factors in lung cancer: a retrospective observational study. PLoS One. 2017;12(8):e0182168. doi:10.1371/journal.pone.0182168
26. Li B, Li C, Guo M, et al. Predictive value of LDH kinetics in bevacizumab treatment and survival of patients with advanced NSCLC. Onco Targets Ther. 2018;11:6287–6294. doi:10.2147/OTT.S171566
27. Deng T, Zhang J, Meng Y, Zhou Y, Li W. Higher pretreatment lactate dehydrogenase concentration predicts worse overall survival in patients with lung cancer. Medicine (Baltimore). 2018;97(38):e12524. doi:10.1097/MD.0000000000012524
28. Taniguchi Y, Tamiya A, Isa SI, et al. Predictive factors for poor progression-free survival in patients with non-small cell lung cancer treated with nivolumab. Anticancer Res. 2017;37(10):5857–5862. doi:10.21873/anticanres.12030
29. Giatromanolaki A, Sivridis E, Arelaki S, Koukourakis MI. Expression of enzymes related to glucose metabolism in non-small cell lung cancer and prognosis. Exp Lung Res. 2017;43(4–5):167–174. doi:10.1080/01902148.2017.1328714
30. Vanzandwijk N, Mooi WJ, Rodenhuis S. Prognostic factors in Nsclc – recent experiences. Lung Cancer. 1995;12:S27–S33.
31. Gray AL, Minutolo F, Cardelli JA. A role for lactate dehydrogenase in the c-Met/HGF signaling axis. Cancer Res. 2012;72.
32. Sanders HR, Li HR, Bruey JM, et al. Exon scanning by reverse transcriptase-polymerase chain reaction for detection of known and novel EML4-ALK fusion variants in non-small cell lung cancer. Cancer Genet-Ny. 2011;204(1):45–52. doi:10.1016/j.cancergencyto.2010.08.024
33. Li YJ, Ye XF, Liu JF, Zha JP, Pei L. Evaluation of EML4-ALK fusion proteins in non-small cell lung cancer using small molecule inhibitors. Neoplasia. 2011;13(1):1–U14. doi:10.1593/neo.101120
34. Chun SG, Choe KS, Iyengar P, Yordy JS, Timmerman RD. Isolated central nervous system progression on Crizotinib: an Achilles heel of non-small cell lung cancer with EML4-ALK translocation? Cancer Biol Ther. 2012;13(14):1376–1383. doi:10.4161/cbt.22255
35. Costa DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol. 2011;29(15):E443–E445. doi:10.1200/JCO.2010.34.1313
36. Costa DB, Shaw AT, Ou SHI, et al. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J Clin Oncol. 2015;33(17):1881–1888. doi:10.1200/JCO.2014.59.0539
37. Zhang ZG, Guo HW, Lu YL, Hao W, Han L. Anaplastic lymphoma kinase inhibitors in non-small cell lung cancer patients with brain metastases: a meta-analysis. J Thorac Dis. 2019;11(4):
38. Soria JC, Tan DSW, Chiari R. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study (vol 389, pg 917, 2017). Lancet. 2017;389(10072):908. doi:10.1016/S0140-6736(17)30123-X
39. Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. New Engl J Med. 2017;377(9):829–838. doi:10.1056/NEJMoa1704795
40. Gettinger SN, Bazhenova LA, Langer CJ, et al. Activity and safety of brigatinib in ALK-rearranged non-small-cell lung cancer and other malignancies: a single-arm, open-label, phase 1/2 trial. Lancet Oncol. 2016;17(12):1683–1696. doi:10.1016/S1470-2045(16)30392-8
41. Shaw AT, Martini JF, Besse B, et al. Efficacy of lorlatinib in patients (pts) with advanced ALK-positive non-small cell lung cancer (NSCLC) and ALK kinase domain mutations. Cancer Res. 2018;78:13.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
