Back to Journals » OncoTargets and Therapy » Volume 13
Elevated Expression of miR-629 Predicts a Poor Prognosis and Promotes Cell Proliferation, Migration, and Invasion of Osteosarcoma
Authors Li X, Li N, Niu Q, Zhu H, Wang Z, Hou Q
Received 25 September 2019
Accepted for publication 21 November 2019
Published 2 March 2020 Volume 2020:13 Pages 1851—1857
DOI https://doi.org/10.2147/OTT.S232479
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Carlos E Vigil
Xuesen Li,1 Na Li,2 Qinghui Niu,3 Haibin Zhu,4 Zhijie Wang,1 Qingxian Hou1
1Department of Spine Surgery, The Affiliated Hospital of Qingdao University, Qingdao, Shandong 266555, People’s Republic of China; 2Department of Ophthalmology, The Affiliated Hospital of Qingdao University, Qingdao, Shandong 266555, People’s Republic of China; 3Department of Hepatic Medicine, The Affiliated Hospital of Qingdao University, Qingdao, Shandong 266555, People’s Republic of China; 4Department of Orthopedics and Traumatology, Traditional Chinese Medical Hospital of Huangdao District, Qingdao, Shandong 266500, People’s Republic of China
Correspondence: Qingxian Hou
Department of Spine Surgery, The Affiliated Hospital of Qingdao University, No. 1677, Wutaishan Road, Qingdao, Shandong 266555, People’s Republic of China
Tel/Fax +86 532 82919525
Email [email protected]
Purpose: Osteosarcoma (OS) is an invasive bone tumor that primarily affects children and adolescents. MicroRNA-629 (miR-629) acts as an oncogene involved in the development of various cancers. This study aims to reveal the clinical significance and biological function of miR-629 in OS.
Patients and Methods: The levels of miR-629 expression in tissues and cells were detected through quantitative real-time polymerase chain reaction (qRT-PCR). Chi-square test was used to evaluate the relationship between miR-621 expression and clinical parameters in patients with OS. Survival analysis was performed by the Kaplan–Meier method. Cox regression analysis of the effect of miR-629 expression on the prognosis of OS patients. CCK-8 and Transwell experiments were used to demonstrate the effect of miR-629 on OS cell function.
Results: Compared with the controls, miR-629 levels were significantly elevated in patients with OS (P < 0.001), Furthermore, miR-629 upregulation showed significantly associated with clinical stage (P = 0.011), distant metastasis (P = 0.003) and poor survival (log rank test, P = 0.013) in OS patients. miR-629 might be a potential prognostic biomarker for OS (HR = 2.890, 95% CI = 1.126– 7.416, P = 0.027). Cell function experiments proved that the high expression of miR-629 promoted cell proliferation, migration, and invasion of OS.
Conclusion: All experimental results demonstrated that miR-629 as an oncogene promotes the tumor cell growth, migration and invasion of OS, and miR-629 may act as a novel prognostic biomarker and therapeutic target for patients with this malignant tumor.
Keywords: miR-629, prognosis, progression, osteosarcoma
Introduction
Osteosarcoma (OS) is the most common primary malignant tumor of bone tissue, occurring mainly in adolescents and young adults.1 Due to the characteristics of early metastasis and poor prognosis of OS, this aggressive malignant tumor has become a major cause of death-threatening adolescents and young adults. Although great progress in the diagnosis and treatment of OS in surgery, radiotherapy, chemotherapy and other aspects, the 5-year overall survival of OS patients has significantly improved to approximately 60–70%.2 However, due to the metastatic nature of OS, the incidence is growing at a rate of 1.4% per year, and the prognosis of patients with recurrence and metastasis remains poor. Therefore, it is urgent to search for new reliable early diagnosis and prognostic markers to improve the clinical efficacy of OS.3
MicroRNAs (miRNAs) are non-coding RNA molecules composed of 22–25 nucleotides. They can regulate gene expression at the post-transcriptional level by blocking the translation of mRNAs, changing the translation of mRNA, or changing the stability of mRNA by binding the 3ʹ-untranslated region (UTR) of the mRNAs.4 Studies have demonstrated that miRNAs are involved in a variety of physiological and pathological processes, such as the proliferation and apoptosis of developmental and differentiated cells.5,6 In recent years, evidence of miRNA dysregulation has emerged in malignant tumors (including OS). Such as miR-21, miR-221, miR-106a has been shown to be deregulated in OS.7,8
miR-629, as one of the numerous miRNAs, is located on human chromosome 15q23 and is abnormally expressed in gastric cancer,9 esophageal cancer10 and other cancers. Meanwhile, it can be a potential diagnostic and prognostic biomarker in many cancer. A recent study by Shi et al showed that miR-629 was significantly up-regulated in pancreatic cancer, which may be a potential biomarker for diagnosis and prognosis of pancreatic cancer.11 However, the clinical and biological role of miR-629 in OS remains unclear.
The purpose of this study was to evaluate miR-629 expression and clinical relevance in OS and study its regulatory role in tumor cell biological behaviors. Our data indicated that miR-629 may act not only as a novel prognostic marker but also as a potential target for molecular therapy of OS patients.
Materials and Methods
Patients and Tissue Specimens
The Ethics Research Committee of The Affiliated Hospital of Qingdao University of China approved this study. All patients participating in the study provided written informed consent. All tissue samples taken from patients with OS were treated and anonymized according to ethical and legal standards. This study was carried out in accordance with the principles of the Declaration of Helsinki.
This study collected the tissues of 110 patients diagnosed with OS and the corresponding non-cancerous bone tissues in The Affiliated Hospital of Qingdao University from May 2009 to August 2013. None of these patients received blood transfusions, radiotherapy, or chemotherapy. All tissue samples were reassessed by 2 pathologists according to WHO classification. During follow-up, overall survival was measured from diagnosis to death or at the end of follow-up. Clinical information and pathological characteristics of the sample, such as gender, age, tumor size, tumor site, clinical stage, and distant metastasis were collected in Table 1.
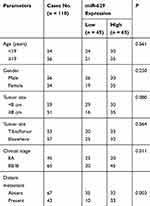 |
Table 1 Correlation Between miR-629 Expression Levels and Clinical Features in Osteosarcoma Patients |
Cell Lines and Transfection
Human fetal osteoblastic cell line hFOB1.19 and the OS cell lines MG63, HOS, SaOS2, and U2OS were obtained from the American Type Culture Collection (ATCC). All cells were cultured in DMEM (Life Technologies) supplemented with 10% fetal bovine serum (FBS) and were kept at 5% CO2 in a humidified incubator at 37°C. Transfection of miR-629 mimic, mimic negative (NC), miR-629 inhibitor, inhibitor NC (RiboBio) into OS cell lines for in vitro function study of miR-629. The cell transfection reagent was Lipofectamine 2000 (Invitrogen), and the culture medium was changed after 6 h of transfection.
RNA Extraction and qRT-PCR Analysis
Total RNA was extracted from the tissues and cells applying Trizol reagent (Invitrogen) following the manufacturer’s instruction. PrimeScript TM RT Master Mix Kit (Takara) was used to reverse transcribe the extracted RNA into cDNA. qRT-PCR reaction was conducted in the 7300 Real-Time PCR System (Applied Biosystems) using the SYBR Green I Master Mix kit (Invitrogen). Primer sequences were presented as following: miR-629 forward 5ʹ-CGTGGGTTTACGTTGGG-3ʹ and reverse 5ʹ-CTCGCTTCGGCAGCACA-3ʹ; and U6 forward 5ʹ-AACGCTTCACGAATTTGCGT-3ʹ and reverse 5ʹ-CTCGCTTCGGCAGCACA-3ʹ. The relative expression level of miR-629 was calculated using the 2−ΔΔCt method, with the CT values normalized using U6 as an internal control.
Cell Proliferation Assay
Cell proliferation was detected by CCK-8 assay (Signal way antibody). OS cell lines were plated into 96-well culture plates at a density of 5 × 103 cells/well after transfection with miR-629 mimics or inhibitors. Every 24 h, 10 μL CCK-8 reagents were added to each well with future 2-hr incubation at 37°C, then measured the absorption at 450 nm light (Molecular Devices).
Cell Migration and Invasion Assay
OS cells were transfected with miR-629 mimics or inhibitors. After 24 h, the transfected cells in serum-free media were seeded into the upper chambers which were coated with matrigel (BD Bioscience) for invasion assay, in the meanwhile, 1×105 cells were placed in the upper chambers for migration and invasion assay. The lower chamber was added with 500 μL medium with 10% FBS. After incubation for 24 h, the matrigel, uninvaded, and unmigrated cells in the upper chamber were wiped off with a cotton swab. The cells adhered to the lower membrane were fixed with methanol for 10 mins, and 0.1% crystal violet stained for 15 mins. The number of cells in the field was counted by randomly taking 5 fields under a microscope.
Statistical Analysis
All statistical analysis in this study was conducted by SPSS 22.0 software and GraphPad Prism 7.0 software. The difference of miR-629 expression between the OS patients and the control group was analyzed by Students’ t test. Chi-square test was used to evaluate the relationship between miR-629 and clinicopathological characteristics. The relationship between miR-629 and overall survival was estimated by Kaplan-Meier analysis and Cox regression analysis. Results with P < 0.05 were considered statistically significant.
Results
Expression of miR-629 in OS Tissues and Cell Lines
In order to determine the expression of miR-629 in OS, qRT-PCR was performed in 110 patients. As shown in Figure 1A, miR-629 expression in OS tissue was higher than that in healthy tissues (P < 0.001). We then examined the expression of miR-629 in OS cell lines MG63, HOS, SaOS2, U2OS, and the human fetal osteoblastic cell line hFOB1.19. As shown in Figure 1B, the expression levels of miR-629 in all four OS cell lines were higher than that of human osteoblasts (P < 0.001).
miR-629 Was Correlated with Clinicopathological Characteristics of OS Patients
In order to explore the relationship between miR-629 and the clinicopathological characteristics, the OS patients were divided into patients with high miR-629 expression group (n = 65) and low miR-629 expression group (n = 45). The relationship between miR-629 expression and various clinicopathological characteristics in OS was shown in Table 1. The results of chi-square analysis indicated that miR-629 overexpression was significantly associated with clinical stage (P = 0.031), and distant metastasis (P = 0.012). However, miR-629 expression was not correlated with age, gender, tumor size or tumor site (P > 0.05).
miR-629 Was Correlated with Poor Prognosis in OS Patients
The Kaplan–Meier method and Log-rank test were used to analyze the relationship between miR-629 expression and the survival time of OS patients, and to explore the prognostic value of miR-629 in OS. The results demonstrated that the overall survival time of patients with lower miR-629 expression was longer than that of patients with higher miR-629 expression levels (log-rank P = 0.013, Figure 2). Moreover, multivariate Cox regression analysis results indicated miR-629 can be used as an independent prognostic factor in OS (HR = 2.890, 95% CI = 1.126–7.416, P = 0.027. Table 2).
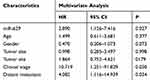 |
Table 2 Multivariate Cox Analysis of miR-629 and Clinical Parameters in Relation to Overall Survival |
 |
Figure 2 The Kaplan-Meier analysis of miR-629 in OS patients. As shown in the curve, OS patients with low miR-629 expression had longer survival time than those with high levels (log-rank P = 0.013). |
miR-629 Regulated Cell Proliferation, Migration, and Invasion in vitro
In addition to studying the clinical significance of miR-629 in OS, we further verified whether miR-629 was involved in tumor progression of OS cells by in vitro functional detection. MG63 and U2OS were transfected with miR-629 inhibitor, inhibitor NC, miR-629 mimic, mimic NC. Transfection efficiency was verified by qRT-PCR for miR-629 expression. Results indicated that miR-629 mimics successfully up-regulated the expression of miR-629, while miR-629 inhibitors down-regulated the expression of miR-629 (P < 0.001, Figure 3A).
The effect of miR-629 on cell proliferation ability was examined by CCK-8 assay. The results showed that overexpression of miR-629 promoted cell proliferation, while decreased miR-629 significantly inhibited cell proliferation (P < 0.01, Figure 3B). This study also demonstrated by Transwell assays that overexpression of miR-629 could promote the migration and invasion ability of OS cells, while the reduction of miR-629 could inhibit the migration and invasion ability of OS cells, which once again verified the correlation between the high expression of miR-629 in clinical and distant metastasis (P < 0.001, Figure 3C and D). In conclusion, the overexpression of miR-629 significantly promoted proliferation, migration, and invasion of OS cells in vitro, whereas knocking down miR-629 results in opposite results.
Discussion
OS is the most common bone malignancy, accounting for about 44.60% of bone tumors. Due to its high degree of malignancy, high rate of metastasis, and high mortality, it has become a research hotspot and clinical treatment difficulty.12–14 In the past few decades, the prognosis of OS patients has improved significantly with the development of surgery, radiotherapy, chemotherapy, and neoadjuvant therapy. However, the survival rate of metastatic patients is still low due to metastasis and recurrence, so there is an urgent need for a prognostic marker or gene therapy target to predict or inhibit the proliferation and metastasis of OS.15
Clearly, miRNAs are considered to be oncogenes or tumor suppressor genes in a variety of cancers and participate in the regulation of a range of complex cancer-related events, including cancer occurrence, development, differentiation, invasion, drug-resistant, and prognosis.16–22 For example, miR-21 and miR-125b are considered as novel, noninvasive prognostic markers for breast cancer.23 miR-29c exerts a tumor suppressor by targeting VEGFA, which may be a promising prognostic biomarker and a potential target for the treatment of lung adenocarcinoma.24 Increased miR-19a expression predicted poor prognosis in patients with osteosarcoma.3 The upregulation of miR-221 can predict clinical outcomes in patients with OS.25
miR-629 has been shown to be abnormally expressed in a variety of cancers and can be used as a prognostic and diagnostic marker for tumors. miR-629 can be used as a molecular marker for the diagnosis and prognosis of pancreatic cancer.11 miR-629 may be a new biomarker and potential therapeutic target for lung metastasis of triple-negative breast cancer.26 By directly targeting low-density lipoprotein receptor-related protein 6 (LRP6), the expression of microRNA-629-5p in colorectal cancer was down-regulated to prevent malignant phenotypes.27
In the current study, we firstly confirmed that miR-629 was overexpressed in OS and OS cell lines, suggesting that miR-629 may serve as an oncogene role in OS. In order to verify our hypothesis, we explored the relationship between miR-629 and the clinicopathological characteristics of OS patients, and the results showed that miR-629 was significantly correlated with clinical stage and distant metastasis. Therefore, we believed that miR-629 may be involved in the development of this malignant tumor as an oncogene in OS. In addition, Kaplan-Meier survival curve showed that the overall survival time of patients with lower miR-629 expression was longer than that of patients with higher miR-629 expression levels, suggesting that the upregulation of miR-629 was associated with poor overall survival. More importantly, miR-629 was identified as an independent prognostic factor for patients with OS by the multivariate Cox model. To our knowledge, this is the first study to investigate the expression pattern and clinical significance of miR-629 in patients with OS and to demonstrate that miR-629 can be used as a biomarker for prognosis of OS.
Since miR-629 plays an important role in a variety of tumors, we detected the role of miR-629 at the cellular level. such as CircSMAD2 inhibits the migration, invasion, and EMT of hepatocellular carcinoma cells by targeting miR-629.28 miR-629 can promote the proliferation, migration, and invasion of ovarian cancer by inhibiting the expression of TSPYL5, and the inhibition of miR-629 can be used as a therapeutic target for the treatment of ovarian cancer.29 Inhibition of miR-629 by regulating RUS1 can enhance the sensitivity of cervical cancer cells to 1ʹ-acetoxavicol acetate.30 We proved that low expression of miR-629 in OS cell lines significantly inhibited cell proliferation, migration, and invasion. The results have the same effect as miR-629 in other cancer, suggesting that miR-629 is a potential oncogene miRNA during OS progression. Recently, Zheng et al found that miR-629 promotes the proliferation, migration, and invasion of nasopharyngeal carcinoma cells by targeting PDCD4 regulation.31 This is consistent with our findings. Sun et al reported in 2017 that miR-433 regulates apoptosis of OS cells through targeted regulation of PDCD4.32 Meanwhile, Yang et al reported in 2017 that NBAT1 affected the growth and metastasis of OS by regulating the downstream target genes of miR-21, such as PDCD4 and PTEN.33 Therefore, we speculated that miR-629 may affect the prognosis of OS and cell biological function by targeting the regulation of PDCD4. However, the specific mechanism of miR-629’s role in OS still needs further study, which is the focus of our next study.
Conclusion
In summary, through a series of experiments, we proved that miR-629 is an oncogene, which may promote the development of OS by promoting the proliferation, migration, and invasion of OS cells. Meanwhile, miR-629 was correlated with the prognosis of OS, it provides a new prognostic biomarker and therapeutic target for OS.
Ethical Statement
The study was carried out in accordance with the principles of the Declaration of Helsinki. The Ethics Research Committee of The Affiliated Hospital of Qingdao University of China approved this study. All patients participating in the study provided written informed consent.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zhang Y, He Z, Li Y, et al. Selection of surgical methods in the treatment of Upper Tibia osteosarcoma and prognostic analysis. Oncol Res Treat. 2017;40(9):528–532. doi:10.1159/000477251
2. Marina N, Gebhardt M, Teot L, Gorlick R. Biology and therapeutic advances for pediatric osteosarcoma. Oncologist. 2004;9(4):422–441. doi:10.1634/theoncologist.9-4-422
3. Zou P, Ding J, Fu S. Elevated expression of microRNA-19a predicts a poor prognosis in patients with osteosarcoma. Pathol Res Pract. 2017;213(3):194–198. doi:10.1016/j.prp.2016.12.020
4. Stark A, Brennecke J, Russell RB, Cohen SM. Identification of drosophila microRNA targets. PLoS Biol. 2003;1(3):E60. doi:10.1371/journal.pbio.0000060
5. Ambros V. microRNAs: tiny regulators with great potential. Cell. 2001;107(7):823–826. doi:10.1016/S0092-8674(01)00616-X
6. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi:10.1016/S0092-8674(04)00045-5
7. Yang Q, Yu H, Yin Q, Hu X, Zhang C. lncRNA-NEF is downregulated in osteosarcoma and inhibits cancer cell migration and invasion by downregulating miRNA-21. Oncol Lett. 2019;17(6):5403–5408. doi:10.3892/ol.2019.10276
8. Nakka M, Allen-Rhoades W, Li Y, et al. Biomarker significance of plasma and tumor miR-21, miR-221, and miR-106a in osteosarcoma. Oncotarget. 2017;8(57):96738–96752. doi:10.18632/oncotarget.v8i57
9. Shin VY, Ng EK, Chan VW, Kwong A, Chu KM. A three-miRNA signature as promising non-invasive diagnostic marker for gastric cancer. Mol Cancer. 2015;14(202). doi:10.1186/s12943-015-0473-3
10. Chan CM, Lai KKY, Ng EKO, et al. Serum microRNA-193b as a promising biomarker for prediction of chemoradiation sensitivity in esophageal squamous cell carcinoma patients. Oncol Lett. 2018;15(3):3273–3280. doi:10.3892/ol.2017.7698
11. Shi W, Lu Y, Gong R, Sun JJ, Liu G. Serum miR-629 is a novel molecular marker for diagnosis and the prognosis of pancreatic cancer. Eur Rev Med Pharmacol Sci. 2018;22(16):5187–5193. doi:10.26355/eurrev_201808_15715
12. Dong J, Liu Y, Liao W, et al. miRNA-223 is a potential diagnostic and prognostic marker for osteosarcoma. J Bone Oncol. 2016;5(2):74–79. doi:10.1016/j.jbo.2016.05.001
13. Wang M, Xie R, Si H, Shen B. Integrated bioinformatics analysis of miRNA expression in osteosarcoma. Artif Cells Nanomed Biotechnol. 2017;45(5):936–943. doi:10.1080/21691401.2016.1196456
14. Di Fiore R, Drago-Ferrante R, Pentimalli F, et al. Let-7d miRNA shows both antioncogenic and oncogenic functions in osteosarcoma-derived 3AB-OS cancer stem cells. J Cell Physiol. 2016;231(8):1832–1841. doi:10.1002/jcp.v231.8
15. He M, Wang G, Jiang L, et al. miR-486 suppresses the development of osteosarcoma by regulating PKC-delta pathway. Int J Oncol. 2017;50(5):1590–1600. doi:10.3892/ijo.2017.3928
16. Chen X, Wang YW, Xing AY, et al. Suppression of SPIN1-mediated PI3K-Akt pathway by miR-489 increases chemosensitivity in breast cancer. J Pathol. 2016;239(4):459–472. doi:10.1002/path.4743
17. Wang YW, Shi DB, Chen X, Gao C, Gao P. Clinicopathological significance of microRNA-214 in gastric cancer and its effect on cell biological behaviour. PLoS One. 2014;9(3):e91307. doi:10.1371/journal.pone.0091307
18. Lin G, Sheng H, Xie H, et al. circLPAR1 is a novel biomarker of prognosis for muscle-invasive bladder cancer with invasion and metastasis by miR-762. Oncol Lett. 2019;17(3):3537–3547. doi:10.3892/ol.2019.9970
19. Galasso M, Sandhu SK, Volinia S. MicroRNA expression signatures in solid malignancies. Cancer J. 2012;18(3):238–243. doi:10.1097/PPO.0b013e318258b5f4
20. Caporali S, Amaro A, Levati L, et al. miR-126-3p down-regulation contributes to dabrafenib acquired resistance in melanoma by up-regulating ADAM9 and VEGF-A. J Exp Clin Cancer Res. 2019;38(1):272. doi:10.1186/s13046-019-1238-4
21. Godbole M, Togar T, Patel K, et al. Up-regulation of the kinase gene SGK1 by progesterone activates the AP-1-NDRG1 axis in both PR-positive and -negative breast cancer cells. J Biol Chem. 2018;293(50):19263–19276. doi:10.1074/jbc.RA118.002894
22. Yang ZQ, Wu CA, Cheng YX. Prognostic value of microRNA-133a expression and its clinicopathologic significance in non-small cell lung cancer: a comprehensive study based on meta-analysis and the TCGA database. Oncol Res Treat. 2018;41(12):762–768. doi:10.1159/000492343
23. Liu B, Su F, Chen M, et al. Serum miR-21 and miR-125b as markers predicting neoadjuvant chemotherapy response and prognosis in stage II/III breast cancer. Hum Pathol. 2017;64:44–52. doi:10.1016/j.humpath.2017.03.016
24. Liu L, Bi N, Wu L, et al. MicroRNA-29c functions as a tumor suppressor by targeting VEGFA in lung adenocarcinoma. Mol Cancer. 2017;16(1):50. doi:10.1186/s12943-017-0620-0
25. Gong N, Gong M. MiRNA-221 from tissue may predict the prognosis of patients with osteosarcoma. Medicine (Baltimore). 2018;97(29):e11100. doi:10.1097/MD.0000000000011100
26. Wang J, Song C, Tang H, et al. miR-629-3p may serve as a novel biomarker and potential therapeutic target for lung metastases of triple-negative breast cancer. Breast Cancer Res. 2017;19(1):72. doi:10.1186/s13058-017-0865-y
27. Yan G, Li C, Zhao Y, Yue M, Wang L. Downregulation of microRNA6295p in colorectal cancer and prevention of the malignant phenotype by direct targeting of low-density lipoprotein receptor-related protein 6. Int J Mol Med. 2019. doi:10.3892/ijmm.2019.4245
28. Zhang X, Luo P, Jing W, et al. circSMAD2 inhibits the epithelial-mesenchymal transition by targeting miR-629 in hepatocellular carcinoma. Onco Targets Ther. 2018;11:2853–2863. doi:10.2147/OTT.S158008
29. Shao L, Shen Z, Qian H, Zhou S, Chen Y. Knockdown of miR-629 inhibits ovarian cancer malignant behaviors by targeting testis-specific Y-like protein 5. DNA Cell Biol. 2017;36(12):1108–1116. doi:10.1089/dna.2017.3904
30. Phuah NH, Azmi MN, Awang K, Nagoor NH. Suppression of microRNA-629 enhances sensitivity of cervical cancer cells to 1’S-1ʹ-acetoxychavicol acetate via regulating RSU1. Onco Targets Ther. 2017;10:1695–1705. doi:10.2147/OTT.S117492
31. Zheng YQ, Bai YF, Yang S, et al. MircoRNA-629 promotes proliferation, invasion and migration of nasopharyngeal carcinoma through targeting PDCD4. Eur Rev Med Pharmacol Sci. 2019;23(1):207–216. doi:10.26355/eurrev_201901_16766
32. Sun Y, Wang F, Wang L, et al. MicroRNA-433 regulates apoptosis by targeting PDCD4 in human osteosarcoma cells. Oncol Lett. 2017;14(2):2353–2358. doi:10.3892/ol.2017.6441
33. Yang C, Wang G, Yang J, Wang L. Long noncoding RNA NBAT1 negatively modulates growth and metastasis of osteosarcoma cells through suppression of miR-21. Am J Cancer Res. 2017;7(10):2009–2019.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


