Back to Journals » Drug Design, Development and Therapy » Volume 14
Electrospun PLA Fibers Containing Metronidazole for Periodontal Disease
Authors Budai-Szűcs M, Léber A, Cui L , Józó M , Vályi P, Burián K, Kirschweng B , Csányi E , Pukánszky B
Received 19 September 2019
Accepted for publication 22 December 2019
Published 16 January 2020 Volume 2020:14 Pages 233—242
DOI https://doi.org/10.2147/DDDT.S231748
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sukesh Voruganti
Mária Budai-Szűcs, 1 Attila Léber, 1 Lu Cui, 2, 3 Muriel Józó, 2, 3 Péter Vályi, 4 Katalin Burián, 5 Balázs Kirschweng, 2, 3 Erzsébet Csányi, 1 Béla Pukánszky 2, 3
1Institute of Pharmaceutical Technology and Regulatory Affairs, Faculty of Pharmacy, University of Szeged, Szeged, Hungary; 2Laboratory of Plastics and Rubber Technology, Department of Physical Chemistry and Materials Science, Budapest University of Technology and Economics, Budapest H-1521, Hungary; 3Institute of Materials and Environmental Chemistry, Research Centre for Natural Sciences, Hungarian Academy of Sciences, Budapest H-1519, Hungary; 4Department of Periodontology, Faculty of Dentistry, University of Szeged, Szeged, Hungary; 5Institute of Clinical Microbiology, Faculty of Medicine, University of Szeged, Szeged, Hungary
Correspondence: Mária Budai-Szűcs
Institute of Pharmaceutical Technology and Regulatory Affairs, Faculty of Pharmacy, University of Szeged, Eötvös utca 6, Szeged 6720, Hungary
Tel +36 62 545-573
Fax +36 62 545-571
Email [email protected]
Purpose: Electrospun PLA fiber devices were investigated in the form of fiber mats and disks. Metronidazole was used as an active agent; its concentration was 12.2 and 25.7 wt% in the devices.
Methods: The structure was studied by X-ray diffraction and scanning electron microscopy, drug release by dissolution measurements, while the antimicrobial efficiency was tested on five bacterial strains.
Results: The XRD study showed that the polymer was partially crystalline in both devices, but a part of metronidazole precipitated and was in the form of crystals among and within the fibers. Liquid penetration and dissolution were different in the two devices, they were faster in disks and slower in fiber mats, due to the morphology of the device and the action of capillary forces. Disks released the drug much faster than fiber mats. Although the release study indicated fast drug dissolution, the concentration achieved a plateau value in 24 hrs for the disks; the inhibition effect lasted much longer, 13 days for bacteria sensitive to metronidazole. The longer inhibition period could be explained by the slower diffusion of metronidazole located inside the fibers of the device.
Conclusion: The results suggest that the devices may be effective in the treatment of periodontitis.
Keywords: drug release devices, fiber mats, disks, morphology, dissolution, capillary forces, diffusion, antimicrobial, inhibition
Introduction
Periodontitis is an inflammatory disease resulting from the overgrowth of subgingival polymicrobial community in susceptible hosts affecting the tissues surrounding the teeth. The inflammation of periodontal tissue causes bone destruction by osteoclastic resorption together with tissue destruction, and the detachment of junctional epithelium result in periodontal pocket formation. Conditions in the periodontal pocket are suitable for bacterial proliferation and result in dysbiosis owing to a breakdown in host-microbe homeostasis.1,2 This disease is the most prevalent reason for tooth loss among adults.3
The elimination of biofilm and/or hard deposits (mineralized biofilm) is the cornerstone of periodontal treatment. The management of periodontal disease includes the mechanical removal of biofilm with or without the adjunctive use of systemic antibiotics.4 Systemically administered antibiotics may not be present in sufficient concentration in the periodontal pocket; therefore, they are often ineffective, while the common side effects of antimicrobial therapy could occur.5
Numerous publications published in the open literature report investigations on local delivery systems containing antimicrobial drugs, which – alone or in combination with other dental procedures – may result in a more efficient treatment.5–10 The local administration of delivery systems with incorporated antibiotics is a promising approach to treating periodontitis. The introduction of devices containing antibiotics into the periodontal pockets could result in efficient therapy with limited side effects and reduced risk of developing drug-resistant microbes. The comparison of local to systemic drug delivery shows that 100-fold larger concentrations of the antimicrobial agents can be achieved at subgingival sites, which can lead to a more efficient therapy.11 Numerous local drug delivery systems such as fibers, strips, films, injectable gels, micro- and nanoparticulate systems, vesicular systems, and in-situ forming implants were developed for this purpose during the last decades.5–10
The electrospinning technique has received considerable attention lately because it is an affordable, cost-effective and easy-to-use technology for creating nanofibrous scaffolds for tissue engineering purposes, as well as drug delivery systems and wound dressings.12–15 The advantages of electrospun drug delivery systems are numerous: i) large drug loading capacity (up to 60%),16 ii) different polymers may be applied depending on the compatibility of the active pharmaceutical ingredients, iii) the process is simple and cost-effective,17 and iv) the modulation of drug release can be achieved as well.18,19
Sustained drug release can be achieved by both non-biodegradable and biodegradable polymers. Biodegradable polyesters such as PLA, polyglycolic acid (PGA), poly(lactic-co-glycolic) acid (PLGA), and polycaprolactone (PCL) have already been applied18 for this purpose. Information about research describing polymer or biopolymer-based electrospun delivery systems containing different antimicrobial agents, which may be applied with success for the treatment of periodontitis, is available in the literature.20–25 Fiber mats fabricated using the electrospinning method may be suitable for the treatment of periodontal disease and they could provide prolonged drug release.20
The goal of the current work was to develop and investigate locally administrable, PLA-based, metronidazole-containing devices for the treatment of periodontal disease. The devices were composed of electrospun fiber mats as received from the spinning process and compressed disks prepared from the mats. The drug used was metronidazole, which is known to be an active pharmaceutical ingredient (API) with an undesirable bitter taste, hindering its local application as a drug for periodontal treatment. It is expected that the encapsulation of metronidazole into a hydrophobic polymer matrix, sustained release, and probably a lower effective concentration in the oral cavity might eliminate this problem. We assumed that mats enable the free flow of the exudate in the periodontal pocket. On the other hand, disks provide drug delivery devices with a standardized geometry (surface, width) and equal drug content and facilitate the treatment of tight pockets. Accordingly, we analyzed the effect of drug concentration and the form of the delivery device on the kinetics of drug release in detail. Practical consequences are briefly mentioned in the final section of the paper.
Materials and Methods
Materials
Spinning solution was made by dissolving polylactic acid (PLA) (Ingeo 4032D type, NatureWorks LLC., Minnetonka, MN, USA) in a mixture of dichloromethane and dimethyl sulfoxide (Molar Chemicals Ltd., Halásztelek, Hungary). For the spinning of fibers containing an active agent, metronidazole (Ph. Eur. 8., Hungaropharma Plc., Budapest, Hungary) was also dissolved in the same solvent mixture in different concentrations.
For in vitro drug diffusion measurements, a pH=7.4 phosphate-buffered saline (PBS) solution was used. The buffer solution was prepared by dissolving 8 g/dm3 NaCl, 0.2 g/dm3 KCl, 1.44 g/dm3 Na2HPO4 · 2 H2O, and 0.12 g/dm3 KH2PO4 (Ph. Eur. 8., Hungaropharma Plc., Budapest, Hungary) in distilled water. The pH was adjusted to 7.4 by adding an adequate amount of 0.1 M HCl to the solution.
Methods
Fiber Spinning
PLA fibers containing various amounts of metronidazole were prepared by coelectrospinning, which is a widely used and cost-effective method to produce nanofibers especially for tissue engineering and regenerative medicine.26 The fibers were spun at ambient temperature at 15-kV voltage, 10-cm collector distance, and a feeding rate of 3 cm3/hr. The solvent used was the 80/20 vol% mixture of dichloromethane and dimethyl sulfoxide. The solution contained the polymer in 10 wt%, while the amount of the active component was changed from 0 to 3 wt% of the solution. The random array of fibers was compressed to disks of 13-mm diameter for further testing.
Sample Preparation
The metronidazole concentration of the fibers was 12.2 and 25.7 wt%, the latter corresponding to the metronidazole concentration of the saturated spinning solution. Neat electrospun fiber mats taken directly from the aluminum collector film and round-shaped, compressed disks were used for the analysis of release kinetics and microbial activity. The disks were obtained by compressing approximately 10–15 mg of the neat fiber under 1 kN pressure for 30 s in a pellet die of 13-mm diameter (Specac Atlas Manual Hydraulic Press 15T and Specac 13 mm Pellet Press Die, Specac Ltd., Orpington, Kent, UK).
Scanning Electron Microscopy
The appearance of the disks and fibers and the diameter of the latter were investigated by scanning electron microscopy using a Jeol JSM 6380 LA apparatus. The micrographs were recorded at different magnifications using 15-kV acceleration voltage. Before viewing, the fibers were sputtered with gold. The determination of fiber thickness was done with the Image Pro Plus 6 software.
X-Ray Diffraction Analysis
A Bruker D8 Advance diffractometer (Bruker AXS GmbH, Karlsruhe, Germany) with CuKα radiation (λ = 1.5406 Å) was used for the XRD analysis. The samples were scanned at 40 kV and 20 mA from 3° to 40° 2θ angle at a scanning rate of 0.1 °/s and a step size of 0.01°.
Penetration of the Aqueous Medium, Wetting
The behavior of the devices in contact with water was studied with an OCA Contact Angle System (Dataphysics OCA 20, Dataphysics Inc., GmbH, Germany). The contact angle of water droplets was determined on the neat electrospun fibers, the disks prepared from them as described above, and on a metronidazole pastille of the same size. The measurement was done with a water droplet of 10 μL volume, which was dropped from a calibrated syringe onto the surface of the various devices. Contact angle was measured instantly after the placement of the droplet, as well as 10 and 20 mins afterward. Five parallel measurements were done on each sample.
In vitro Drug Release Study
The in vitro drug release profiles of the nanofibers were determined by the measurement of dissolution followed by UV-Vis spectroscopy. Disks (0.014–0.017 g) and neat fiber mats (0.012–0.016 g) were weighed and put into 7.5 mL of pH=7.4 PBS solution thermostated at 37°C. Samples of 1.0 mL volume were taken at 0.5, 1, 4, 6, 10, 24, 30, 48, 72, 96, and 196 hrs and replaced with 1.0 mL of fresh PBS solution. Drug release was followed for 7 days. The concentration of the released metronidazole was determined by UV-Vis spectrophotometry (Helios α Thermospectronic UV-spectrophotometer v4.55, Unicam: Thermo Fisher Scientific, Waltham, MA) at 318 nm. Blank samples (PLA fiber mats and disks) were also treated in the same way as reference, but in their case no absorbance was detected in the wavelength range of 200–900 nm with spectrophotometric analysis.
Antimicrobial Test
The antibacterial efficiency of the disks containing 12.2 or 25.7 wt% metronidazole was determined as well. The control strains of five bacteria were used in the study: Fusobacterium nucleatum (ATCC® 25586™), Parvimonas micra (ATCC 33270™), Eikenella corrodens (ATCC 23834™), Aggregatibacter actinomycetemcomitans (ATCC 29524™), and Prevotella intermedia (118710). All measurements were carried out according to the same protocol. A bacterial suspension of one McFarland standard concentration, which is equivalent to approximately 3×108 colony-forming units/mL in the suspension, was freshly prepared with normal saline solution. Equivalent portions of the suspensions were then spread onto a horse blood agar plate and disks of equal weight were placed on it. The diameter of the inhibition zone was measured after 24 hrs of incubation in an anaerobic chamber. The disks were then put again on a new horse blood agar plate, also inoculated with a bacterial suspension of one McFarland standard concentration freshly made from the bacterial strains mentioned above. The plates were then put into an anaerobic chamber for 24 hrs. The procedure was repeated until no inhibition zone could be detected, or for a maximum of 14 days. Three parallel measurements were carried out for every formulation and bacterial strain.
Statistical Analysis
The results of the in vitro drug release study were analyzed statistically with GraphPad Prism version 5 software. Two-way ANOVA analysis was used with Bonferroni post-tests. A level of p ≤ 0.05 was considered as significant, p ≤ 0.01 as very significant, and p ≤ 0.001 as highly significant.
Results and Discussion
The results are discussed in several sections. First, results related to the structure of the devices are presented and then the penetration of the dissolution medium into them is analyzed. Drug release and the results of the microbiological testing are discussed in the last two sections together with a few remarks on practical relevance.
Structure
PLA has a regular molecular structure and can crystallize under appropriate conditions, and metronidazole is also a crystalline material. The solubility of the drug in the polymer and its diffusion into the surrounding medium depend on the structure of the components and on their distribution. Metronidazole is a water-soluble, polar substance, the solubility of which is small in the polymer. Accordingly, some of the drugs can be dissolved, but another part precipitates and is located among or inside the fibers as crystals. Structure determines drug release and its control allows the regulation of efficiency through modified drug release.
The structure was studied by XRD analysis and SEM microscopy. The crystallization of PLA is slow; thus, products prepared from this polymer are usually amorphous. On the other hand, the evaporation rate and the possible presence of residual solvent might result in the crystallization of the polymer. The XRD study showed that the fibers were partially crystalline at the time of the measurements and the release, as well as during the microbiological measurements. As expected, the compression of the mat into disc did not change the structure.
The incorporation of metronidazole may change the structure of the polymer. The drug may act as a nucleating agent, but it can change the rate of crystallization as well. The XRD traces of metronidazole and those of the fiber mat containing 12.2 and 25.7 wt% of the drug are presented in Figure 1. The characteristic reflections of metronidazole can be detected in the traces and their intensity increases with increasing concentration. Obviously, not the entire amount of the drug added to the spinning solution is dissolved in the polymer, but it precipitates during fiber spinning and is dispersed in crystalline form among and/or inside the fibers. The location and physical form of metronidazole may influence drug release significantly, thus determining both the efficiency of the device and its lifetime, the length of the active period.
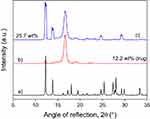 |
Figure 1 Influence of metronidazole content on the structure of PLA fiber mats, where: a) neat metronidazole, b) 12.2 wt%, and c) 25.7 wt% drug. |
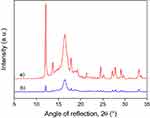
The XRD traces of disks containing 25.7 wt% metronidazole are presented in Figure 2 before and after drug release (dissolution). The intensity of characteristic peaks belonging to metronidazole decreases during the dissolution experiment as an effect of drug release. It can also be seen that some metronidazole remains in the device even after the dissolution experiment, which confirms our earlier assumption that some of the drug precipitates during fiber spinning and crystals are located not only among, but also within the fibers. The release of this latter part of the drug is quite slow and does not take place in the timescale of the dissolution experiment.
The structure of the fiber mat and the disks was also studied by scanning electron microscopy. Micrographs showing the differences in structure are presented in Figure 3. The mat consists of loose fibers with considerable space among individual fibers (Figure 3A). One would expect fast penetration and easy flow of the fluid used for dissolution and thus very fast release of the drug. The scrutiny of micrographs recorded on mats reveals the presence of metronidazole crystals among the fibers. The structure of a disk is shown in the micrograph of Figure 3B. The disk has a much more compact structure, voids are smaller, and the fibers are close to each other. The presence of metronidazole crystals among the fibers is more obvious in this case. The SEM study verified the conclusions drawn from the XRD measurements and proves that a part of the drug is distributed in crystal form among and probably also within the fibers.
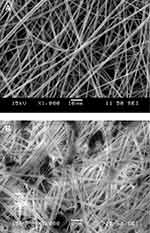 |
Figure 3 SEM micrographs recorded on the PLA devices studied. A) fiber mat, B) compressed disk. The black dots are crystalline metronidazole particles in Figure 3B. |
Penetration of the Aqueous Medium, Wetting
In order to obtain some idea about the penetration of the dissolution medium into the devices prepared, the contact angle of water was measured on them as a function of time. The results are presented in Table 1. Interpretation is difficult because of the complexity of the system and the related processes. Contact angle is usually measured by placing a droplet of a liquid onto a smooth, stable surface. In our case, the surface used for the measurement is neither stable nor smooth. Metronidazole dissolves in water, while both the fiber mats and the compressed disks have rough, porous surfaces (see Figure 3) into which the liquid may penetrate. Accordingly, the contact angle measured depends on the rate of dissolution in the case of metronidazole, and on surface tension and surface morphology in the case of the two devices. Pore size and capillary forces also play an important role in the determination of the value of the contact angle, but also in drug release. Since the surface tension of the components is constant and we do not expect large changes in the surface tension of PLA as an effect of the dissolution of some metronidazole, the major factors must be morphology, porosity, and capillary forces.
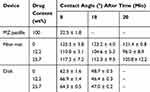 |
Table 1 Contact Angles Measured (Mean and SD Values) on a Metronidazole Pastille and on Drug Release Devices (Fiber Mats, Disks) at Various Times |
According to the results of Table 1, the contact angle of water on the metronidazole pastille is small, which is not very surprising since metronidazole dissolves in water. Dissolution is confirmed by the fact that the contact angle could not be measured after 10 mins of forming the droplet. Contrary to metronidazole, the contact angle of the droplet placed onto the fiber mat is quite large, indicating poor wetting and penetration. Contact angle does not change with metronidazole content; the differences are caused by changes in morphology and the standard deviation of the measurements. Contact angles showed just a slight systematic decrease with time for the neat fiber mats, indicating that water can slowly penetrate into the mat.
The contact angle measured on the compressed disks is much smaller and it is independent of the concentration of metronidazole as well. It decreases considerably with time and could not be measured after 10 mins at all, showing that the water droplet placed onto the disk disappeared in this time interval. The difference in the contact angles measured on the fiber mat and the compressed disk proves that the primary factor determining aqueous media penetration is morphology and this factor is expected to determine the extent and rate of drug release as well.
The apparent contradiction that water cannot penetrate so fast into the loose fiber mats, while it does into the discs, should be considered. Capillary forces depend on the interaction of the liquid and the capillary and also on the size of the capillary, i.e., on pore size, in our case. Water climbs in a glass capillary, but the level of mercury decreases in it. The surface tension of water is 72 mJ/m2, while that of mercury is 486 mJ/m2; thus, the first wets glass, while the other does not. In our case, the surface tension of water is still the same (72 mJ/m2) as mentioned above and that of PLA is around 40 mJ/m2.27–29 Accordingly, water does not wet PLA fibers and cannot penetrate into the devices easily. Compression obviously changed the pore structure of the device (see Figure 3), which decreases capillary forces and helps penetration. As an effect of changing morphology and penetration, we may expect a faster release of the drug from the disk than from the fiber mat.
In vitro Drug Release
The release of the drug incorporated into the devices prepared from electrospun fibers is a complex process and depends on several factors. As the results of the XRD measurements (Figures 1 and 2) and the SEM micrographs (Figure 3) showed the drug, metronidazole in this case, is incorporated into the devices in various forms: as precipitated crystals within and among the fibers and as dissolved molecules in PLA. The dissolution of these forms must be different. The PBS solution must penetrate the device, dissolve the crystals and diffuse out into the surrounding medium. On the other hand, dissolved metronidazole must diffuse out of the PLA fibers into the surrounding medium. The solubility of metronidazole is small in PLA, and diffusion is driven by concentration difference, which is also small or even negative due to the large drug concentration of the surrounding solution because of the dissolution of the crystals. Consequently, the main factor determining the dissolution of metronidazole from the devices, i.e., drug release, is the penetration and flow of the PBS solution. This is different for the two devices, mats and disks; thus dissimilar drug release is expected from them.
The time dependence of dissolution is presented in Figure 4 for the fiber mats and the disks at two different concentrations. In view of the observations presented in Section 3.2, some of the results were expected. Compressed disks release the drug much faster than fiber mats because of the larger and faster penetration of the aqueous medium into the pores of the device. However, the fact that dissolution is independent of the initial concentration of metronidazole in the device is somewhat surprising. The fast penetration and dissolution of metronidazole in the PBS solution can result in the independence of concentration. The diffusion of the liquid containing the dissolved drug into the surrounding medium may be the rate-determining step of dissolution in this case.
In the case of the fiber mats, the rate of dissolution depends on concentration, however, not as expected, i.e., faster rate at larger concentration, but in the opposite way. The slower release of the drug from the mats can be understood easily if we consider the difference in the penetration of the aqueous medium (see Table 1). The effect of concentration, on the other hand, is difficult to explain. Obviously, the dissolution of the drug in the PBS solution and its diffusion into the surrounding medium are the rate-determining steps in this case. However, the presence of the drug cannot influence the diffusion rate much; thus, dissolution must be dissimilar at the two concentrations of metronidazole. The larger drug concentration probably results in larger precipitated crystals, which leads to slower dissolution and release.
The rate of dissolution can be estimated qualitatively from the correlations presented in Figure 4. According to this evaluation, plateau concentrations are reached after 24 hrs for the disks, and after 48 or 96 hrs for the fiber mats. However, time dependence can be evaluated quantitatively if appropriate functions are fitted to the experimental data. The dissolution and the diffusion of the drug are determined by Fick’s laws. Fick’s equations can be solved numerically or they can be expressed analytically using simplifications.30 Two main approaches are used in practice, those describing the first part of the function plotting experimental results as the function of the square root of time, or those which use an exponential function. This approach gives a more accurate estimate at long times and it allows the estimation of the overall rate of dissolution and the maximum amount of dissolved material at infinite time (if the shape and structure of the device do not change, e.g.: fiber degradation). We followed the latter approach and fitted the function of Equation 1 to the experimental results:
(1)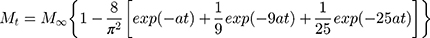
where Mt and M∞ are the dissolved amount of drug at time t and at infinite time, respectively, and a is the overall rate of dissolution. The fitted functions are presented in Figure 4 as solid lines. The parameters calculated from the fitting are collected in Table 2.
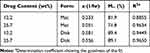 |
Table 2 Parameters Characterizing the Kinetics of Dissolution Determined by Fitting Equation 1 to the Experimental Results |
Results presented in Table 2 confirm our qualitative evaluation and show that dissolution is much faster from the disk than from the fiber mat (see parameter a). It also confirms the composition dependence observed. The comparison of the predicted amount of drug dissolved at infinite time (M∞) indicates that a considerable amount of drug, 10–30% remains in the devices after the dissolution experiment even in the case of the disks. Moreover, a closer comparison of the fitted lines and the measured values indicates that dissolution cannot be described with a single process; it consists of at least two steps, a faster one at the beginning of the experiment and another one proceeding at a slower rate. This two-step process is demonstrated especially well by the results obtained on the fiber mat containing 25.7 wt% metronidazole (see ∆ series in Figure 4). The two steps demonstrated by the broken line in the figure might be explained by the dissolution of the different forms of the drug; crystals located among the fibers dissolve much faster than dissolved metronidazole or crystals precipitated within the fibers. In this case, significant differences could be observed (from 2.5 to 6 hrs: p ≤ 0.001; from 6 to 30 hrs: p ≤ 0.01; and from 30 to 72 hrs: p ≤ 0.05) between the drug release of fiber mats and disks. The different rates allow the regulation of the amount of the drug as a function of time and also the active lifetime of the device. Controlling the form of the drug in the device and the rate of water diffusion into the electrospun porous fiber network might be an efficient strategy to control drug release.31,32
Antimicrobial Activity
The oral cavity and/or various dental surfaces may be inhabited by numerous strains of pathogen bacteria. Personal features, dietary habits, or even habitation could have an influence on the composition of the oral microbiota.33,34 The contribution of different bacterial strains to the initiation and progression of periodontitis is under serious investigation.33–41 However, the task seems to be almost impossible because of the large number of various microorganism species, difficulties of cultivation, and the fact that only a small part of the bacteria present in the subgingival cavity could be linked to the pathogenesis of the disease.42 Pathogens playing a role in the formation and progression of periodontal disease can be subdivided into three groups. The first category strains are able to stick to dental surfaces, the bacteria in the second group form a bridge between the first and the third category, while the bacteria of the third group are responsible for bacterial biofilm formation (Socransky and Haffajee, 2002). The following strains may initiate disease formation: Porphyromonas gingivalis, P. intermedia, Bacteroides forsythus, A. actinomycetemcomitans, Treponema denticola, Tannerella forsythia, F. nucleatum, Fusobacterium periodonticum, Prevotella nigrescens, P. micra, Campylobacter gracilis, Campylobacter rectus, Campylobacter showae, Eubacterium nodatum, Streptococcus constellatus, E. corrodens, Streptococcusspp., etc.33,42–44
Only disks were included in the microbiological study, as uniform shape, weight, and thickness could not have been achieved with the fiber mats. The delivery systems evaluated contained 12.2 and 25.7 wt% metronidazole. Five different bacterial strains were used in this investigation: E. corrodens, P. intermedia, P. micra, F. nucleatum, and A. actinomycetemcomitans. E. corrodens is a facultative anaerobic, gram-negative bacterium and a human (mostly oral) pathogen. E. corrodens is reported to be unsusceptible to metronidazole.45 P. micra is a gram-positive, anaerobic coccus and part of the normal human gastrointestinal flora.46 Literature data suggest that this strain is not susceptible to metronidazole either.47 P. intermedia is a black pigmented, gram-negative anaerobic bacterium. It is often connected with oral and subgingival diseases. Metronidazole is efficient against P. intermedia.48 F. nucleatum, which is a mostly oral and periodontal anaerobic pathogen bacterium, can be related to several human diseases. This strain seems to be sensitive to metronidazole.49,50
A facultatively anaerobic gram-negative bacterium, A. actinomycetemcomitans, can often be linked to periodontal disease and oral infections. Susceptibility to metronidazole is reported to be weak.51,52
The results of the microbiological study are presented in Figure 5. The duration of the antimicrobial effect is apparently independent of concentration; it is the same for disks with 12.2 and 25.7 wt% metronidazole content in most cases. A one-day difference appeared in growth inhibition for P. micra at 12.2 and 25.7 wt% metronidazole contents. In the other cases, disks with a larger metronidazole content provided slightly larger inhibition zones than systems containing less drug on most days. Bacterial growth inhibition is shorter, only 2–3 days for A. actinomycetemcomitans, E. corrodens and P. micra, while the growth of F. nucleatum and P. intermedia was affected for a longer time, for 13 days.
The results of our measurements agree well with those published in the literature, suggesting that F. nucleatum and P. intermedia are more susceptible to metronidazole than A. actinomycetemcomitans, E. corrodens and P. micra, which may be completely resistant or minimally sensitive to the antimicrobial drug used. The diameter of the inhibition zone increases slightly with increasing metronidazole concentration, probably because of longer diffusion paths resulting in enhanced inhibition. The differences in the diameter of the inhibition zone are more pronounced for strains with larger susceptibility to metronidazole and they increase with time as well. Inhibition was observed in the growth of susceptible bacteria for as long as almost 2 weeks, indicating that our devices can be efficient for a long time. This fact, however, needs some consideration, since the dissolution study indicated that most of the drug is released from the disks in 24 hrs. The contradiction might be explained by the difference in the conditions, but also in the presence of metronidazole located within the polymer in the form of dissolved molecules or precipitated crystals. The diffusion, thus the release rate of metronidazole is much slower in this latter case than for the drug located among the fibers in crystal form. A slower rate leads to prolonged inhibition times, which could result in greater patient compliance and better results of the periodontitis treatment.
Conclusion
The XRD study of drug release devices prepared from electrospun PLA fibers showed that the polymer was partially crystalline in both devices, i.e., in fiber mats and disks. A part of metronidazole precipitated and was located in the form of crystals among the fibers. The penetration of the aqueous medium and dissolution were different in the two devices, they were faster in disks and slower in fiber mats, due to the morphology of the device and because of the action of capillary forces. Disks released the drug much faster than fiber mats. The microbiological study carried out with five bacterial strains confirmed results published in the open literature that some strains are insensitive to metronidazole (A. actinomycetemcomitans, E. corrodens, P. micra), while the drug is very efficient against others (F. nucleatum, P. intermedia). Although the release study indicated the fast dissolution of the drug, the concentration achieved a plateau value in 24 hrs for the disks; the inhibition effect was much longer, 13 days for bacteria sensitive to metronidazole. The longer inhibition period could be explained by the slower diffusion of metronidazole located inside the fibers of the devices and the slow penetration of the aqueous medium into them. The results indicated that in all probability the devices prepared may be effective in the treatment of periodontitis.
Acknowledgments
Zita Zuba is acknowledged for carrying out preliminary experiments and optimization of fiber spinning for the devices tested. We are grateful to Judit Rebeka Molnar for the preparation of the fibers used in the experiments and to Péter Polyák for the fitting of dissolution results. The National Research, Development and Innovation Office (NKFIH, Grant No. K 120039) is acknowledged for the financial support of the research. The University of Szeged Open Access Fund (FundRef, Grant No. 4383) is also acknowledged for funding the open-access publication. This research was supported by the ÚNKP-19-3-SZTE-175 New National Excellence Program of the Ministry for Innovation and Technology.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bosshardt DD. The periodontal pocket: pathogenesis, histopathology and consequences. Periodontol 2000. 2018;76(1):43–50. doi:10.1111/prd.12153
2. Lamont RJ, Koo H, Hajishengallis G. The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol. 2018;16(12):745–759. doi:10.1038/s41579-018-0089-x
3. Sheiham A, Netuveli GS. Periodontal diseases in Europe. Periodontol 2000. 2002;29(1):104–121. doi:10.1034/j.1600-0757.2002.290106.x
4. Eick S, Nydegger J, Bürgin W, Salvi GE, Sculean A, Ramseier C. Microbiological analysis and the outcomes of periodontal treatment with or without adjunctive systemic antibiotics—a retrospective study. Clin Oral Investig. 2018;22(9):3031–3041. doi:10.1007/s00784-018-2392-3
5. Jain N, Jain GK, Javed S, et al. Recent approaches for the treatment of periodontitis. Drug Discov Today. 2008;13(21–22):932–943. doi:10.1016/j.drudis.2008.07.010
6. Schwach-Abdellaoui K. Local delivery of antimicrobial agents for the treatment of periodontal diseases. Eur J Pharm Biopharm. 2000;50(1):83–99. doi:10.1016/S0939-6411(00)00086-2
7. Southard GL, Godowski KC. Subgingival controlled release of antimicrobial agents in the treatment of periodontal disease. Int J Antimicrob Agents. 1998;9(4):239–253. doi:10.1016/S0924-8579(98)00004-1
8. Yar M, Farooq A, Shahzadi L, et al. Novel meloxicam releasing electrospun polymer/ceramic reinforced biodegradable membranes for periodontal regeneration applications. Mater Sci Eng C. 2016;64:148–156. doi:10.1016/j.msec.2016.03.072
9. Do MP, Neut C, Metz H, et al. Mechanistic analysis of PLGA/HPMC-based in-situ forming implants for periodontitis treatment. Eur J Pharm Biopharm. 2015;94:273–283. doi:10.1016/j.ejpb.2015.05.018
10. Tyagi P, Vaish S, Dodwad V. Clinical efficacy of subgingivally delivered 0.5% controlled release azithromycin gel in the management of chronic periodontitis. Indian J Med Sci. 2011;65(6):223–230. doi:10.4103/0019-5359.107017
11. Rams TE, Slots J. Local delivery of antimicrobial agents in the periodontal pocket. Periodontol 2000. 1996;10(1):139–159. doi:10.1111/j.1600-0757.1996.tb00072.x
12. Liu M, Duan X-P, Li Y-M, Yang D-P, Long Y-Z. Electrospun nanofibers for wound healing. Mater Sci Eng C. 2017;76:1413–1423. doi:10.1016/j.msec.2017.03.034
13. Zahedi P, Rezaeian I, Ranaei-Siadat SO, Jafari SH, Supaphol P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym Adv Technol. 2010;21(2):77–95. doi:10.1002/pat.1625
14. Thakkar S, Misra M. Electrospun polymeric nanofibers: new horizons in drug delivery. Eur J Pharm Sci. 2017;107(May):148–167. doi:10.1016/j.ejps.2017.07.001
15. Sill TJ, von Recum HA. Electrospinning: applications in drug delivery and tissue engineering. Biomaterials. 2008;29(13):1989–2006. doi:10.1016/j.biomaterials.2008.01.011
16. Zamani M, Prabhakaran MP, Ramakrishna S. Advances in drug delivery via electrospun and electrosprayed nanomaterials. Int J Nanomedicine. 2013;8:2997–3017. doi:10.2147/IJN.S43575
17. Asmatulu R, Khan WS. Introduction to electrospun nanofibers. Synth Appl Electrospun Nanofibers. 2019;1–15. doi:10.1016/B978-0-12-813914-1.00001-8
18. Chou SF, Carson D, Woodrow KA. Current strategies for sustaining drug release from electrospun nanofibers. J Control Release. 2015;220:584–591. doi:10.1016/j.jconrel.2015.09.008
19. Chou SF, Woodrow KA. Relationships between mechanical properties and drug release from electrospun fibers of PCL and PLGA blends. J Mech Behav Biomed Mater. 2017;65(July2016):724–733. doi:10.1016/j.jmbbm.2016.09.004
20. Reise M, Wyrwa R, Müller U, et al. Release of metronidazole from electrospun poly(l-lactide-co-d/l-lactide) fibers for local periodontitis treatment. Dent Mater. 2012;28(2):179–188. doi:10.1016/j.dental.2011.12.006
21. Schkarpetkin D, Reise M, Wyrwa R, et al. Development of novel electrospun dual-drug fiber mats loaded with a combination of ampicillin and metronidazole. Dent Mater. 2016;32(8):951–960. doi:10.1016/j.dental.2016.05.002
22. Monteiro APF, Rocha CMSL, Oliveira MF, et al. Nanofibers containing tetracycline/β-cyclodextrin: physico-chemical characterization and antimicrobial evaluation. Carbohydr Polym. 2017;156:417–426. doi:10.1016/j.carbpol.2016.09.059
23. Khan G, Yadav SK, Patel RR, Kumar N, Bansal M, Mishra B. Tinidazole functionalized homogeneous electrospun chitosan/poly (ε-caprolactone) hybrid nanofiber membrane: development, optimization and its clinical implications. Int J Biol Macromol. 2017;103:1311–1326. doi:10.1016/j.ijbiomac.2017.05.161
24. Kopytynska-Kasperczyk A, Dobrzynski P, Pastusiak M, Jarzabek B, Prochwicz W. Local delivery system of doxycycline hyclate based on -caprolactone copolymers for periodontitis treatment. Int J Pharm. 2015;491(1–2):335–344. doi:10.1016/j.ijpharm.2015.06.034
25. Ranjbar-Mohammadi M, Zamani M, Prabhakaran MP, Bahrami SH, Ramakrishna S. Electrospinning of PLGA/gum tragacanth nanofibers containing tetracycline hydrochloride for periodontal regeneration. Mater Sci Eng C. 2016;58:521–531. doi:10.1016/j.msec.2015.08.066
26. Amani H, Arzaghi H, Bayandori M, et al. Controlling cell behavior through the design of biomaterial surfaces: a focus on surface modification techniques. Adv Mater Interfaces. 2019;6(13):1900572. doi:10.1002/admi.201900572
27. Li ZQ, Zhou XD, Pei CH. Synthesis of PLA-co-PGMA copolymer and its application in the surface modification of bacterial cellulose. Int J Polym Mater. 2010;59(9):725–737. doi:10.1080/00914037.2010.483214
28. Jordá-Vilaplana A, Fombuena V, García-García D, Samper MD, Sánchez-Nácher L. Surface modification of polylactic acid (PLA) by air atmospheric plasma treatment. Eur Polym J. 2014;58:23–33. doi:10.1016/j.eurpolymj.2014.06.002
29. Biresaw G, Carriere CJ. Interfacial tension of poly(lactic acid)/polystyrene blends. J Polym Sci B Polym Phys. 2002;40(19):2248–2258. doi:10.1002/polb.10290
30. Kenyó C, Kajtár DA, Renner K, Kröhnke C, Pukánszky B. Functional packaging materials: factors affecting the capacity and rate of water adsorption in desiccant composites. J Polym Res. 2013;20(11):294. doi:10.1007/s10965-013-0294-2
31. Zhang JJ, Liu J, Yu H, Zhang Y, Zhu MF, Chen YM. Crosslinked electrospun UPM/PHBV/PVP fibers for sustained drug release. Mater Sci Forum. 2009;610–613:1331–1334. doi:10.4028/www.scientific.net/MSF.610-613.1331
32. Yohe ST, Colson YL, Grinstaff MW. Superhydrophobic materials for tunable drug release: using displacement of air to control delivery rates. J Am Chem Soc. 2012;134(4):2016–2019. doi:10.1021/ja211148a
33. Moore WEC, Moore LVH. The bacteria of periodontal diseases. Periodontol 2000. 1994;5(1):66–77. doi:10.1111/j.1600-0757.1994.tb00019.x
34. Costalonga M, Herzberg MC. The oral microbiome and the immunobiology of periodontal disease and caries. Immunol Lett. 2014;162(2):22–38. doi:10.1016/j.imlet.2014.08.017
35. Darby I, Curtis M. Microbiology of periodontal disease in children and young adults. Periodontol 2000. 2001;26:33–53. doi:10.1034/j.1600-0757.2001.2260103.x
36. Tsai C-Y, Tang CY, Tan T-S, Chen K-H, Liao K-H, Liou M-L. Subgingival microbiota in individuals with severe chronic periodontitis. J Microbiol Immunol Infect. 2016;1–9. doi:10.1016/j.jmii.2016.04.007
37. Wang J, Qi J, Zhao H, et al. Metagenomic sequencing reveals microbiota and its functional potential associated with periodontal disease. Sci Rep. 2013;3(1):1843. doi:10.1038/srep01843
38. Laksmana T, Kittichotirat W, Huang Y. Metagenomic analysis of subgingival microbiota following non-surgical periodontal therapy: a pilot study. Open Dent J. 2012;6(1):255–261. doi:10.2174/1874210601206010255
39. Chen H, Liu Y, Zhang M, et al. A filifactor alocis-centered co-occurrence group associates with periodontitis across different oral habitats. Sci Rep. 2015;5(1):9053. doi:10.1038/srep09053
40. Liu B, Faller LL, Klitgord N, et al. Deep sequencing of the oral microbiome reveals signatures of periodontal disease. Highlander SK, ed. PLoS One. 2012;7(6):e37919. doi:10.1371/journal.pone.0037919
41. Könönen E, Müller H-P. Microbiology of aggressive periodontitis. Periodontol 2000. 2014;65(1):46–78. doi:10.1111/prd.12016
42. Kinane DF. Causation and pathogenesis of periodontal disease. Periodontol 2000. 2001;25(1):8–20. doi:10.1034/j.1600-0757.2001.22250102.x
43. Socransky SS, Haffajee AD. Dental biofilms: difficult therapeutic targets. Periodontol 2000. 2002;28(1):12–55. doi:10.1034/j.1600-0757.2002.280102.x
44. Sanz M, Beighton D, Curtis MA, et al. Role of microbial biofilms in the maintenance of oral health and in the development of dental caries and periodontal diseases. Consensus report of group 1 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal disease. J Clin Periodontol. 2017;44:S5–S11. doi:10.1111/jcpe.12682
45. Chen CKC, Wilson ME. Eikenella corrodens in human oral and non-oral infections: a review. J Periodontol. 1992;63(12):941–953. doi:10.1902/jop.1992.63.12.941
46. Baghban A, Gupta S. Parvimonas micra: a rare cause of native joint septic arthritis. Anaerobe. 2016;39:26–27. doi:10.1016/j.anaerobe.2016.02.004
47. Rams TE, Feik D, Listgarten MA, Slots J. Peptostreptococcus micros in human periodontitis. Oral Microbiol Immunol. 1992;7(1):1–6. doi:10.1111/j.1399-302X.1992.tb00011.x
48. Santos FA, Bastos EMA, Rodrigues PH, et al. Susceptibility of prevotella intermedia/prevotella nigrescens (and porphyromonas gingivalis) to propolis (bee glue) and other antimicrobial agents. Anaerobe. 2002;8(1):9–15. doi:10.1006/anae.2002.0411
49. Han YW. Fusobacterium nucleatum: a commensal-turned pathogen. Curr Opin Microbiol. 2015;23(3):141–147. doi:10.1016/j.mib.2014.11.013
50. Jacinto RC, Montagner F, Signoretti FGC, Almeida GC, Gomes BPFA. Frequency, microbial interactions, and antimicrobial susceptibility of fusobacterium nucleatum and fusobacterium necrophorum isolated from primary endodontic infections. J Endod. 2008;34(12):1451–1456. doi:10.1016/j.joen.2008.08.036
51. Raja M. Aggregatibacter actinomycetemcomitans – a tooth killer? J Clin Diagn Res. 2014;8(8):13–16. doi:10.7860/JCDR/2014/9845.4766
52. Oettinger-Barak O, Dashper SG, Catmull DV, et al. Antibiotic susceptibility of aggregatibacter actinomycetemcomitans JP2 in a biofilm. J Oral Microbiol. 2013;5(1):20320. doi:10.3402/jom.v5i0.20320
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.