Back to Journals » Patient Preference and Adherence » Volume 16
Electronic Smart Blister Packages to Monitor and Support Medication Adherence: A Usability Study
Authors Izzah Z , Zijp TR , Åberg C , Touw DJ , van Boven JF
Received 17 May 2022
Accepted for publication 29 July 2022
Published 13 September 2022 Volume 2022:16 Pages 2543—2558
DOI https://doi.org/10.2147/PPA.S374685
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Johnny Chen
Zamrotul Izzah,1– 3,* Tanja R Zijp,1,* Christoffer Åberg,3 Daan J Touw,1,3,4 Job FM van Boven1,4
1Department of Clinical Pharmacy and Pharmacology, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands; 2Department of Pharmacy Practice, Faculty of Pharmacy, Universitas Airlangga, Surabaya, Indonesia; 3Department of Pharmaceutical Analysis, Groningen Research Institute of Pharmacy, University of Groningen, Groningen, the Netherlands; 4Medication Adherence Expertise Center of the Northern Netherlands (MAECON), Groningen, the Netherlands
*These authors contributed equally to this work
Correspondence: Job FM van Boven, Department of Clinical Pharmacy and Pharmacology, University Medical Center Groningen, Hanzeplein 1 (Internal Postcode AP50), Groningen, 9713 GZ, the Netherlands, Tel +31 50 361 7893, Fax +31 50 361 4087, Email [email protected]
Purpose: An electronic version of the Dosepak® (EDP) which records date and time of dosing events has been developed to monitor adherence to medication packaged in blisters. This study aimed to evaluate its usability and acceptance and to monitor dose-taking adherence for optimal implementation in future clinical trials and practice.
Methods: Healthy volunteers aged over 18 years were asked to dispense placebo tablets twice daily from EDPs equipped with a re-usable electronic module for a total duration of four weeks. Afterwards, subjects were asked to complete an online questionnaire and partake in a short one-on-one interview. The usability of the EDP was assessed using the System Usability Scale (SUS), while dose-taking adherence was monitored by EDP records, pill counting, and self-report. The short interview explored user experiences in more detail.
Results: Twenty subjects with median [IQR] age 41.5 [32– 49.8] years, 55% female, 45% healthcare professionals, and 20% chronic medication users completed the study and found the EDP easy to use, with a mean [SD] SUS score of 78.0 [11.2]. Median [IQR] dose-taking adherence was 89% [82– 95%] based on EDP records, 96.5% [89– 100%] based on pill counting, 92% [91– 96%] based on self-report, and the levels differed significantly (p < 0.05). Four themes emerged from the interviews: user preference, experience, patient burden, and ideas for improvement. Most participants preferred smaller sized blisters. They found the EDP simple to use and did not see any patient burden for its use in trials or clinical practice. Some reported forgetfulness and suggested reminders built into the blister or sent to their mobile phones. Adequate information or instruction should also be provided for older people and polypharmacy patients.
Conclusion: EDP had good perceived usability, was well accepted, and differed significantly from other adherence measurement methods. This study provides input to further guide scale-up of the blister packages.
Keywords: compliance, digital health, e-health, patient preference, real-time monitoring, smart packaging
Introduction
Medication non-adherence is a global problem with half of the people taking medication for a chronic condition being non-adherent, which highly varies per study and disease.1–3 Non-adherence hinders disease control, placing a burden on patient quality of life as well as on healthcare systems. The World Health Organization (WHO) concludes that an improvement in patients’ adherence may have a higher effect on people’s health than any other revision in therapy.1 Therefore, improving adherence and increasing the accuracy of adherence data may in turn enhance health outcomes and decrease healthcare utilization and costs.4
While it is generally challenging to objectively measure, monitor and improve adherence, some complex interventions, including digitally supported patient education (eg, with real-time data, sending of reminders, and packaging of medications) have been shown to improve adherence successfully.5–8 A meta-analysis of 48 studies revealed that blister packaging interventions when given out by pharmacies were the most effective intervention to increase medication adherence.9 For example, calendar blister packages may support and improve adherence, as they provide a visual record, thereby helping to identify whether doses have been taken or missed.10
The addition of smart features that record medication intake behavior provides granular data on non-adherence. This could be used to individualize patients’ adherence management, support treatment decisions, and support patient self-management.11 Multiple smart packaging solutions have been developed and include multidose dispensing systems, smart pill bottles, and pill-tracers.12 However, there is still a gap between the development of smart packaging solutions and their actual end-use in clinical practice. Indeed, for digital adherence monitoring devices to be optimally implemented in clinical drug trials and daily clinical practice, their usability and acceptance should be systematically evaluated.13–17
Recently, an electronic version of the Dosepak® (EDP) has been developed to monitor adherence. It consists of a customizable blister package with a re-usable electronic module that records when the package is opened and closed. To inform future large-scale implementation, this study aimed to assess the usability and acceptance of the EDP and to monitor dose-taking adherence.
Materials and Methods
Study Design
An observational single-arm study with 20 healthy volunteers was performed in July and August 2021. The study observed the use of an EDP twice daily for four weeks. After inclusion, all participants had two site visits; one at initiation and one after four weeks of use. Prior to the first visit, all subjects attended a 30-minute digital kick-off meeting during which information on the study and how to use the EDP were given, including an EDP explanatory video provided by the manufacturer. In their first visit, subjects received the EDPs containing placebo tablets and written instructions for use. Prior to the follow-up visit, all subjects were requested to complete an online survey. During the follow-up visit, subjects returned the EDPs and took part in a short one-on-one interview, lasting 5–10 minutes.
This study, albeit not being a formal clinical trial, was registered at the Netherlands Trial Register (Trial NL9535). According to Dutch law, the Medical Ethics Review Board of the University Medical Center Groningen (METc UMCG) waived the study protocol (number: 2021/363) from formal review as they found the study was not clinical research with human subjects as meant in the Medical Research Involving Human Subjects Act (WMO) and there was no infringement of the physical and/or psychological integrity of the subjects. This study did, however, follow the declaration of Helsinki and the General Data Protection Regulation. No clinical data on the subjects were collected or used during this study, and there were no changes to subjects’ care routines other than the use of the EDPs. All participants gave written informed consent (IC) before the start of the study.
Participants
The healthy volunteers were recruited directly by the investigators, ZI and TRZ, through the University Medical Center Groningen in the Netherlands. They were in good general health and had no known significant health problems or illnesses relevant to the proposed study as defined by the WHO and the Royal College of Physicians.18 Subjects were employees from different departments at the study site. They were approached personally, and a participant information sheet was provided. Subjects were eligible if they were aged over 18 years and provided written IC. Exclusion criteria after enrolment were unavailability to participate in the study and withdrawal of IC. After signing the IC, potential subjects provided demographic information including age, gender, highest education level, and whether they were taking chronic medication (in which contraceptive use could also be regarded as chronic medication). As there were more potential subjects that provided IC than EDPs at our disposal, a random selection was made based on the demographic characteristics (gender, age, education levels) to acquire a diverse group. The sample size was determined pragmatically and in line with previous similar studies.13,19,20 Study materials were provided in English, and all participants stated that they had sufficient proficiency in the English language to understand these. Furthermore, no incentives were provided to the participants.
Smart Blister Package
The electronic Dosepak® or EDP (Westrock Healthcare Packaging, Atlanta, United States) is a smart blister package first developed in 2017 and equipped with a medication event monitoring system (MEMS®) in the form of an electronic module (AARDEX Group, Belgium) to automatically compile dosing events (hereafter referred to as EDP). Figure 1 illustrates the application of the EDP. The EDP contains medication information on the outer carton with placebo tablets packaged in a calendar blister, which included labels indicating the day of the week and symbols indicating morning/evening, and a re-usable electronic module (e-module) affixed to the card. The blister packages with a twice-daily regimen were provided by the manufacturer; however, the packages are customizable for different dosing regimens, different package sizes, and a variety of pill sizes and numbers. The e-module has a battery life of three years.
It requires two steps to open the blister package: first, the left button should be pressed and held gently, and second, the medication card should be pulled out and flipped open. Each blister contains 14 tablets for a twice daily regimen of one week. Upon each opening and closing of the pulled-out medication card, the date and time are registered by the e-module. Therefore, the e-module should be inserted into the plastic rail of the blister package prior to taking the first dose. After pushing out the last dose, the e-module should be removed and inserted into a new package.
To access the data stored in the e-module, the e-module is to be read out by MEMS® USB Reader, which is equipped with a near field communication (NFC) interface, with a computer connected to the internet and with the Electron Reader 0.9.3 software installed. The data may then be uploaded to the web-based MEMS® Adherence Software v4, which is accessible to the study investigators. The software calculates adherence from the number of registered events that followed the predefined regimen, and graphically shows the subject’s dose-taking behavior over time.
Study Procedures Related to EDP
Before providing the EDPs to the subjects, initialization was done for each e-module through the MEMS® Adherence Software v4. Patient number, time zone, monitoring starting date, and predefined regimen of twice daily were set up to correctly identify subjects. The subjects were instructed to start the use of EDP on a Monday as the first day of the study. During the first visit, each subject received four blister packages (one for each week) and an e-module. They practiced opening and closing the blister packages in front of the study investigators. During the study period, the use of the blister packages was not directly observed by the investigators. However, an instruction sheet was provided to guide subjects through all the steps necessary to use the blister packages. Subjects could also reach the investigators, ZI or TRZ, by e-mail or phone if they encountered any problems using the blister packages. During the follow-up visit, the study investigators scanned the e-module with the reader device to download the dosing history data.
Data Collection
Data were collected through the e-module, an online questionnaire, and in a short one-on-one interview at the end of the study. Data were stored digitally in a research electronic data capture (REDCap®) database,21,22 hosted by the University Medical Center Groningen.
The online questionnaire consisted of the validated System Usability Scale (SUS),23 ten additional questions (see Supplementary Table S1), and the validated 5-item Medication Adherence Report Scale (MARS-5).24,25 The SUS is used to assess usability for a wide range of products and consists of ten statements with answers given on a 5-point Likert scale, ranging from “Strongly disagree” (1) to “Strongly agree” (5). The SUS score ranges from 0 to 100, with a higher score indicating higher perceived usability, whereas a score of 68 is considered the average.23 The ten additional questions concerned the usability and acceptability of EDP blister package use. The answers were also given on a 5-point Likert scale, ranging from “Strongly disagree” (1) to “Strongly agree” (5). The MARS-5 consists of five statements about non-adherent behavior. The statements reflected on the use of smart blister packages (with placebo tablets). Subjects indicated to what extent each statement applied to them on a 5-point Likert scale, ranging from “Always” (1) to “Never” (5).24,25 The MARS-5 score ranges from 5 to 25 and higher scores indicate higher self-reported adherence.
Interviews were conducted by two investigators (ZI and TRZ). The interview was audio recorded, for which all participants gave consent. Subjects were asked a selection of open-ended and closed questions following a predefined interview guide (see Supplementary Material S1), aiming to receive additional feedback and elaboration on their experience, usability, and acceptance of the EDPs. Interview questions included what the participants experienced during use of the EDP and their preferences, ideas for improvements, and any other comments. All questions were asked in English, while participants were given the opportunity to respond in Dutch when preferred. After the interview, the study investigators read out the dosing history data from the e-module and discussed the documented dose taking behavior with the participants.
Study Outcomes
The primary outcome was perceived usability and acceptance of the EDP. The secondary outcome included dose-taking adherence from the EDP records, to be compared with the widely used measurement methods of pill counting and self-reported adherence.
Data Analysis
Descriptive statistics were used to describe characteristics of the study population, usability of EDP, and dose-taking adherence. SUS and MARS-5 scores were calculated as described previously.23–25 All questionnaire data were tabulated using Microsoft Excel (2016), and results are presented using bar graphs. Data are presented in numbers, percentages, mean (standard deviation, SD), or median (interquartile range, IQR) when appropriate. Whether the data were normally distributed or not were determined visually with histograms and statistically with the Kolmogorov–Smirnov test.
While no gold standard exists and mixed-methods for adherence measurement are advised,17,26,27 differences in dose-taking adherence levels as measured by pill counting, self-reported adherence, and EDP records were compared. The Kruskal–Wallis test was used, with the null hypothesis that there were no differences among the three measurement methods. As the EDP was hypothesized to report more granular data that might introduce less bias, differences of adherence estimates with pill counting and self-report were graphically displayed through Bland-Altman plots28 using Analyse-it software (version 5.50, Leeds, UK) in Microsoft Excel (2016). All statistical analyses were performed using IBM® SPSS® Statistics 28.0 for Windows with p < 0.05 considered significant.
Dose-taking adherence was defined as the percentage of registered package openings, in which placebo tablets were pushed out from the EDPs as scheduled. Dose-taking adherence measured by EDP records was expressed as percentage of recorded dose-takings on schedule by the following formula: (total number of tablets recorded as taken on schedule)/(total number of tablets provided) × 100%. Dose-taking adherence by pill counting was calculated by the formula: (total number of tablets provided – total number of tablets left)/(total number of tablets provided) × 100%. The total number of tablets provided for each subject was 56 (14 tablets times four weeks). Self-reported adherence level was aggregated as the percentage of the MARS-5 score, eg, a MARS-5 score of 20 is equivalent to 80%.29
Tablets were considered to have been taken on schedule when the blister package was opened twice daily (between 3:00 a.m. and 3:00 a.m. on the next day) and when these openings were recorded by the e-module. Filters were applied to the MEMS® Adherence Software v4, where ≥15 minutes between openings were considered to be different recordings. Also, a somewhat more precise definition of adherence was tested where a tablet was considered to have been taken on schedule if it was taken between 3:00 a.m. and 3:00 p.m. or between 3:00 p.m. and 3:00 a.m. the next day for the morning and evening dose, respectively (see Supplementary Figure S1).
Recorded interviews were transcribed in full by ZI and TRZ using F4transkript (Dr. Dresing & Pehl GmbH, Germany). Interview questions answered in Dutch were translated using Google Translate, with the translation checked and, where necessary, corrected by TRZ who is a native Dutch speaker. The transcribed versions (with possible translations) were shared with the participants for their approval, and no disagreements were noted. The transcripts were all uploaded in ATLAS.ti 9 Windows (ATLAS.ti Scientific Software Development GmbH, Germany) and further analyzed with this software.
Qualitative data were analyzed by ZI and TRZ using inductive thematic analysis in six steps as described by Braun and Clarke.30 Units of analyses were generated that were related to the main questions asked during the interview, which included explicit content on user experience, preference, patient burden, possible changes, and improvements (see Supplementary Material S1). All interviews were either transcribed or read by each of the investigators to become familiar with the data. Next, open coding was performed to develop the codes revealed in the data. Generated codes were either keywords or descriptions of answers given by the participants, or one-word summaries for answers given to closed questions, and the definitive code list was established by agreement between the investigators. Then, all transcripts were re-read independently and cross-checked for the assigned codes, and any discrepancies were discussed. Codes were then grouped into subthemes, where some codes were discarded due to being irrelevant or not being mentioned enough times. The subthemes were categorized within the main themes, which reflected the units of analysis: (i) user experience, (ii) user preference, (iii) patient burden, and (iv) ideas for improvements. Selected quotes were provided as examples of identified subthemes, which were discussed by the investigators. Answers to closed questions were quantified for the number of participants per category.
Results
All 20 subjects completed the study. Characteristics of the study population are described in Table 1.
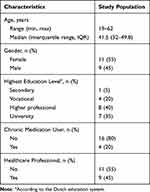 |
Table 1 Baseline Characteristics of Study Population (N = 20) |
Usability of EDP
The mean (standard deviation, SD) SUS score was 78.0 (11.2), ranging from 57.5 to 95.0. Usability was above average (SUS score >68) for 75% (n = 15) of the participants. The outcomes of the SUS scores are provided in Figure 2. Ninety percent (n = 18) of the subjects responded that most people would learn to use the EDP very quickly and that the EDP was not unnecessarily complex. All participants felt very confident using the EDP.
The outcomes of the additional questions concerning usability and acceptability are given in Figure 3. Ninety percent (n = 18) of the subjects agreed the EDP was simple to use and that the instructions on the package were clear. Half of the participants (n = 10) mentioned that the blister package was not easy to carry around and found it difficult to remember taking the placebo tablets at the correct time. Ninety percent (n = 18) of the subjects found that it was easy to transfer the e-module, and 70% (n = 14) found it clear when to change the e-module. Moreover, half of the participants (n = 10) would have liked to get feedback on their “medication” taking behavior, in terms of their performance on pushing out the placebo tablets during the study period on schedule.
Dose-Taking Adherence
All participants transferred the e-module to all blister packages, as there were reports on pressed-out tablets from each blister package. There was no extra event recorded by the EDPs for checking the habit of dose-taking or so-called “curiosity openings”. It was later confirmed during the interviews that no subjects had opened the blisters without taking tablets out.
Differences in dose-taking adherence levels are shown in Supplementary Figure S2. Adherence levels measured by pill counting, self-report, and EDP records differed significantly (p < 0.05). The median (IQR) adherence levels for EDP records were 89% (82–95%), ranging from 48% to 100%. A perfect adherence (100%) was seen in one subject. The median (IQR) dose-taking adherence for pill counting was 96.5% (89–100%), range 52–100%. The pill counting showed that 40% (n = 8) of the subjects brought back fully empty blister packages; in this group, a wide range of adherence behavior was observed by the EDP record, ie, between 82% and 100%. The median (IQR) self-reported adherence was 92% (91–96%), ranging from 80% to 100%. Only 20% (n = 4) of the subjects self-reported no non-adherent behavior. These subjects had adherence levels of 93–96% as observed by the EDP records.
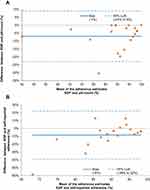
Furthermore, differences in adherence as measured by EDP records and pill counting were observed in 75% (n = 15) of the participants. Differences between EDP records and pill counting or self-reported adherence estimates are shown in Figure 4. Observed discrepancies in adherence level can be the results of differences in reliability and precision of the measurement methods (see Discussion section below).
Chronology plots of tablet taking as recorded by the EDPs for two example participants are presented in Figure 5. The blue dots indicate the opening and closing dates and times when placebo tablets were removed from the blister packages. The upper plot (Figure 5A) shows a subject with an adherence level of 96% (taking 54 out of 56 tablets) and a pill counting adherence of 100%. Two skipped doses were recorded during the study period; however, one of those was not exactly skipped, but rather on day 3 the subject reported taking a previous (day 2) evening tablet a few hours before the morning tablet and took the next evening tablet on schedule. The lower plot (Figure 5B) represents a subject with an adherence level of 48% (taking 27 out of 56 tablets) and a 52% pill counting adherence. This subject reported forgetfulness in taking the tablets on schedule, particularly in the evening. Furthermore, on day 2 the subject took two doses in the evening, which by the used definition of adherence was still adherent behavior (see the definitions discussed in Supplementary Figure S1).
Reasons for non-adherence reported by the study participants are shown in Figure 6. Most subjects reported taking the placebo tablets from the blister packages as instructed. However, some participants disclosed forgetfulness in taking their tablets, rarely (n = 6), sometimes (n = 7), or often (n = 3). The statements related to altering the dose, stopping for a while, deciding to miss out a dose, and taking less than instructed were indicated by n ≤ 3 participants.
 |
Figure 6 Self-reported (non)adherence to the smart blister package assessed with MARS-5. None of the participants reported “always” for all questions. MARS-5 5-item medication adherence report scale. |
Qualitative Assessment
The one-on-one interview further explored the usability and acceptability of the EDPs. The analysis of the interview content is provided in Table 2. The thematic analysis found four themes with 21 subthemes.
 |
Table 2 Results of Interview Content Analysis |
Theme 1: User Experience
The theme “user experience” of the EDP was further categorized into six subthemes: easy to use, size of package, hard to remember, opening of package, e-module transfer, and application in combination with regular medication. When asked about the experience of the participants with the EDP, most of them responded that the EDP was easy to use. They expressed their concerns about the size of the EDP, that it was hard to remember to take the medication, and the opening of the package. Subjects’ experience with the change of the e-module at the end of the week was mixed. They described that they could combine the use of the blister package with their regular medications as well.
Theme 2: User Preference
User preference of the EDP comprised seven subthemes: being monitored, week days/pictures on the blister, push-out flexibility, child-safe packaging, adherence check, cost, and eco-friendly packaging. A total of seven subjects preferred using the EDP over standard blisters, seven did not know, and four observed no difference. Two participants preferred using the standard blisters. The main reason for choosing the EDP would be the idea of being monitored or to check their own adherence.
Features of the blister package itself were also mentioned, such as the labels for weekdays and pictures displayed on the package, and that there was a two-step opening procedure or child-safe packaging. Some participants remarked that as the medication should only be taken out at the moment of intake, they could not combine the EDP with (week) boxes or prepare them in advance. Also, the possible cost of the package and environmental burden were mentioned by the subjects.
Theme 3: Patient Burden
Sixteen out of 20 participants did not regard the EDP to be a patient burden. The remaining four gave an unclear answer to the question regarding this topic. Some reasons that were given for a possible patient burden were patient groups (elderly people, patients with arthritis, polypharmacy patients) and the package size.
Theme 4: Ideas for Improvement
Improvements to the EDP were addressed in seven subthemes: reminder, size, direct access to data, app/website connection, adding e-module to each package, adding signal for data log, and transparent packaging. Subjects mostly addressed the incorporation of a reminder and the size of the EDP. Some mentioned that they would like to have direct access to the data and suggested that a connection to an app or website would improve the system. Other suggestions were to add an e-module to each package and a light signal if the data were logged or if the module ran out of battery and to exchange the e-module for a logger with electronic wires. Two subjects suggested to make the package transparent, as they were asked not to open the package without pushing a dose out, but they wanted to check if they took the dose correctly.
Discussion
Study Findings
This study is the first to report on the usability of an EDP before being implemented in clinical trials or real-world practice. The study results elucidated that the EDP is perceived as a valued and usable tool to monitor medication adherence. The adherence level as registered by the EDP was comparable with the other adherence measures, although some differences between the measures were observed. Qualitative data indicated that the EDP was perceived as easy to use, child-safe, and giving no patient burden. Recommendations were to change the package size, to incorporate reminders, and to give the participants direct insight into their performance.
Interpretation
The perceived usability of the EDP was good, as reflected by the mean SUS score of 78, which is well above the average score of 68.31,32 Usability has been scarcely reported for other smart blister packages, which differ in technology and intention of use, making direct comparisons difficult. The SUS score in our study was similar to a previous study on usability of a smart blister package with event-detection microcircuitry (a mean SUS score of 82.6 ± 14.8).33 In another study, most users of a smart blister package connected to a web-based portal through an NFC-connected phone also found it was easy to use in a questionnaire (47/52 reported it was easy to use).34 As the SUS score does not cover all usability aspects, such as personal experience and uncontrolled environment, additional questions were asked. The additional questions showed participants’ difficulties with carrying the system around and with remembering to take the medication, which were further addressed in the qualitative assessment.
Significant differences were observed in adherence levels as measured by pill counting, self-report, and the EDP records, the reasons for which could not be detected within the scope of this study. However, these differences in results were expected as differences in reliability and precision of the methods have been demonstrated in other studies.27 For use in clinical trials, pill counting is subject to an upward desirability bias and only gives an idea on the average adherence (not day-to-day variability), while self-report is subjective and tends to overestimate adherence with a desirability and recall bias.27 Electronic monitoring is considered the most objective method in measuring adherence and gives more in-depth information on medication taking behavior.35 Still, opening and closing the blister package does not actually reflect dose ingestion, especially when so-called “curiosity openings” occur, or multiple pills are taken out in advance. Data exist that openings are a good proxy for drug intake,36 but “true” pill ingestion measurement may only be possible by more complex or invasive methods, such as directly observed therapy, using digital pills, or measuring drug concentrations in body fluids or tissue.20,37–39
Medication event recording devices could improve adherence in patient-centered care, providing subjects with feedback in terms of a graphical display of adherence performance.14 The performance records could enable healthcare professionals to tailor interventions based on subject’s individual adherence patterns. However, before use in clinical trials or daily health care, smart adherence monitoring tools are advised to be checked systematically15,16 and through usability testing by end-users. Monitoring equipment that does not function properly has been reported previously,15 including in trials with human immunodeficiency virus (HIV)-positive patients40 and transplant recipients.41 Reports on smart blister testing give more insight into functionality, usability, and robustness and contribute to developing the technology further.13
The main three recommendations given by the participants in this study were to decrease the package size, to incorporate reminders and to give the participants direct insight into their performance. First, half of the participants commented on the size of the blister package. Most preferred smaller portable packages, as they brought their medications to the workplace or other locations during their holiday (the study period overlapped with the summer holiday period). This finding is consistent with previous studies, which revealed that users prefer devices that are small in size and those that do not attract attention when used in public.7,35,42 In this study, package size was arbitrarily chosen as a standard size and can be adjusted by the manufacturer, which was recommended by the participants in this study. When the package has to be combined with other medication, eg, in patients with polypharmacy, multi-dose systems or electronic pillboxes may be more acceptable to reduce the total size of the medication.
Second, subjects suggested to incorporate alarms in the system to remind them when to take medication. Because this study looked not only at current usability but also potential for improvement and new features in the future, the reminders fitted with the last objective. As forgetfulness is the most reported reason for non-adherence, reminders can be beneficial for modifying the behavior of unintentionally non-adherent subjects who are willing to take the dose but forget it or are inaccurate in their intake behavior.35 When alarm systems could efficiently help them to achieve their goal of being adherent, which is a definition of usability, this knowledge is of added value. The reminder system could either be built into the blister itself or be prompted by a (mobile) application. Blister wallets such as the Helping Hand® can register openings and provide reminders through color-coded light signals or beeping signals.15,43,44 In systems incorporating data transfer to an online platform, reminders can be sent via SMS or “push notifications” if the blister was not opened or when data transmission failed.45,46 Although an alarm or reminder function is important, the effort required to set up the alarm on the smart blister system, the risk of faulty signals (due to data transmission delay) and signal fatigue should also be considered.42
Third, the participants would prefer to directly access their own data. In the EDP tested in this study, data transfer was performed with a specific reading device, also used in other reported studies.14,27 Connection to a mobile phone was also an option in other previous studies.45,46 In the latter case, data can then be stored on the patient’s phone and transmitted to an online database.45 If the blister packages are connected to a mobile/web-based application, patients have direct insight into their medication taking behavior, even without having their medication close by. Real-time data could also be beneficial for remote adherence monitoring, as healthcare providers have direct access and the participant does not need to bring the packages to a location with a reader device. To our knowledge, no smart blister packages with real-time data have been reported to enable the user to directly see their own data during the implementation stage.
Strengths and Limitations
This study sheds light on user experience from the questionnaires and interviews, where the interview gives more detailed insight into user preferences and EDP-related issues. As with (phase I) trials for new medicines, before providing patients with an intervention, it needs to be tested in “healthy” volunteers first. This may minimize the risk of severe adverse events in real patients, who are usually more vulnerable. The recommendations given by the participants can be used for further development to increase end-user satisfaction. This study recruited a heterogeneous group that included different age and education level groups, healthcare professionals and non-healthcare professionals as well as a mix of chronic medication users and non-medication users. Non-users reflected first time medication users, so they could add value with their (naive) experience of blister package usage.
The results should be considered in the light of the following limitations. This study could not explain the gap in human/system discrepancies and was not designed to assess robustness and full accuracy. Furthermore, the study design was limited by the short length of the study (one month) while many medications are intended for chronic use. Moreover, this study was prone to the Hawthorne effect, which may contribute to an artificially higher adherence rate.7 The standard twice-daily regimen chosen in this study might be easier for people to be adherent to, compared to a three or four times daily regimen. The sample size was small, and the number of participants was chosen pragmatically and in line with numbers used in similar studies.13,19,20 However, a sample of 20 people should be able to find a minimum of 95% and an average of 98% of the problems.47 Moreover, the study population was limited with respect to extrapolation to the end-user population due to (i) the lack of experience from elderly patients aged 65+ years because of technical difficulties during the recruitment and concerns regarding mobility for attending the study visits; (ii) the distribution of education level that did not fully reflect the general population (as there were more highly educated subjects among the study’s participants); and (iii) the limited number of chronic medication users (while individuals without previous experience that start using medication may also be part of the target population). A selection bias existed in the study population, which consisted of employees of an academic healthcare facility that may have higher health literacy and other preferences than the population of end-users. However, as healthcare professionals will have an important role in implementation, they add value in the development phase of monitoring devices. Their experiences could advocate for benefits to other healthcare practitioners in the use of the device. Further studies could be conducted to distinguish features that appeal to different subgroups, eg, healthcare professionals and patients. Furthermore, as with all qualitative studies, organizing and ordering comprehensive interview data for analysis is complex and more prone to selection and observational biases.
Future Perspectives
The study results may stimulate future research in features to improve adherence, such as adding reminders and direct data access, whereas the robustness and accuracy might be further investigated. Future studies could focus on drug stability of the medication inside the blisters and cost–benefit analyses. Smart blister packages require equipment for data transmission and supporting infrastructure (eg, computer, internet connection). On the one hand, both this equipment and the smart blisters themselves come with additional costs compared to current practice. On the other hand, improved adherence to medications is associated with reduced healthcare costs (eg, hospital admissions) and may compensate the cost incurred from the electronic monitoring devices.48,49 Further cost-effectiveness research in this field is therefore warranted. For a wider use in clinical practice, there is a need for further fine-tuning of the smart packages to optimally fit patients’ needs and usability. Trials should be performed with patients using real medication to determine long-term adherence either to chronic medication, eg, for hypertension, HIV, or post-transplant care, or to short-term medication, eg, antibiotic, antifungal, or analgesic treatment. Inclusion of elderly patients should be considered in order to respect the needs of the elderly in the packaging design as age and comorbidities may impact the usability of drug products.34 As the adherence measurement methods used in this study are not able to objectively confirm real drug intake, biochemical drug measurements are recommended to further validate adherence data, preferably through non-invasive methods.39
When deemed sufficiently validated, the use of smart blister packages may be further scaled-up in clinical trials as well as daily practice. In real medication studies, the adherence data could be used to interpret clinical outcomes or pharmacokinetics. The smart blister packages could also be applied in self-management programs, to discuss adherence patterns with the patient, for instance, before adding medication or switching therapies. Furthermore, remote monitoring could support patient follow-up, especially when face-to-face appointments are limited, such as during a global pandemic.50
Conclusion
The participants perceived the EDP usability as good, being an acceptable tool to monitor adherence. The dose-taking adherence levels measured by EDP, pill counting, and self-reported adherence differed significantly. Through the interviews, recommendations were given which can be used to guide scale-up of the EDP. Further investigations may be conducted on the robustness and accuracy.
Abbreviations
EDP, electronic Dosepak®; HIV, human immunodeficiency virus; IC, informed consent; IQR, interquartile range; MARS-5, 5-item medication adherence report scale; MEMS, medication event monitoring system; NFC, near field communication; SD, standard deviation; SUS, system usability scale; WHO, World Health Organization.
Data Sharing Statement
De-identified data are available upon reasonable request to the corresponding author of the study.
Acknowledgments
The authors thank all healthy volunteers who participated in the study, Prof. Rob Horne (Centre for Behavioural Medicine, Department of Practice and Policy, UCL School of Pharmacy, London, UK) for the permitted use of the MARS-5 questionnaire in this study, and Iris Nijmeijer, who has worked as a translator at the University of Groningen’s Translation and Correction Department, the Netherlands, for English editing.
Author Contributions
ZI and TRZ contributed equally to this work and share first authorship. All authors made a significant contribution to the work reported, whether in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all of the following areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
ZI is supported by the LPDP scholarship (The Indonesian Endowment Fund for Education, Ministry of Finance of Republic of Indonesia, grant no. 20193220414030), and TRZ is supported by a grant of Chiesi Pharmaceutici (contract no. PA2019-7108). The authors declare that the study received funding from AARDEX Group. An unrestricted grant from AARDEX Group was provided to the Department of Clinical Pharmacy and Pharmacology to perform this study and the blister packages were provided at no costs (study no. 11059). AARDEX Group was offered the possibility to assess the article before submission on technical accuracy but incorporation of comments remained at the discretion of the authors. Comments included were related to the description of the EDP and the reference to the discussion on observed discrepancies in adherence level within the results section. The funding bodies had no role in the study design, data collection, data analysis, writing of the article, and the decision to submit it for publication.
Disclosure
DJT reports grants from Chiesi, outside the submitted work; and Advisory board from PureIMS and Sanquin, not related to this project; DSMB of the FORMAT trial, not related to this project. The authors report no other conflicts of interest in this work.
References
1. World Health Organization. Adherence to Long-Term Therapies - Evidence for Action. Geneva; 2003.
2. Yeam CT, Chia S, Tan HCC, Kwan YH, Fong W, Seng JJB. A systematic review of factors affecting medication adherence among patients with osteoporosis. Osteoporos Int. 2018;29(12):2623–2637. doi:10.1007/s00198-018-4759-3
3. Krass I, Schieback P, Dhippayom T. Adherence to diabetes medication: a systematic review. Diabet Med. 2015;32(6):725–737. doi:10.1111/dme.12651
4. Cutler RL, Fernandez-Llimos F, Frommer M, Benrimoj C, Garcia-Cardenas V. Economic impact of medication non-adherence by disease groups: a systematic review. BMJ Open. 2018;8(1):e016982. doi:10.1136/bmjopen-2017-016982
5. Simpson RJ. Challenges for improving medication adherence. J Am Med Assoc. 2006;296(21):2614–2616. doi:10.1001/JAMA.296.21.JED60074
6. Mahtani KR, Heneghan CJ, Glasziou PP, Perera R. Reminder packaging for improving adherence to self-administered long-term medications. Cochrane Database Syst Rev. 2011;9:CD005025. doi:10.1002/14651858.cd005025.pub3
7. Checchi KD, Huybrechts KF, Avorn J, Kesselheim AS. Electronic medication packaging devices and medication adherence: a systematic review. J Am Med Assoc. 2014;312(12):1237–1247. doi:10.1001/jama.2014.10059
8. Pouls BPH, Vriezekolk JE, Bekker CL, et al. Effect of interactive eHealth interventions on improving medication adherence in adults with long-term medication: systematic review. J Med Internet Res. 2021;23(1):1–16. doi:10.2196/18901
9. Conn VS, Ruppar TM, Chan KC, Dunbar-Jacob J, Pepper GA, Geest De S. Packaging interventions to increase medication adherence: systematic review and meta-analysis. Curr Med Res Opin. 2015;31(1):145–160. doi:10.1185/03007995.2014.978939
10. Zedler BK, Kakad P, Colilla S, Murrelle L, Shah NR. Does packaging with a calendar feature improve adherence to self-administered medication for long-term use? A systematic review. Clin Ther. 2011;33(1):62–73. doi:10.1016/J.CLINTHERA.2011.02.003
11. Zijp TR, Mol PGM, Touw DJ, van Boven JFM. Smart medication adherence monitoring in clinical drug trials: a prerequisite for personalised medicine? EClinicalMedicine. 2019;15:3–4. doi:10.1016/j.eclinm.2019.08.013
12. van Boven JFM, Tsiligianni I, Potočnjak I, et al. European network to advance best practices and technology on medication adherence: mission statement. Front Pharmacol. 2021;12:1–6. doi:10.3389/fphar.2021.748702
13. Zijp TR, Touw DJ, van Boven JFM. User acceptability and technical robustness evaluation of a novel smart pill bottle prototype designed to support medication adherence. Patient Prefer Adherence. 2020;14:625–634. doi:10.2147/PPA.S240443
14. van Onzenoort HA, Neef C, Verberk WW, van Iperen HP, de Leeuw PW, van der Kuy P-HM. Determining the feasibility of objective adherence measurement with blister packaging smart technology. Am J Health Pharm. 2012;69(10):872–879. doi:10.2146/AJHP100592
15. Bleser De L, Geest De S, Vandenbroeck S, Vanhaecke J, Dobbels F. How accurate are electronic monitoring devices? A laboratory study testing two devices to measure medication adherence. Sensors. 2010;10(3):1652–1660. doi:10.3390/S100301652
16. Denhaerynck K, Schäfer-Keller P, Young J, Steiger J, Bock A, De Geest S. Examining assumptions regarding valid electronic monitoring of medication therapy: development of a validation framework and its application on a European sample of kidney transplant patients. BMC Med Res Methodol. 2008;8(1):5. doi:10.1186/1471-2288-8-5
17. Faisal S, Ivo J, Lee C, Carter C, Patel T. The usability, acceptability, and functionality of smart oral multidose dispensing systems for medication adherence: a scoping review. J Pharm Pract. 2020;1–14. doi:10.1177/0897190020977756
18. Breithaupt-Groegler K, Coch C, Coenen M, et al. Who is a ‘healthy subject’?—consensus results on pivotal eligibility criteria for clinical trials. Eur J Clin Pharmacol. 2017;73(4):409–416. doi:10.1007/s00228-016-2189-8
19. De Bruin M, Hospers HJ, Van Den Borne HW, Kok G, Prins JM. Theory- and evidence-based intervention to improve adherence to antiretroviral therapy among HIV-infected patients in the Netherlands: a pilot study. AIDS Patient Care STDS. 2005;19(6):384–394. doi:10.1089/apc.2005.19.384
20. Daar ES, Rosen MI, Wang Y, et al. Real-time and wireless assessment of adherence to antiretroviral therapy with co-encapsulated ingestion sensor in HIV-infected patients: a pilot study. Clin Transl Sci. 2020;13(1):189–194. doi:10.1111/CTS.12701
21. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi:10.1016/j.jbi.2008.08.010
22. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:e103208. doi:10.1016/j.jbi.2019.103208
23. Lewis JR. The system usability scale: past, present, and future. Int J Hum Comput Interact. 2018;34(7):577–590. doi:10.1080/10447318.2018.1455307
24. Horne R, Weinman J. Self-regulation and self-management in asthma: exploring the role of illness perceptions and treatment beliefs in explaining non-adherence to preventer medication. Psychol Health. 2002;17(1):17–32. doi:10.1080/08870440290001502
25. Chan AHY, Horne R, Hankins M, Chisari C. The medication adherence report scale: a measurement tool for eliciting patients’ reports of nonadherence. Br J Clin Pharmacol. 2020;86(7):1281–1288. doi:10.1111/bcp.14193
26. Lam WY, Fresco P. Medication Adherence Measures: an Overview. Biomed Res Int. 2015;2015:1–12. doi:10.1155/2015/217047
27. Bruxvoort K, Festo C, Cairns M, et al. Measuring patient adherence to malaria treatment: a comparison of results from self-report and a customised electronic monitoring device. PLoS One. 2015;10(7):1–18. doi:10.1371/journal.pone.0134275
28. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;327(8476):307–310. doi:10.1128/AAC.00483-18
29. Al Bawab AQ, Al-Qerem W, Abusara O, Alkhatib N, Mansour M, Horne R. What are the factors associated with nonadherence to medications in patients with chronic diseases? Healthc. 2021;9(9):1–12. doi:10.3390/healthcare9091237
30. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101. doi:10.1191/1478088706qp063oa
31. Bangor A, Kortum P, Miller J. Determining what individual SUS scores mean: adding an adjective rating scale. J Usability Stud. 2009;4(3):114–123. doi:10.5555/2835587.2835589
32. Brooke J. SUS: a retrospective. J Usability Stud. 2013;8(2):29–40. doi:10.5555/2817912.2817913
33. Arnet I, Rothen J-P, Albert V, Hersberger KE. Validation of a novel electronic device for medication adherence monitoring of ambulatory patients. Pharmacy. 2019;7(4):155. doi:10.3390/pharmacy7040155
34. Mühlfeld L, Langguth P, Häusler H, Hagels H. Influence of blister package design on usability among older adults. Int J Clin Pharm. 2012;34(4):553–560. doi:10.1007/s11096-012-9643-1
35. Vervloet M, Linn AJ, van Weert JCM, de Bakker DH, Bouvy ML, van Dijk L. The effectiveness of interventions using electronic reminders to improve adherence to chronic medication: a systematic review of the literature. J Am Med Informatics Assoc. 2012;19(5):696–704. doi:10.1136/amiajnl-2011-000748
36. Vrijens B, Tousset E, Rode R, Bertz R, Mayer S, Urquhart J. Successful projection of the time course of drug concentration in plasma during a 1-year period from electronically compiled dosing-time data used as input to individually parameterized pharmacokinetic models. J Clin Pharmacol. 2005;45(4):461–467. doi:10.1177/0091270004274433
37. Peters-Strickland T, Hatch A, Adenwala A, Atkinson K, Bartfeld B. Human factors evaluation of a novel digital medicine system in psychiatry. Neuropsychiatr Dis Treat. 2018;14:553–565. doi:10.2147/NDT.S157102
38. Chai PR, Carreiro S, Innes BJ, et al. Digital pills to measure opioid ingestion patterns in emergency department patients with acute fracture pain: a pilot study. J Med Internet Res. 2017;19(1):e19. doi:10.2196/jmir.7050
39. Zijp TR, Izzah Z, Åberg C, et al. Clinical value of emerging bioanalytical methods for drug measurements: a scoping review of their applicability for medication adherence and therapeutic drug monitoring. Drugs. 2021;81(17):1983–2002. doi:10.1007/S40265-021-01618-7
40. Bova CA, Fennie KP, Knafl GJ, Dieckhaus KD, Watrous E, Williams AB. Use of electronic monitoring devices to measure antiretroviral adherence: practical considerations. AIDS Behav. 2005;9(1):103–110. doi:10.1007/S10461-005-1685-0
41. Weng FL, Israni AK, Joffe MM, et al. Race and electronically measured adherence to immunosuppressive medications after deceased donor renal transplantation. J Am Soc Nephrol. 2005;16(6):1839–1848. doi:10.1681/ASN.2004121059
42. Faisal S, Ivo J, McDougall A, Patel T. Stakeholder feedback of electronic medication adherence products: qualitative analysis. J Med Internet Res. 2020;22(12):e18074. doi:10.2196/18074
43. Dobbels F, De Bleser L, Berben L, et al. Efficacy of a medication adherence enhancing intervention in transplantation: the MAESTRO-Tx trial. J Heart Lung Transplant. 2017;36(5):499–508. doi:10.1016/J.HEALUN.2017.01.007
44. Christensen A, Christrup LL, Fabricius PE, et al. The impact of an electronic monitoring and reminder device on patient compliance with antihypertensive therapy: a randomized controlled trial. J Hypertens. 2010;28(1):194–200. doi:10.1097/HJH.0b013e328331b718
45. Brath H, Morak J, Kästenbauer T, et al. Mobile health (mHealth) based medication adherence measurement – a pilot trial using electronic blisters in diabetes patients. Br J Clin Pharmacol. 2013;76(S1):47–55. doi:10.1111/BCP.12184
46. Morak J, Schwarz M, Hayn D, Schreier G. Feasibility of mHealth and near field communication technology based medication adherence monitoring.
47. Faulkner L. Beyond the five-user assumption: benefits of increased sample sizes in usability testing. Behav Res Meth Instrum Comput. 2003;35(3):379–383. doi:10.1109/DCABES.2011.32
48. Tan BY, Shafie AA, Hassali MAA, Saleem F. Assessment of medication adherence and the costs associated with a calendar blister pack intervention among hypertensive patients in Malaysia: a randomized controlled trial. SAGE Open Med. 2017;5:1–9. doi:10.1177/2050312117709189
49. Haberer JE, Garrison L, Tumuhairwe JB, et al. Factors affecting the implementation of electronic antiretroviral therapy adherence monitoring and associated interventions for routine HIV care in Uganda: qualitative study. J Med Internet Res. 2020;22(9):1–12. doi:10.2196/18038
50. Kardas P, van Boven JFM, Pinnock H, et al. Disparities in European healthcare system approaches to maintaining continuity of medication for non-communicable diseases during the COVID-19 outbreak. Lancet Reg Health - Eur. 2021;4:0–2. doi:10.1016/j.lanepe.2021.100099
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.