Back to Journals » Journal of Pain Research » Volume 13
Electroacupuncture Regulates Pain Transition by Inhibiting the mGluR5-PKCϵ Signaling Pathway in the Dorsal Root Ganglia
Authors Wang S , Du J, Shao F, Wang W, Sun H, Shao X , Liang Y , Liu B , Fang J, Fang J
Received 29 February 2020
Accepted for publication 27 May 2020
Published 19 June 2020 Volume 2020:13 Pages 1471—1483
DOI https://doi.org/10.2147/JPR.S251948
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Robert B. Raffa
Sisi Wang,* Junying Du,* Fangbing Shao, Wen Wang, Haiju Sun, Xiaomei Shao, Yi Liang, Boyi Liu, Jianqiao Fang, Junfan Fang
Department of Neurobiology and Acupuncture Research, The Third Clinical Medical College, Zhejiang Chinese Medical University, Key Laboratory of Acupuncture and Neurology of Zhejiang Province, Hangzhou 310053, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jianqiao Fang; Junfan Fang Email [email protected]; [email protected]
Background: Acute pain can transition to chronic pain, presenting a major clinical challenge. Electroacupuncture (EA) can partly prevent the transition from acute to chronic pain. However, little is known about the mechanisms underlying the effect of EA. This study investigated the effect of EA on pain transition and the activation of metabotropic glutamate receptor 5 (mGluR5)–protein kinase C epsilon (PKCϵ) signaling pathway in the dorsal root ganglia (DRG).
Methods: The hyperalgesic priming model was established by the sequential intraplantar injection of carrageenan (1%, 100 μL) and prostaglandin E2 (PGE2) into the left hind paw of rats. EA treatment (2/100 Hz, 30 min, once/day) was applied at bilateral Zusanli (ST36) and Kunlun (BL60) acupoints in rats. Von Frey filaments were used to investigate the mechanical withdrawal threshold (MWT) at different time points. The protein expression levels of mGluR5 and PKCϵ in the ipsilateral L4-L6 DRGs of rats were detected by Western blot. Some pharmacological experiments were performed to evaluate the relationship between mGluR5, PKCϵ and the MWT. It was also used to test the effects of EA on the expression levels of mGluR5 and PKCϵ and changes in the MWT.
Results: Sequential injection of carrageenan and PGE2 significantly decreased the MWT of rats and up-regulated the expression level of mGluR5 and PKCϵ in the ipsilateral L4-L6 DRGs. EA can reverse the hyperalgesic priming induced by sequential injection of carrageenan/PGE and down-regulate the protein expression of mGluR5 and PKCϵ. Glutamate injection instead of PGE2 can mimic the hyperalgesic priming model. Pharmacological blocking of mGluR5 with specific antagonist MTEP can prevent the hyperalgesic priming and inhibit the activation of PKCϵ in DRGs. Furthermore, EA also produced analgesic effect on the hyperalgesic priming rats induced by carrageenan/mGluR5 injection and inhibited the high expression of PKCϵ. Sham EA produced none analgesic and regulatory effect.
Conclusion: EA can regulate pain transition and it may relate with its inhibitory effect on the activation of mGluR5-PKCϵ signaling pathway in the DRGs.
Keywords: electroacupuncture, pain transition, hyperalgesic priming, mGluR5, PKCϵ, DRGs
Introduction
Acute and chronic pain are the most common clinical symptoms.1–3 When acute pain transitions to chronic pain (pain transition), adverse effects are induced at both the physiological and psychological levels and cause unbearable suffering to the patient. However, most analgesics fail to produce robust effects on chronic pain and are accompanied by obvious side effects.4,5 Many studies have demonstrated that the mechanisms of pain change dramatically when the pain transitions from acute to chronic.6–8 thus, the therapeutic effects of various drugs are greatly weakened, causing patients to suffer from chronic pain. Therefore, understanding the mechanisms of pain transition is of great significance.
Protein kinase C epsilon (PKCε) is distributed in most neurons of the dorsal root ganglion (DRG).9,10 It has been shown that the activation of PKCε in the peripheral neuron system is key to the generation and maintenance of chronic pain.11,12 Recent studies have found that PKCε is also involved in the process of pain transition.13–16 PKCε inhibitors can effectively prevent the occurrence of pain transition and modulate the long-term and severe chronic pain caused by this process, which is clearly separate from tissue damage.14,17,18 However, the upstream pathway that is involved in pain transition remains an open question.
Metabotropic glutamate receptor 5 (mGluR5) is one of the glutamate receptors expressed in small-diameter neurons in the DRGs.19,20 Previous studies have found that damaged peripheral tissue can release glutamate21,22 and activate primary afferent neurons that express mGluR5, causing hyperalgesia.23,24 The use of selective inhibitors of mGluR5 can significantly reduce hyperalgesia.25,26 In addition, the process of hyperalgesia induced by mGluR5 may be achieved through PKCε.23,27 Therefore, mGluR5 may be involved in pain transition by activating PKCε in the DRGs.
Electroacupuncture (EA) is one of the most common methods for treating pain in Chinese medical clinics and has a therapeutic effect on acute and chronic pain.28,29 Previous studies have shown that EA can partly prevent the transition from acute to chronic pain.30,31 However, the mechanisms underlying its preventive effect remain unclear. Related studies have shown that the therapeutic effect of EA on pain is closely related to its intervention in the peripheral nervous systems.32–34 A previous study demonstrated that the therapeutic effect of EA on chronic pain is partially achieved by regulating the PKCε signal transduction pathway.35,36 EA can also exert its analgesic effect by regulating the expression of mGluR5 in the DRG.37 All of the above findings suggest that the effect of EA stimulation in pain transition may be related to the regulation of the mGluR5-PKCε pathway in the DRGs.
In this experiment, the hyperalgesic priming model was established to explore the mechanism underlying pain transition.38 The model was designed to separate the acute pain from chronic pain.6,39 The chronic pain (hyperalgesia) was induced by sequential injection of carrageenan and PGE2 in the model. However, PGE2 can only induce an acute pain without the carrageenan. This feature given the opportunity to observe the mechanism of pain transition. In addition, the regulatory effect of EA on the MWT and mGluR5-PKCε pathway in hyperalgesic priming model rats was observed. Finally, mGlu5R was inhibited to confirm the role mGluR5-PKCε pathway played in the model and was activated to further explore the effect and mechanism of EA on pain transition.
Materials and Methods
Animals
Male Sprague-Dawley rats weighing 160 to 180 g were purchased from the Experimental Animal Center of Zhejiang Chinese Medical University. All rats were fed standard rodent food and water and housed at a constant room temperature of 23 to 25 °C and at a relative humidity of 55% ± 5% with a 12/12 h light-dark cycle. The entire experimental procedures were in line with the Animal Care and Welfare Committee of Zhejiang Chinese Medical University, Zhejiang, China (approval no. IACUC-20180319-12). We have minimized the animals’ suffering.
Drugs
Carrageenan, prostaglandin E2 (PGE2) and glutamate were purchased from Sigma-Aldrich (Sigma-Aldrich, Saint Louis, MO, USA). They were all dissolved in sterile saline and then diluted to appropriate concentrations before being injected into the plantar surface of the left hind paw (carrageenan (1 mg, 100 μL), PGE2 (100 ng, 25 μL) and glutamate (0.17 mg, 25 μL)). MTEP, an inhibitor of mGluR5, was purchased from Tocris Bioscience (Tocris Bioscience, Minnesota, USA) and diluted to 10 mM in sterile physiological saline.
Experimental Design
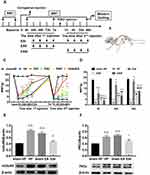
This study was divided into three parts. In the first part, we studied the changes in pain threshold and protein expression in the DRGs during pain transition. Rats were randomly assigned to three groups (n=18/group): (1) the Normal (N) group, (2) the sham hyperalgesic priming (sham HP) group, and (3) the hyperalgesic priming (HP) group. The mechanical withdrawal threshold (MWT) and the expression levels of mGluR5 and PKCε were investigated by Western blot according to the experimental schedule (Figure 1A).
In the second part, the different effects of EA administered for different periods on pain transition were observed. The effects of EA on mGluR5 and PKCε were also investigated. Rats were randomly assigned to six groups (n=6/group): (1) the sham HP group, (2) the HP group, (3) the EA I group, (4) the EA II group, (5) the EA III group, and (6) the sham EA group.
In the third part, the involvement of mGluR5 in pain transition and the analgesic effect of EA were explored. This part of the experiment was divided into three sub-parts. In the first subpart, 8 rats were randomly assigned to three groups (n=6/group): (1) the N group, (2) the sham HP group, and (3) the Glutamate (Glu) group. In the Glu group, glutamate was administered subcutaneously instead of PGE2 at 7 d after carrageenan injection. In the second subpart, 12 rats were randomly assigned to two groups (n=6/group): (1) the HP group and (2) the MTEP (mGluR5 inhibitor) group. In the MTEP group, MTEP was subcutaneously injected into the left paw 5 min before PGE2 injection. In the third subpart, EA was used to regulate the pain transition induced by carrageenan/glutamate injection. 24 rats were randomly assigned to four groups (n=6/group): (1) the sham HP group, (2) the Glu group, (3) the EA I group and (4) the sham EA group.
Hyperalgesic Priming Model
The hyperalgesic priming model was established by injecting 100 μL of 1% carrageenan (1st injection) into the left hind paw of each rat and 100 ng/25 μL of PGE2 (2nd injection) into the same paw after 7 days.
In the sham HP group, 100 μL of 0.9% saline (1st injection) was injected subcutaneously into the left hind paw of each rat, and 100 ng/25 μL of PGE2 was injected into the same paw after 7 days (2nd injection). In the N group, 0.9% saline was injected subcutaneously into the left hind paw (100 μL for the 1st injection and 25 μL for the 2nd injection). In the Glu group, 100 μL of 1% carrageenan was injected into the left hind paw of each rat for the 1st injection, and 0.17 mg/25 μL glutamate was injected into the same paw for the 2nd injection. In the MTEP group, 10 mM/25 μL of MTEP was preinjected into the left hind paw of each rat 5 min before the 2nd injection.
Rats were briefly anesthetized with 2.5% isoflurane to facilitate the intraplantar injection of carrageenan, PGE2 or the other drugs used in this study.
Mechanical Withdrawal Threshold (MWT)
The up-down method was used in this experiment.40 Von Frey hairs (Stoelting Co, Thermo, Gilroy, CA, USA) with forces of 0.4, 0.6, 1, 2, 4, 6, 8, 15, and 26 g were selected. The rats were placed into individual cages for 30 min before measurement to allow acclimation to the environment. A von Frey hair with a force of 4 g was first applied to the central surface of the hind paw (avoiding the footpad) until the hair bent into an “S” shape, and it was maintained there for 6 s. If the rat did not exhibit a positive avoidance response, the result was recorded as “O”, and the von Frey hair was replaced with a hair of higher force. Conversely, in the case of a avoidance response, the result was recorded as “X”, and the von Frey hair was replaced with a hair of lower force. The stimulation interval was at least 2 min. After a “OX” or “XO” combination of responses, 4 more measurements were performed and recorded as above (for example, “OXOXOO”). The pain threshold was calculated according to the following formula: MWT (g) = (10[Xf + κδ])/10,000, where “Xf” is the force of the last hair test, the “κ” value is obtained from the k-value table, and “δ” is the average value of the difference between the logarithm of hairs of each force, which is approximately equal to 0.231. Here, the maximum stimulation intensity was 26 g, and the minimum stimulation intensity was 0.4 g.
EA Treatment
All rats in the EA group were treated with EA stimulation. The EA stimulation procedure was performed as described below. The intervention began after the completion of the behavioral test 4 h after the 1st injection. The rats were gently immobilized in a cotton retainer. Stainless-steel needles (0.18 mm × 13 mm) were inserted to a depth of 5 mm into the bilateral Zusanli (ST36) and Kunlun (BL60) acupoints. The needles were connected to a HANS Acupuncture Point Nerve Stimulator (LH-202H Huawei Co, Ltd, Beijing, China), the intensity was set at 0.5 mA, 1.0 mA, and 1.5 mA (the intensity increased every 10 min), and EA was administered once per day at 2/100 Hz for 30 min until the end of the experiment or as otherwise noted in specific cases. Here, 2/100 Hz meant that the stimulator alternatively administered electrical stimulation at 2 Hz and 100 Hz every 3 seconds. In the EA II group, EA stimulation was only administered once per day between 4 h after the 1st injection and before the 2nd injection. In the EA III group, EA stimulation was only administered once per day after the 2nd injection. Other parameters were consistent with the parameters described above.
Sham EA was also administered. The same needles were inserted subcutaneously into the ST36 and BL60 acupoints (at a depth of 1 mm) of the animals. The needles were connected to the same stimulator, but no electrical stimulation was administered.
Western Blot Analysis
Animals were anesthetized with 2% pentobarbital sodium and euthanized at 4, 24 or 48 h after the 2nd injection once MWT testing was completed. They were quickly perfused with 150 mL of 0.9% NaCl (4 °C). Then, the DRGs were extracted rapidly and stored in a −80 °C freezer for Western blot experiments. The protein expression of mGluR5 and PKCε in the L4-L6 DRGs was detected by Western blot. The DRGs were pulverized in RIPA buffer and centrifuged at 14,000 rpm for 5 min at 4 °C. The supernatant was placed in a clean EP tube. The protein concentration of the tissue lysates was determined by the BCA method. Fifteen micrograms of the lysates were denatured and loaded and then transferred to polyvinylidene difluoride (PVDF) membranes (Merck KGaA, Darmstadt, Germany) by 5% SDS-PAGE electrophoresis. Then, 5% skim milk powder was used for blocking at room temperature for 1 h. Rabbit anti-PKCε (ab124806, Abcam, Cambridge, UK) and rabbit anti-mGluR5 (ab53090, Abcam, Cambridge, UK) were used as the primary antibodies, and mouse anti-β-actin (HRP) (ab20272, Abcam, Cambridge, UK) was used as the internal control. The membranes were incubated overnight at 4 °C (18 h). Subsequently, the immunoblots were incubated with secondary antibody for 2 h at room temperature. An ECL kit (Pierce, Rockford, lL, USA) was used for development. The blots were photographed after color development, the average optical density values of the bands were calculated.
Statistical Analysis
All data are presented as the mean ± standard error of the mean (x̄ ± SEM). For behavioral testing, the data were analyzed using repeated-measures ANOVA followed by LSD post hoc test. When the interaction between the time points and groups resulted in a P-value less than 0.05, one-way ANOVA followed by LSD post hoc test was used to analyze the data. Western blot was analyzed by one-way ANOVA followed by LSD post hoc test. P < 0.05 was considered statistically significant.
Result
MWT Gets Lower in Hyperalgesic Priming Model Rats
Repeated measures ANOVA was conducted to compare the effect of time on the MWT in the N, sham HP and HP groups. The results did not meet Mauchly’s test of Sphericity (P<0.01) and the within-subjects effects revealed that there was a significant difference over time (P<0.01) and between groups (P<0.01) and there was also an interaction between time points and groups (P<0.01). The data were normally distributed and analyzed using a one-way ANOVA. The overall experimental design is shown in Figure 1A. The hyperalgesic priming model was established by sequential intraplantar injection of carrageenan/PGE2 into the left hind paw. As shown in Figure 1B, before the modeling, there was no significant difference in the MWT between the groups (P>0.05). The MWT of the HP group was significantly lower than that of the N group and sham HP group 4, 24, 48, and 72 h after the 1st injection (P<0.01). Then, the MWT gradually recovered and returned to the initial level approximately 7 d after the 1st injection. The MWT of the sham HP group and the HP group decreased to the lowest level 1 h after the 2nd injection and was significantly lower than that of the N group (P<0.01). The MWT of the rats in the sham HP group returned to the original level and was not different than that of the N group 4 h after the 2nd injection. However, the MWT of the HP group was still at a low level and was significantly lower than that of the N and sham HP groups (P<0.01, Figure 1B). The results indicated that the hyperalgesic priming model was successfully established.
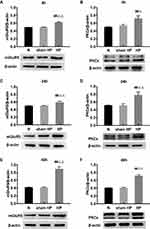
Protein Levels of mGluR5 and PKCε in the DRGs at Different Time Points in Hyperalgesic Priming Rats
The expression levels of mGluR5 and PKCε in the L4-L6 DRGs were investigated by Western blot 4, 24, and 48 h after the 2nd injection. As shown in Figure 2, the protein levels of mGluR5 (Figure 2A, C and E) and PKCε (Figure 2B, D and F) in the DRGs of the sham HP group were not significantly different from those in the DRGs of the N group 4, 24 and 48 h after the 2nd injection. However, the protein levels of mGluR5 and PKCε in the HP group were significantly increased compared with those in the N group and the sham HP group 4 h (P<0.05) (Figure 2A and B), 24 h (P<0.01) (Figure 2C and D) and 48 h (P<0.01) (Figure 2E and F) after the 2nd injection. These results indicate that both mGluR5 and PKCε may be involved in the process of hyperalgesic priming.
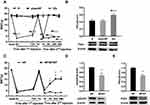
EA Stimulation Alleviates Pain and Decreases the Protein Expression of mGluR5 and PKCε in the DRGs Induced by Hyperalgesic Priming
EA was administered for 3 different time periods to investigate its regulatory effect on pain transition. The entire EA stimulation procedure and the location of ST36 and BL60 are respectively shown in Figure 3A and B. The results of repeated measures ANOVA did not meet Mauchly’s test of Sphericity (P=0.009) and the within-subjects effects indicated that there was a significant difference over time (P<0.01) and between the different groups (P<0.01). There was a significant interaction between time and group (P<0.01). The data were normally distributed and analyzed using a one-way ANOVA. The MWTs of the EA I group and EA II group increased rapidly when compared with that of the HP group during the period from the 1st injection to the 2nd injection (P<0.05) (Figure 3C). However, the three different doses of EA produced varying effects on the MWTs of model rats after the 2nd injection, especially those in the EA I group and EA III group. The MWT of the EA I group was significantly higher than that of the EA II and EA III groups 4, 24 and 48 h after the 2nd injection. In addition, the MWT of the EA III group was significantly higher than that of the EA II group 48 h after the 2nd injection (P<0.05) (Figure 3D). According to the above results, EA I was used in the subsequent experiments. Then, Western blot was used to detect the expression levels of mGluR5 and PKCε in the L4-L6 DRGs. The results showed that the expression levels of mGluR5 and PKCε in the L4-L6 DRGs of the HP group were greatly increased 48 h after the 2nd injection compared with those in the L4-L6 DRGs of the N group. EA stimulation significantly inhibited the increase in mGluR5 and PKCε in the L4-L6 DRGs, which was significantly lower than that in the HP group and was not significantly different from that in the N group. However, sham EA had no effect on the expression levels of mGluR5 or PKCε, and the expression levels were not different from those of the HP group (P<0.05) (Figure 3E and F).
mGluR5 Is Involved in Pain Transition by Activating PKCε in the DRGs
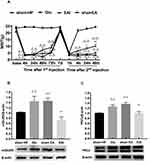
To verify whether mGluR5 plays an important role in hyperalgesic priming, glutamate was used in place of PGE2 for the 2nd injection. The results of repeated measures ANOVA met Mauchly’s test of Sphericity (P>0.05) and the within-subjects effects indicated that there was a significant difference over time (P<0.01) and between the different groups (P<0.01). There was a significant interaction between time and group (P<0.01). The data were normally distributed and analyzed using a one-way ANOVA. As shown in Figure 4A, the MWT of the rats in the Glu group was significantly decreased 1, 4, 24 and 48 h after glutamate injection (P<0.01). In addition, compared with that in the L4-L6 DRGs of the N group, the protein expression level of PKCε in the L4-L6 DRGs of the Glu group was significantly increased 48 h after the 2nd injection. The expression level of PKCε in the sham HP group was not different from that in the N group (P<0.01) (Figure 4B). All the above results indicated that mGluR5 may be involved in hyperalgesic priming by promoting the expression of PKCε. However, the relationship between mGluR5 and PKCε is unknown. MTEP, a selective inhibitor of mGluR5, was used to modulate the MWT of the model rats in an attempt to confirm the role mGluR5 plays in hyperalgesic priming and PKCε activation. MTEP was injected into the ipsilateral hind paw 5 min before the 2nd injection. The results of repeated measures ANOVA met Mauchly’s test of Sphericity (P>0.05) and the within-subjects effects indicated that there was a significant difference over time (P<0.01) and between the different groups (P<0.05). There was a significant interaction between time and group (P<0.01). The data were normally distributed and analyzed using a one-way ANOVA. It increased the MWT of the model rats compared to the HP group rats 4, 24 and 48 h after the 2nd injection (P<0.05) (Figure 4C). In addition, the protein expression levels of mGluR5 and PKCε in the L4-L6 DRGs of the HP+MTEP group were significantly decreased compared with those in the L4-L6 DRGs of the HP group (P<0.01) (Figure 4D and E). The above results suggested that the mGluR5-PKCε pathway plays a pivotal role in the process of pain transition.
EA Stimulation Exerts Regulatory Effects on Pain Transition Caused by Activating the mGluR5-PKCε Pathway in the DRGs
Finally, we observed whether EA stimulation has an effect on glutamate-induced hyperalgesic priming. The results of repeated measures ANOVA did not meet Mauchly’s test of Sphericity (P=0.006) and the within-subjects effects indicated that there was a significant difference over time (P<0.01) and between the different groups (P<0.01). There was a significant interaction between time and group (P<0.01). The data were normally distributed and analyzed using a one-way ANOVA. As shown in Figure 5A, there was an increase in the MWT of the EA I group compared with the Glu group 24 and 48 h after the 2nd injection (P<0.05) (Figure 5A). In addition, the Western blot results also confirmed that EA stimulation significantly inhibited the high expression of mGluR5 and PKCε in the L4-L6 DRGs of glutamate-induced hyperalgesic priming rats (P<0.01) (Figure 5B and C). The data suggested that EA stimulation can inhibit the transition from acute pain to chronic pain by inhibiting the mGluR5-PKCε pathway in the L4-L6 DRGs.
Discussion
In this study, the rat hyperalgesic priming model was established to investigate the potential mechanisms of pain transition. We found that the mGluR5 and PKCε were highly expressed in the ipsilateral L4-L6 DRGs. By screening the EA treatment options with different time points, we identified an optimized EA protocol to be the option for relieving hyperalgesia of model rats. In the terms of mechanisms, we found EA reduced the overexpression of mGluR5 and PKCε in DRGs of hyperalgesic priming rats. Besides, Glutamate can replace PGE2 in the 2nd injection to mimics the hyperalgesic priming model. Pharmacological blocking of mGluR5 with specific antagonist MTEP can reverse the hyperalgesic priming and decrease the protein expression of PKCε in DRGs. EA also reverse the hyperalgesic priming induced by mGluR5 injection and down-regulate the overexpression of PKCε in the DRGs. These results indicate that EA prevents the pain transition may involve in inhibiting the mGluR5-PKCε signaling pathway in DRGs.
Chronic pain is one of the major challenges in the clinic. Although many studies have focused on chronic pain, few effective treatment strategies without side effects have been developed in the last decade. Previous studies have proposed that preventing the transition from acute to chronic pain may be a new strategy for controlling chronic pain.41,42 In a previous study, we observed that EA can regulate pain transition in hyperalgesic priming model rats.30,31 Here, we further investigated the potential mechanisms underlying the analgesic and regulatory effects of EA on hyperalgesic priming. We found that EA not only produced an analgesic effect on chronic pain but also prevented pain transition. Furthermore, the analgesic and preventive effects of EA were cumulative. In addition, we found that the activation of mGluR5 was involved in hyperalgesic priming by activating PKCε in the DRGs. In addition, EA prevented pain transition via mechanisms that may involve inhibiting the mGluR5-PKCε signaling pathway in the DRGs.
Previous studies have demonstrated that PKCε is involved in sensitizing nociceptors and inducing mechanical hyperalgesia.43,44 In the current study, PKCε was increased in the DRGs of hyperalgesic priming rats, which is consistent with previous publications showing that PKCε plays an important role in the process of hyperalgesic priming.6,15,30,45 However, previous studies have specifically focused on changes in the expression of PKCε and its downstream targets in the DRGs upon hyperalgesic priming. In this study, we mainly evaluated whether upstream molecules of PKCε are involved in the process of hyperalgesic priming.
As a type of glutamate receptor expressed in small-diameter neurons in the DRGs,19,20 mGluR5 contributes to the regulation of neuronal growth, neuroprotection and excitotoxicity.46 It has been demonstrated that mGluR5 can be activated by glutamate released from damaged peripheral tissue and is involved in hyperalgesia. In addition, many researchers have found that there is a certain relationship between mGluR5 and PKCε.27,47 A previous study found that PKCε contributes to the process by which mGluR5 causes hyperalgesia.22 In this study, glutamate and MTEP were chosen to examine whether mGluR5 is involved in the process of hyperalgesic priming and PKCε activation. Our study found that glutamate can mimic hyperalgesic priming and increase PKCε expression in the DRGs. Furthermore, an mGluR5 antagonist reversed the hyperalgesic priming and PKCε expression in the DRGs induced by PGE2 injection. Therefore, mGluR5 is upstream of PKCε and induces the expression of PKCε in the DRGs of hyperalgesic priming rats.
Clinical and basic studies have confirmed that 2/100 Hz EA can treat various types of pain. Our previous study demonstrated that 2/100 Hz EA can regulate the MWT of hyperalgesic priming rats and inhibit the increasing expression of PKCε in the DRGs. In this study, we further investigated whether EA can regulate higher PKCε expression and the MWT. First, we found that the analgesic effect of EA can be divided into two phases. EA partly prevented pain transition when it was only administered during the period between carrageenan and PGE2 injection. EA produced a significant analgesic effect on the MWT of hyperalgesia priming rats when it was only given after PGE2 injection. In the current study, we ultimately used a classical strategy in which EA was administered after carrageenan injection until the end of the experiment. We observed that EA administration throughout the experimental period produced the greatest analgesic effect on hyperalgesic priming rats. This indicated that EA stimulation may have a cumulative effect on regulating pain transition. We further studied the effect of EA on PKCε and mGluR5 expression in the lumbar DRGs. EA significantly decreased the overexpression of mGluR5 and PKCε in the DRGs of hyperalgesic priming rats. Because mGluR5 induced hyperalgesic priming by activating PKCε, we observed the effect of EA on the mimic model established by carrageenan/glutamate injection. EA also significantly depressed the expression of mGluR5 and PKCε in the DRGs. All of the above results suggested that EA may regulate hyperalgesic priming by inhibiting the activation of mGluR5-PKCε in the DRGs. However, the phase during which EA administration mainly contributes to its effect and whether the administration of EA before PGE2 injection can prevent pathway activation are still open questions. We will further study them.
Conclusion
Our study suggests that the activation of the mGluR5-PKCε pathway in the DRGs plays a pivotal role in the pain transition. And EA can regulate pain transition that maybe related with the effect on inhibiting the activation of the mGluR5-PKCε signaling pathway in the DRGs.
Abbreviations
ANOVA, Analysis of variance; DRG, Dorsal root ganglia; EA, Electroacupuncture; Glu, Glutamate; HP, hyperalgesic priming; HRP, Horseradish peroxidase; mGluR5, Metabotropic Glutamate Receptors 5; MWT, Mechanical withdrawal threshold; N, normal; PKCε, Protein Kinase C epsilon; PGE2, Prostaglandin E2; PVDF, polyvinylidene difluoride.
Data Sharing Statement
Data and materials are available upon request to corresponding author.
Ethics Approval and Consent to Participate
All animal care and experimental studies were approved by the Animal Care and Welfare Committee of Zhejiang Chinese Medical University, Zhejiang, China (approval no. IACUC-20180319-12).
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bouhassira D, Lanteri-Minet M, Attal N, et al. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain. 2007;136(3):380–387. doi:10.1016/j.pain.2007.08.013
2. Saastamoinen P, Leino-Arjas P, Laaksonen M, et al. Socio-economic differences in the prevalence of acute, chronic and disabling chronic pain among ageing employees. Pain. 2005;114(3):364–371. doi:10.1016/j.pain.2004.12.033
3. Chen B, Li L, Donovan C, et al. Prevalence and characteristics of chronic body pain in China: a national study. Springerplus. 2016;5(1):938–944. doi:10.1186/s40064-016-2581-y
4. Lanas A, Carrera-Lasfuentes P, Arguedas Y, et al. Risk of upper and lower gastrointestinal bleeding in patients taking nonsteroidal anti-inflammatory drugs, antiplatelet agents, or anticoagulants. Clin Gastroenterol Hepatol. 2015;13(5):2034. doi:10.1016/j.cgh.2014.11.007
5. Harirforoosh S, Asghar W, Jamali F. Adverse effects of nonsteroidal antiinflammatory drugs: an update of gastrointestinal, cardiovascular and renal complications. J Pharm Pharm Sci. 2013;16(5):821–847. doi:10.18433/J3VW2F
6. Reichling DB, Levine JD. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci. 2009;32(12):611–618. doi:10.1016/j.tins.2009.07.007
7. Huang WY, Dai SP, Chang YC, et al. Acidosis mediates the switching of Gs-PKA and Gi-PKCepsilon dependence in prolonged hyperalgesia induced by inflammation. PLoS One. 2015:10. doi:10.1371/e0125022
8. Sun WH, Chen CC. Roles of proton-sensing receptors in the transition from acute to chronic pain. J Dent Res. 2016;95(2):135–142. doi:10.1177/0022034515618382
9. Gu Y, Li G, Huang LYM. Inflammation induces Epac-protein kinase C alpha and epsilon signaling in TRPV1-mediated hyperalgesia. Pain. 2018;159(11):2383–2393. doi:10.1097/j.pain.0000000000001346
10. Wang W, Ma X, Luo L, et al. Exchange factor directly activated by cAMP-PKCepsilon signalling mediates chronic morphine-induced expression of purine P2X3 receptor in rat dorsal root ganglia. Br J Pharmacol. 2018;175(10):1760–1769. doi:10.1111/bph.14191
11. Malek N, Pajak A, Kolosowska N, et al. The importance of TRPV1-sensitisation factors for the development of neuropathic pain. Mol Cell Neurosci. 2015;65:1–10. doi:10.1016/j.mcn.2015.02.001
12. Summer GJ, Puntillo KA, Miaskowski C, et al. TrkA and PKC-epsilon in thermal burn-induced mechanical hyperalgesia in the rat. J Pain. 2006;7(12):884–891. doi:10.1016/j.jpain.2006.04.009
13. Araldi D, Bogen O, Green PG, et al. Role of nociceptor toll-like receptor 4 (TLR4) in opioid-induced hyperalgesia and hyperalgesic priming. J Neurosci. 2019;39(33):6414–6424. doi:10.1523/JNEUROSCI.0966-19.2019
14. Ferrari LF, Levine E, Levine JD. Role of a novel nociceptor autocrine mechanism in chronic pain. Eur J Neurosci. 2013;37(10):1705–1713. doi:10.1111/ejn.12145
15. Ferrari LF, Bogen O, Levine JD. Role of nociceptor alphaCaMKII in transition from acute to chronic pain (hyperalgesic priming) in male and female rats. J Neurosci. 2013;33(27):11002–11011. doi:10.1523/JNEUROSCI.1785-13.2013
16. Aley KO, Messing RO, Mochly-Rosen D, et al. Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the isozyme of protein kinase C. J Neurosci. 2000;12(12):4680–4685.
17. Parada CA, Yeh JJ, Reichling DB, et al. Transient attenuation of protein kinase Cepsilon can terminate a chronic hyperalgesic state in the rat. Neuroscience. 2013;120(1):219–226. doi:10.1016/S0306-4522(03)00267-7
18. Araldi D, Ferrari LF, Levine JD. Repeated mu-opioid exposure induces a novel form of the hyperalgesic priming model for transition to chronic pain. J Neurosci. 2015;35(36):12502–12517. doi:10.1523/JNEUROSCI.1673-15.2015
19. Walker K, Reeve A, Bowes M, et al. mGlu5 receptors and nociceptive function II. mGlu5 receptors functionally expressed on peripheral sensory neurones mediate inflammatory hyperalgesia. Neuropharmacology. 2001;40(1):10–19. doi:10.1016/S0028-3908(00)00114-3
20. Dong L, Quindlen JC, Lipschutz DE, et al. Whiplash-like facet joint loading initiates glutamatergic responses in the DRG and spinal cord associated with behavioral hypersensitivity. Neurosci Res. 1997;28(1):49–57. doi:10.1016/s0168-0102(97)01175-9
21. deGroot J, Zhou S, Carlton SM. Peripheral glutamate release in the hindpaw following low and high intensity sciatic stimulation. Neuroreport. 2000;11(3):497–502. doi:10.1097/00001756-200002280-00014
22. Honda K, Shinoda M, Kondo M, et al. Sensitization of TRPV1 and TRPA1 via peripheral mGluR5 signaling contributes to thermal and mechanical hypersensitivity. Pain. 2017;158(9):1754–1764. doi:10.1097/j.pain.0000000000000973
23. Gan X, Wu J, Ren C, et al. Potentiation of acid-sensing ion channel activity by peripheral group I metabotropic glutamate receptor signaling. Pharmacol Res. 2016;107:19–26. doi:10.1016/j.phrs.2016.02.018
24. Masuoka T, Kudo M, Yoshida J, et al. Long-term activation of group i metabotropic glutamate receptors increases functional TRPV1-expressing neurons in mouse dorsal root ganglia. Front Cell Neurosci. 2016;10.
25. Shueb SS, Erb SJ, Lunzer MM, et al. Targeting MOR-mGluR5 heteromers reduces bone cancer pain by activating MOR and inhibiting mGluR5. Neuropharmacology. 2019;160:107690.
26. Hudson LJ, Bevan S, McNair K, et al. Metabotropic glutamate receptor 5 upregulation in A-fibers after spinal nerve injury: 2-methyl-6-(phenylethynyl)-pyridine (MPEP) reverses the induced thermal hyperalgesia. J Neurosci. 2002;22(7):2660–2668. doi:10.1523/JNEUROSCI.22-07-02660.2002
27. Vergouts M, Doyen PJ, Peeters M, et al. PKC epsilon-dependent calcium oscillations associated with metabotropic glutamate receptor 5 prevent agonist-mediated receptor desensitization in astrocytes. J Neurochem. 2017;141(3):387–399. doi:10.1111/jnc.14007
28. Liu Q, Liu Y, Bian J, et al. The preemptive analgesia of pre-electroacupuncture in rats with formalin-induced acute inflammatory pain. Mol Pain. 2019;15:174480691986652. doi:10.1177/1744806919866529
29. Yen CM, Wu TC, Hsieh CL, et al. Distal electroacupuncture at the LI4 acupoint reduces CFA-induced inflammatory pain via the brain TRPV1 signaling pathway. Int J Mol Sci. 2019;20(18):4471. doi:10.3390/ijms20184471
30. Wang SS, Sun HJ, Du JY, et al. Effect of electroacupuncture on pain transition and content of protein kinase cepsilon in dorsal root ganglia in hyperalgesia rats. Zhen Ci Yan Jiu. 2018;43(11):677–681. doi:10.13702/j.1000-0607.180212
31. Hu HY, Ding JW, Wu YQ, et al. Effect of electroacupuncture on pain reaction and content of proteinase-activated receptors 2 in dorsal root ganglion in hyperalgesia rats. Zhen Ci Yan Jiu. 2018;43(1):14–19. doi:10.13702/j.1000-0607.170302
32. Li Y, Yin C, X L, et al. Electroacupuncture alleviates paclitaxel-induced peripheral neuropathic pain in rats via suppressing TLR4 signaling and TRPV1 upregulation in sensory neurons. Int J Mol Sci. 2019;20(23):5917. doi:10.3390/ijms20235917
33. Weng ZJ, Wu LY, Zhou CL, et al. Effect of electroacupuncture on P2X3 receptor regulation in the peripheral and central nervous systems of rats with visceral pain caused by irritable bowel syndrome. Purinergic Signal. 2015;11(3):321–329. doi:10.1007/s11302-015-9447-6
34. Xiang X, Wang S, Shao F, et al. Electroacupuncture stimulation alleviates CFA-induced inflammatory pain via suppressing P2X3 expression. Int J Mol Sci. 2019;20(13):3248. doi:10.3390/ijms20133248
35. Lin YW, Hsieh C. Auricular electroacupuncture reduced inflammation-related epilepsy accompanied by altered TRPA1, pPKCalpha, pPKCepsilon, and pERk1/2 signaling pathways in kainic acid-treated rats. Mediators Inflamm. 2014;1–9.
36. Liu Y, Lin X, Fang J, et al. Involvement of MrgprC in electroacupuncture analgesia for attenuating CFA-induced thermal hyperalgesia by suppressing the TRPV1 pathway. Evid Based Complement Alternat Med. 2018;1–13.
37. Lin D, Kan Y, Qiao LN, et al. Effects of electroacupuncture at “Futu”(LI 18), etc. on pain threshold and cervico-spinal mGlu receptor 5/cAmp/CREB signaling in rats with neck incision pain. Zhen Ci Yan Jiu. 2012;37(3):191–196.
38. Ferrari LF, Bogen O, Reichling DB, et al. Accounting for the delay in the transition from acute to chronic pain: axonal and nuclear mechanisms. J Neurosci. 2015;35(2):495–507. doi:10.1523/JNEUROSCI.5147-13.2015
39. Parada CA, Reichling DB, Levine JD. Chronic hyperalgesic priming in the rat involves a novel interaction between cAMP and PKC? second messenger pathways. Pain. 2005;113(1):185–190. doi:10.1016/j.pain.2004.10.021
40. Chaplan SR, Bach FW, Pogrel JW, et al. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi:10.1016/0165-0270(94)90144-9
41. Pozek JP, Beausang D, Baratta JL, et al. The acute to chronic pain transition: can chronic pain be prevented? Med Clin North Am. 2016;100(1):17–30. doi:10.1016/j.mcna.2015.08.005
42. McGreevy K, Bottros MM, Raja SN. Preventing chronic pain following acute pain: risk factors, preventive strategies, and their efficacy. Eur J Pain Suppl. 2011;5(S2):365–372. doi:10.1016/j.eujps.2011.08.013
43. Wu DF, Chandra D, McMahon T, et al. PKCepsilon phosphorylation of the sodium channel NaV1.8 increases channel function and produces mechanical hyperalgesia in mice. J Clin Invest. 2012;122(4):1306–1315. doi:10.1172/JCI61934
44. Bogen O, Alessandri H, Chu C, et al. Generation of a pain memory in the primary afferent nociceptor triggered by PKCepsilon activation of CPEB. J Neurosci. 2012;32(6):2018–2026. doi:10.1523/JNEUROSCI.5138-11.2012
45. Aley KO, Messing RO, Mochly-Rosen D, et al. Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the epsilon isozyme of protein kinase C. J Neurosci. 2000;20(12):4680–4685. doi:10.1523/JNEUROSCI.20-12-04680.2000
46. Viwatpinyo K, Chongthammakun S. Activation of group I metabotropic glutamate receptors leads to brain-derived neurotrophic factor expression in rat C6 cells. Neurosci Lett. 2009;467(2):127–130. doi:10.1016/j.neulet.2009.10.020
47. Dong L, Quindlen JC, Lipschutz DE, et al. Whiplash-like facet joint loading initiates glutamatergic responses in the DRG and spinal cord associated with behavioral hypersensitivity. Brain Res. 2012;1461:51–63.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.